Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 15
A Review on Cutaneous and Musculoskeletal Manifestations of CLOVES Syndrome
Authors Öztürk Durmaz E , Demircioğlu D, Yalınay Dikmen P, Alanay Y, Alanay A, Demirkesen C, Tokat F, Karaarslan E
Received 29 November 2021
Accepted for publication 17 March 2022
Published 13 April 2022 Volume 2022:15 Pages 621—630
DOI https://doi.org/10.2147/CCID.S351637
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Jeffrey Weinberg
Emel Öztürk Durmaz,1 Deniz Demircioğlu,1 Pınar Yalınay Dikmen,2 Yasemin Alanay,3 Ahmet Alanay,4 Cüyan Demirkesen,5 Fatma Tokat,5 Ercan Karaarslan6
1Department of Dermatology, Acıbadem Mehmet Ali Aydınlar University School of Medicine, İstanbul, Turkey; 2Department of Neurology, Acıbadem Mehmet Ali Aydınlar University School of Medicine, İstanbul, Turkey; 3Department of Pediatrics, Acıbadem Mehmet Ali Aydınlar University School of Medicine, İstanbul, Turkey; 4Department of Orthopedics, Acıbadem Mehmet Ali Aydınlar University School of Medicine, İstanbul, Turkey; 5Department of Pathology, Acıbadem Mehmet Ali Aydınlar University School of Medicine, İstanbul, Turkey; 6Department of Radiology, Acıbadem Mehmet Ali Aydınlar University School of Medicine, İstanbul, Turkey
Correspondence: Emel Öztürk Durmaz, Acıbadem Maslak Hospital, Büyükdere Caddesi 40, Maslak, Istanbul, 34457, Turkey, Tel +90 (212) 304 46 19, Fax +90 (212) 286 15 66, Email [email protected]
Abstract: CLOVES syndrome is a novel sporadic mosaic segmental overgrowth syndrome, currently categorized under the canopy of PROS (PIK3CA-related overgrowth spectrum) disorders. All PROS disorders harbor heterozygous postzygotic activating somatic mutations involving the PIK3CA gene. As an upstream regulator of the PI3K/AKT/mTOR signal transduction pathway, activating mutations of PIK3CA gene commence in uncontrolled growth of cutaneous, vascular (capillaries, veins, and lymphatics), adipose, neural, and musculoskeletal tissues. The excessive growth is segmental, patchy, asymmetric, and confined to body parts affected by the mutation. The term ‘CLOVES’ is an acronym denoting congenital lipomatous overgrowth, vascular malformations, epidermal nevi and spinal (scoliosis) and/ or skeletal anomalies. The syndrome is characterized by an admixture of overgrown tissues, derived mainly from mesoderm and neuroectoderm. Among PROS disorders, CLOVES syndrome represents the extreme end of the spectrum with massive affection of almost the entire body. The syndrome might judiciously be treated with medications hampering with the PI3K/AKT/mTOR signal transduction pathway. This article aims at reviewing the cutaneous and musculoskeletal manifestations of CLOVES syndrome, as the paradigm for PROS disorders. CLOVES syndrome and other PROS disorders are still misdiagnosed, underdiagnosed, underreported, and undertreated by the dermatology community.
Keywords: CLOVES syndrome, PIK3CA-related overgrowth spectrum, cutaneous manifestations, port wine stains, lymphangiomas, lipomas, epidermal nevi
Definition and History
CLOVES syndrome (OMIM number 612918) is a recently described rare, sporadic (non-hereditary) complex mosaic overgrowth syndrome.1–3 It was initially described in 2007 by Sapp et al as a novel overgrowth syndrome and entitled as “CLOVE” syndrome.4 Although “CLOVE” syndrome had overlapping features with other overgrowth syndromes such as Proteus syndrome, the affected patients could not fulfill the established diagnostic criteria for the other disorders.5,6 Upon recognition of skeletal and spinal abnormalities (scoliosis) as part of this new syndrome, the nomenclature has later been revised by Alomari as ‘CLOVES’ syndrome.7 The term “CLOVES” is an acronym denoting congenital lipomatous overgrowth, vascular malformations, epidermal nevi and spinal (scoliosis) and/ or skeletal anomalies.1,2,8–13
Epidemiology
Because of its heterogeneous nature and rarity, the number of published cases of CLOVES syndrome is under 200 hitherto.11,14 The estimated incidence is less than 1:1,000,000.1,11,14,15 There is no gender predilection, and the syndrome has been reported in all races and ethnic groups.11,15 The disorder is congenital and has a prenatal onset; thus, it presents either at birth or in early childhood.3,5,11,15–17 Segmental overgrowth is noticeable in most afflicted patients by one year of age.18
Classification and Genetics
CLOVES syndrome is currently categorized as a disorder within the canopy of PIK3CA-Related Overgrowth Spectrum (PROS) (Box 1).3,5,8,11,15,19–30 The syndrome harbors postzygotic activating somatic mutations in PIK3CA (phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit alpha) gene mapped to chromosome 3q26.32, which encodes a 110-kD catalytic α subunit of PI3K (phosphoinositide 3-kinase).1,8,11,13,23,24,31,32 PI3K is a lipid kinase that converts phosphatidylinositol (4,5)-bisphosphate to phosphatidylinositol (3,4,5)- triphosphate and regulates cell proliferation, growth, and survival.13 A complex signaling pathway paves the way for activation of AKT1 (protein kinase B), and subsequently drives enhanced cell proliferation through mTOR1 (mammalian target of rapamycin).6,13 Activating mutations of PIK3CA commence in uncontrolled growth of predominantly mesoderm-derived (eg, adipose tissue, vascular and lymphatic tissues, muscle, bone) and neuroectoderm-derived tissues (eg, skin, brain cephalic connective tissue).1,3,6,8,18,23,27,32–35 The overgrowth is segmental, patchy, asymmetric, and confined to body parts affected by the mutation.1,14,32
 |
Box 1 PROS (PIK3CA-Related Overgrowth Spectrum) Disorders |
Clinical Findings
CLOVES syndrome displays a potpourri of malformations originating from cutaneous, vascular, lymphatic, fatty and bony tissues.5,24 Phenotypic expressivity and malformation severity might vary.1,5,8 As compared with other PROS disorders, CLOVES syndrome presents with a pleiotropic, divergent and a more severe constellation of findings.3
Cutaneous manifestations of CLOVES syndrome are summarized in Table 1.
 |
Table 1 Cutaneous and Musculoskeletal Manifestations of CLOVES Syndrome |
Congenital Lipomatous Overgrowth
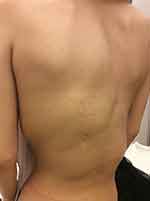
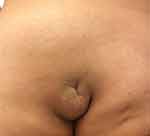
Congenital overgrowth of adipose tissue in all body regions and tissue spaces is typical in CLOVES syndrome.14,29 Asymmetric truncal/ thoracic hypertrophy is present at birth.14,32 The tumefactions, visible on the thoracic and abdominal wall as unilateral or bilateral masses, consist mainly of lipomatous and partly of lymphatic and vascular components.7 The truncal masses are detected commonly in the posterolateral chest wall and flank, but they may show variable contiguous extension to the anterior abdominal wall, groin, scrotum, retroperitoneum, mediastinum, pleura, paraspinal musculature, epidural spaces, gluteal area, and face.5–7,12,13,20,32,36–38 Some reports suggest that truncal overgrowth is predominantly left-sided and that unaffected areas display a pronounced paucity of adipose tissue (lipoatrophy) (Figure 1).39 The truncal lipomatous masses are generally covered by capillary, venous, lymphatic, or arteriovenous malformations and may elicit pain sensation.7,37 The lipomatous overgrowths are often infiltrative, recalcitrant and tend to recur following resection.7,40 Their infiltration into the paraspinal and intraspinal spaces may cause compression of the cord or nerve roots (Figure 2).7,37
Vascular Malformations
The frequency of vascular malformations in PROS disorders varies between 42 to 60%.15 Vascular malformations tend to overlie the lipomatous masses on the trunk, but they may extend to areas of skeletal overgrowth on the extremities as well.1,5,7,10,15,21 There is complex and combined deposition of vascular tissue in CLOVES syndrome with a blended mixture of capillary (low-flow and geographic), venous (superficial phlebectasia), lymphatic (macrocystic, microcystic or mixed), and arteriovenous malformations with or without arteriovenous fistulae (high-flow).1,7,9,12,14,21,27,36,38,39,41,42 The lesions might be solitary or multiple, localized or diffuse, superficial, or deeply located.41 Capillary and lymphatic malformations are the most common type of vascular malformations.15,21
From a dermatological point of view, capillary malformations in the form of port wine stains may be observed on the trunk, extremities, palms, soles, or fingertips as geographic stains.5,7,43 Pink to reddish flat port wine stains gradually acquire a purplish papulonodular appearance (cobble stoning) by adulthood. Although superficial microcystic lymphatic malformations (lymphangiomas/ lymphangiectasias) usually present as translucent blebs and blisters filled with a clear fluid on the skin (Figures 3 and 4), weeping may occur and connection of these lesions to the venous system may lead to intra-lesional bleeding, which may impart the lesions a reddish to purplish hue (hemolymphangiomas) (Figure 5).36,39,41
 |
Figure 3 (A) Translucent blister filled with a clear fluid, typical of superficial lymphangioma/lymphangiectasia. (B) Dermatoscopy revealing yellowish structureless areas. |
 |
Figure 5 (A) Hemolymphangioma/hemolymphangiectasia caused by intra-lesional bleeding into a lymphangioma/lymphangiectasia. (B) Dermatoscopy revealing vascular lacunes. |
Abnormal marginal veins (vein of Servelle/ lateral embryonal vein/ persistent embryonic vein) may be encountered both in Klippel-Trenaunay (KTS) and CLOVES syndromes and represent subdermal overgrowth of venous tissue, rather than signifying noninvoluting embryonic remnants.44 Therefore, the term “anomalous marginal vein” has been suggested as a more appropriate nomenclature.44 These phlebectasias may be detected in central, thoracic, cervical and extremity locations.2,3,17,36,44,45 Anomalous marginal veins possess a high risk of venous hypertension-related morbidity, deep vein thrombosis and potentially fatal pulmonary embolism.3,36,38,44–46 The risk of major thromboembolic events tends to correlate with the extent and severity of the vascular malformations and usually develop in the perioperative setting.2,36
Epidermal Nevi
The frequency of epidermal nevi in PROS disorders ranges from 11.4 to 46.6%.15 Epidermal nevus (particularly linear keratinocytic nevus) is a common, albeit not a universal feature, in CLOVES syndrome.42,47 Some port wine stains accompanying CLOVES syndrome have been erroneously reported as epidermal nevi in the pediatrics literature.40 In contrast to Proteus syndrome, connective tissue nevus (elastoma, collagenoma) is not a component of CLOVES syndrome.47
True epidermal nevi are either congenital or develop early in childhood as brown-gray velvety pigmented thickenings on the neck, abdomen, flank, or limbs.6,36 They may be limited to the areas of bony or lipomatous overgrowth or extend beyond the affected area, conforming to the lines of Blaschko.15 They show a progressive growth during childhood, acquire a papillomatous appearance by puberty and stabilize thereafter.47
Scoliosis and/or Skeletal Abnormalities
Skeletal malformations may be severely deforming and consist of varying degrees of scoliosis and asymmetric enlargement of skeletal structures on the extremities.6,7,13,37 Scoliosis may be congenital, or it may develop during childhood, sometimes because of lower limb asymmetry (Figure 1).36 Bony overgrowth most commonly affects the lower extremities; distal segment is involved more frequently than the proximal segment.7,39 As the disease progresses by time, proximal segment may also be involved.15,39 In contrast to Proteus syndrome, bone hypertrophy is not rapidly progressive or distorting in CLOVES syndrome.1
The list of reported skeletal and spinal abnormalities is shown in Table 1. Scoliosis is prominent and clinically apparent. Wide triangular feet with broad forefoot, splayed feet and toes (wide gaps between metatarsal heads), and sandal gap (widened first toe web) are striking orthopedic manifestations implying foot overgrowth (Figure 6).1,3,5,7,9,10,12,15–18,21,32,36,38–40,46 From a dermatological perspective, the soles and palms may have a furrowed and creased appearance and there may be overgrowth of palmar and plantar skin (Figure 7); however, there is no lobular hyperplasia and firm consistency as seen in cerebriform connective tissue nevus of Proteus syndrome.1,3,7,15,37
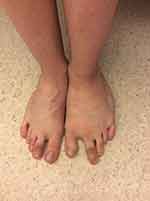 |
Figure 6 Wide triangular left foot, with prominent sandal gap deformity. |
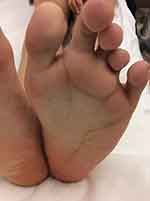 |
Figure 7 Furrowed and creased left sole. |
Others
Other cutaneous abnormalities reported in patients with CLOVES syndrome are presented in Table 1. A long list of systemic abnormalities, predominantly involving the neurological, vascular, visceral, ocular, and dental systems, have also been published. It is of note that Wilms tumor is the single malignancy meaningfully associated with CLOVES syndrome.48
Prognosis
CLOVES syndrome has a serious morbidity and a high mortality rate.2,35,49 The most dreaded clinical sequalae arise from vascular crises and compressive phenomena.50 Depending on the phenotypic expressivity, anatomic site, extent, and severity of the disorder, affected patients may suffer from functional impairment (eg, of walking, swallowing, feeding, breathing), disfigurement, chronic pain, recurrent superficial infections, coagulopathy (thrombotic and hemorrhagic), epilepsy, developmental delay, and organ dysfunction.1,11,18,27,35,36 The lipomatous, vascular and skeletal deformities tend to grow slowly throughout life and attempts at their removal by liposuction or surgical debulking are generally constrained by futile or temporary outcomes; since both lipomatous masses and skeletal structures tend to relapse and become more aggressive, infiltrative, or distortive following such interventions.5,14,15 In addition, debulking surgeries for lipomatous masses can be technically challenging, potentially life-threatening and associated with high morbidity.11,14 Skeletal and soft tissue deformities, along with excessive scarring left by surgeries, ruin social life and decrease life quality.1,51
Diagnosis
The diagnosis of CLOVES syndrome relies on clinical examination, radiologic imaging studies and genetic analysis.1,52 Due to the rarity, complexity, and substantial overlap among PROS disorders, a definitive diagnosis CLOVES syndrome may only be established through identification of the specific PIK3CA mutation in the affected tissues.1,3,11,15,19,22–24,26,31,34,39,46,52,53
Recently established diagnostic criteria for PROS disorders include the presence of somatic PIK3CA mutations, congenital or early disease onset, overgrowth of tissue that appears sporadic and mosaic (patchy, irregular), and features of ≥ 2 of the following: overgrowth of adipose, muscle, nerve, or skeletal tissue; vascular malformations (capillary, venous, arteriovenous malformation, lymphatic); and epidermal nevi.3,18,30 If a PIK3CA mutation could not be identified, then the diagnosis is considered as presumptive.3 Thus, failure to detect a hotspot PIK3CA mutation cannot exclude a diagnosis of a PROS disorder in an afflicted individual with telltale clinical stigmata.38,50
Radiologic imaging studies are crucial for uncovering the extent of deformities and assessing long-term prognosis; cranial, spinal, and skeletal X-rays (conventional radiography), USG of the abdomen (for Wilms tumor), Doppler USG, CT, MRI (for soft-tissue and bony hyperplasia or hypertrophy) and MR angiography (for vascular malformations) are usually performed on a case-by-case basis.1–3,12,15,21,52
Differential Diagnosis
The most important differential diagnostic considerations embrace Proteus syndrome, KTS and other PROS disorders (Box 1).1,7,12,14,20,21,25,27,40,46 The presentations in CLOVES syndrome and Proteus syndrome are strikingly similar and, in the past, many patients with CLOVES syndrome have been inaccurately diagnosed as Proteus syndrome.7,21 CLOVES syndrome and Proteus syndrome genetically harbor different mutation types (PIK3CA vs AKT1) and they may be clinically distinguished by the absence of cerebriform connective tissue nevi and visceral involvement in the former, and a postnatal onset of a severely distorting overgrowth and absence of truncal fatty-vascular overgrowth, paraspinal fast-flow lesions and acral abnormalities in the latter.5,7,12,21,31,38,39,52,54 Overgrowth in CLOVES syndrome is detailed as “ballooning” (gradual increase in the volume of the soft tissue mass), congenital, slowly progressive, mild, symmetric, and proportionate, while that in Proteus syndrome is acknowledged as distorted, postnatal, rapidly progressive, severe, asymmetric, and disproportionate (out of proportion to normal body growth).1,10,14,18,31,40,42,54 CLOVES syndrome and KTS may be distinguished by the absence of truncal involvement and preferential affection of the lower extremities in the latter.14,15,21,46 High-flow AVM and spinal/ paraspinal AVM are features of CLOVES syndrome and Parkes-Weber syndrome, but not that of KTS.55
Treatment
There is no specific cure for CLOVES syndrome.49 Until recently, patients with CLOVES syndrome were managed palliatively by multidisciplinary, staged debulking surgeries for lipomatous and skeletal overgrowths (surgical excision, extremity or finger/ toe amputation, surgical correction of scoliosis), and vascular interventional techniques (sclerotherapy for macrocystic lymphatic malformations, laser therapy for capillary malformations, coil embolization procedures for large venous malformations, superior vena cava filters for central and thoracic phlebectasias).2,12,14,15,18,20,21,35,36,38,40,50 Patients with low-flow malformations were prescribed anticoagulant medications for the prevention of thromboembolic disease.3,21,36
Identification of causative PIK3CA mutations empowered the use of older medications impeding the genetic pathway and the development of novel medications directly targeting the abnormal PIK3CA gene itself.20,34,49 Theoretically, such medications could halt the progressive overgrowth of tissues in PROS disorders.6 Sirolimus (rapamycin) and its congeners (everolimus, ridaforolimus, temsirolimus) are mTOR inhibitors, originally used as immunosuppressant and anti-tumor medications.5,18,21,27,35 Oral sirolimus has been shown to exert anti-angiogenic, anti-lymphangiogenic and anti-proliferative effects in 85% of adults and children with PROS disorders.11,21,27,35,50,56 It is effective in low-flow vascular malformations (microcystic lymphatic malformations, and venous malformations) and it can reduce the volume of lipomatous overgrowths as well.11,21,27,52,55,57 Currently, several PI3K pathway inhibitors, that have originally been developed for the treatment of cancers, are undergoing trials in PROS disorders.20,50 Among these are alpelisib (BYL719, Novartis), taselisib (GDC032, Roche), pictilisib (GDC-0941), copanlisib (BAY 80–6946) and dactolisib (dual PI3K /mTOR inhibitor).5,37,50 Based on limited evidence, a reduction in the size and volume of lipomatous, vascular and skeletal overgrowths might be attained with low doses of PI3K pathway inhibitors in PROS disorders.11,37,49,50,58,59 A pan AKT inhibitor miransertib (MK-7075, formerly ARQ092) is an emerging therapeutic option in the horizon.59–61 As the outcome of these treatments is suppressive, temporary, and partial (rather than curative, persistent, and complete), prolonged, and even life-long administration will be required in affected patients.18,27,50 Thus, despite encouraging clinical efficacy, long-term safety issues of these medications will pose a therapeutic dilemma for the clinician27,37,46,56,57,62 Nevertheless, the future holds promise for therapeutic optimism in CLOVES syndrome, KTS and other PROS disorders.
Funding
There is no funding to report.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Mahajan VK, Gupta M, Chauhan P, Mehta KS. Cloves syndrome: a rare disorder of overgrowth with unusual features - an uncommon phenotype? Indian Dermatol Online J. 2019;10(4):447–452. doi:10.4103/idoj.IDOJ_418_18
2. Alomari AI, Burrows PE, Lee EY, Hedequist DJ, Mulliken JB, Fishman SJ. CLOVES syndrome with thoracic and central phlebectasia: increased risk of pulmonary embolism. J Thorac Cardiovasc Surg. 2010;140(2):459–463. doi:10.1016/j.jtcvs.2010.04.023
3. Keppler-Noreuil KM, Rios JJ, Parker VE, et al. PIK3CA-related overgrowth spectrum (PROS): diagnostic and testing eligibility criteria, differential diagnosis, and evaluation. Am J Med Genet A. 2015;167A(2):287–295. doi:10.1002/ajmg.a.36836
4. Sapp JC, Turner JT, van de Kamp JM, van Dijk FS, Lowry RB, Biesecker LG. Newly delineated syndrome of congenital lipomatous overgrowth, vascular malformations, and epidermal nevi (CLOVE syndrome) in seven patients. Am J Med Genet A. 2007;143A(24):2944–2958. doi:10.1002/ajmg.a.32023
5. Akgumus G, Chang F, Li MM. Overgrowth syndromes caused by somatic variants in the phosphatidylinositol 3-Kinase/AKT/Mammalian target of rapamycin pathway. J Mol Diagn. 2017;19(4):487–497. doi:10.1016/j.jmoldx.2017.04.001
6. Vahidnezhad H, Youssefian L, Uitto J. Molecular genetics of the PI3K-AKT-mTOR pathway in genodermatoses: diagnostic implications and treatment opportunities. J Invest Dermatol. 2016;136(1):15–23. doi:10.1038/JID.2015.331
7. Alomari AI. Characterization of a distinct syndrome that associates complex truncal overgrowth, vascular, and acral anomalies: a descriptive study of 18 cases of CLOVES syndrome. Clin Dysmorphol. 2009;18(1):1–7. doi:10.1097/MCD.0b013e328317a716
8. Hanafusa H, Morisada N, Nomura T, et al. A girl with CLOVES syndrome with a recurrent PIK3CA somatic mutation and pancreatic steatosis. Hum Genome Var. 2019;6:31. doi:10.1038/s41439-019-0063-9
9. Gopal B, Keshava SN, Selvaraj D. A rare newly described overgrowth syndrome with vascular malformations-Cloves syndrome. Indian J Radiol Imaging. 2015;25(1):71–73. doi:10.4103/0971-3026.150166
10. Puvabanditsin S, Memon N, Chekmareva M, Di Stefano V, Mehta R. Cloves syndrome: a case report and perinatal diagnostic findings. Genet Couns. 2014;25(3):265–270.
11. Alomar S, Khedr RE, Alajlan S. CLOVES syndrome in a nine-month-old infant. Cureus. 2019;11(9):e5772. doi:10.7759/cureus.5772
12. Alomari AI, Chaudry G, Rodesch G, et al. Complex spinal-paraspinal fast-flow lesions in CLOVES syndrome: analysis of clinical and imaging findings in 6 patients. AJNR Am J Neuroradiol. 2011;32(10):1812–1817. doi:10.3174/ajnr.A2349
13. Vahidnezhad H, Youssefian L, Baghdadi T, et al. Phenotypic heterogeneity in PIK3CA-related overgrowth spectrum. Br J Dermatol. 2016;175(4):810–814. doi:10.1111/bjd.14618
14. Quinn KE, Infante J, Thorson W, Thorson CM. Unique case of congenital lipomatous overgrowth with vascular malformations, epidermal nevi, and skeletal/spinal anomalies syndrome in a pediatric patient. Cureus. 2020;12(9):e10737. doi:10.7759/cureus.10737
15. Mathew L, George R, Sudhakar S, Keshava SN, Fouzia NA. Clinical profile of overgrowth syndromes consistent with PROS (PIK3CA-Related Overgrowth Syndromes)-A case series. Indian Dermatol Online J. 2020;11(5):738–746. doi:10.4103/idoj.IDOJ_520_19
16. Fernandez-Pineda I, Fajardo M, Chaudry G, Alomari AI. Perinatal clinical and imaging features of CLOVES syndrome. Pediatr Radiol. 2010;40(8):1436–1439. doi:10.1007/s00247-010-1559-0
17. Damian L, Lebovici A, Pamfil C, Belizna C, Vulturar R. Rheumatoid arthritis and CLOVES syndrome: a tricky diagnosis. Diagnostics. 2020;10(7):467. doi:10.3390/diagnostics10070467
18. Keppler-Noreuil KM, Parker VE, Darling TN, Martinez-Agosto JA. Somatic overgrowth disorders of the PI3K/AKT/mTOR pathway & therapeutic strategies. Am J Med Genet C Semin Med Genet. 2016;172(4):402–421. doi:10.1002/ajmg.c.31531
19. Chang F, Liu L, Fang E, et al. Molecular diagnosis of mosaic overgrowth syndromes using a custom-designed next-generation sequencing panel. J Mol Diagn. 2017;19(4):613–624. doi:10.1016/j.jmoldx.2017.04.006
20. Loconte DC, Grossi V, Bozzao C, et al. Molecular and functional characterization of three different postzygotic mutations in PIK3CA-Related Overgrowth Spectrum (PROS) patients: effects on PI3K/AKT/mTOR signaling and sensitivity to PIK3 inhibitors. PLoS One. 2015;10(4):e0123092. doi:10.1371/journal.pone.0123092
21. Bertino F, Braithwaite KA, Hawkins CM, et al. Congenital limb overgrowth syndromes associated with vascular anomalies. Radiographics. 2019;39(2):491–515. doi:10.1148/rg.2019180136
22. Youssefian L, Vahidnezhad H, Baghdadi T, et al. Fibroadipose hyperplasia versus Proteus syndrome: segmental overgrowth with a mosaic mutation in the PIK3CA gene. J Invest Dermatol. 2015;135(5):1450–1453. doi:10.1038/jid.2015.15
23. Michel ME, Konczyk DJ, Yeung KS, et al. Causal somatic mutations in urine DNA from persons with the CLOVES subgroup of the PIK3CA-related overgrowth spectrum. Clin Genet. 2018;93(5):1075–1080. doi:10.1111/cge.13195
24. Kurek KC, Luks VL, Ayturk UM, et al. Somatic mosaic activating mutations in PIK3CA cause CLOVES syndrome. Am J Hum Genet. 2012;90(6):1108–1115. doi:10.1016/j.ajhg.2012.05.006
25. Rodriguez-Laguna L, Ibañez K, Gordo G, et al. CLAPO syndrome: identification of somatic activating PIK3CA mutations and delineation of the natural history and phenotype. Genet Med. 2018;20(8):882–889. doi:10.1038/gim.2017.200
26. Kuentz P, St-Onge J, Duffourd Y, et al. Molecular diagnosis of PIK3CA-related overgrowth spectrum (PROS) in 162 patients and recommendations for genetic testing. Genet Med. 2017;19(9):989–997. doi:10.1038/gim.2016.220
27. de Grazia R, Giordano C, Cossio L, Downey C, Delucchi Á, Kramer D. CLOVES syndrome: treatment with oral rapamycin. Report of two cases. Rev Chil Pediatr. 2019;90(6):662–667. doi:10.32641/rchped.v90i6.1025
28. Alsaedi SA, Qurashi O, Bajunaid M, Altalhi AA, Shawli AM. One of the first cases with PIK3CA-related Overgrowth Spectrum (PROS) in Saudi Arabia: a case report and literature review. Cureus. 2020;12(1):e6586. doi:10.7759/cureus.6586
29. Castiglioni C, Bertini E, Orellana P, et al. Activating PIK3CA somatic mutation in congenital unilateral isolated muscle overgrowth of the upper extremity. Am J Med Genet A. 2014;164A(9):2365–2369. doi:10.1002/ajmg.a.36651
30. Roth GM, Ferguson N, Wanat KA. Segmental epidermal nevus and mucosal neuromas associated with PIK3CA-related overgrowth spectrum disorder. JAAD Case Rep. 2018;4(10):1080–1082. doi:10.1016/j.jdcr.2018.08.023
31. Vahidnezhad H, Youssefian L, Uitto J. Klippel-Trenaunay syndrome belongs to the PIK3CA-related overgrowth spectrum (PROS). Exp Dermatol. 2016;25(1):17–19. doi:10.1111/exd.12826
32. Panteliades M, Silva CM, Gontijo B. What is your diagnosis? An Bras Dermatol. 2016;91(3):378–380. doi:10.1590/abd1806-4841.20165897
33. Collins M, Krochmalnek E, Alsubhi S, Srour M. Teaching NeuroImages: CLOVES syndrome. Neurology. 2021;96(10):e1487–e1488. doi:10.1212/WNL.0000000000010856
34. Lalonde E, Ebrahimzadeh J, Rafferty K, et al. Molecular diagnosis of somatic overgrowth conditions: a single-center experience. Mol Genet Genomic Med. 2019;7(3):e536. doi:10.1002/mgg3.536
35. Parker VER, Keppler-Noreuil KM, Faivre L, et al. Safety and efficacy of low-dose sirolimus in the PIK3CA-related overgrowth spectrum. Genet Med. 2019;21(5):1189–1198. doi:10.1038/s41436-018-0297-9
36. Acosta S, Torres V, Paulos M, Cifuentes I. CLOVES syndrome: severe neonatal presentation. J Clin Diagn Res. 2017;11(4):TR01–TR03. doi:10.7860/JCDR/2017/23801.9719
37. Manor J, Lalani SR. Overgrowth syndromes-evaluation, diagnosis, and management. Front Pediatr. 2020;8:574857. doi:10.3389/fped.2020.574857
38. Mirzaa G, Conway R, Graham JM
39. Keppler-Noreuil KM, Sapp JC, Lindhurst MJ, et al. Clinical delineation and natural history of the PIK3CA-related overgrowth spectrum. Am J Med Genet A. 2014;164A(7):1713–1733. doi:10.1002/ajmg.a.36552
40. Sarici D, Akin MA, Kurtoglu S, Tubas F, Sarici SU. A neonate with CLOVES Syndrome. Case Rep Pediatr. 2014;2014:845074. doi:10.1155/2014/845074
41. Del Pozo J, Gómez-Tellado M, López-Gutiérrez JC. Vascular malformations in childhood. Actas Dermosifiliogr. 2012;103(8):661–678. doi:10.1016/j.ad.2011.12.006
42. Biesbroeck L, Brandling-Bennett HA. Update on epidermal nevi and associated syndromes. Curr Derm Rep. 2012;1:186–194. doi:10.1007/s13671-012-0025-7
43. López-Gutiérrez JC, Redondo P, Ivars M. Fingertip capillary malformation and associated disorders: report of 9 cases. Pediatrics. 2017;140(1):e20162967. doi:10.1542/peds.2016-2967
44. Lim Y, Fereydooni A, Brahmandam A, Dardik A, Choate K, Nassiri N. Mechanochemical and surgical ablation of an anomalous upper extremity marginal vein in CLOVES syndrome identifies PIK3CA as the culprit gene mutation. J Vasc Surg Cases Innov Tech. 2020;6(3):438–442. doi:10.1016/j.jvscit.2020.05.013
45. Abu-Haniyeh A, Alkukhun L, Al-Natour M, Hassan M, Tonelli A. Arteriovenous malformation in CLOVES syndrome. Med Rep Case Stud. 2017;2:135.
46. Pandita A, Panghal A, Gupta G, Naranje KM. Overgrowth syndrome in neonates: a rare case series with a review of the literature. BMJ Case Rep. 2019;12(1):e225640. doi:10.1136/bcr-2018-225640
47. Garcias-Ladaria J, Cuadrado Rosón M, Pascual-López M. Epidermal nevi and related syndromes – part 1: keratinocytic nevi. Actas Dermosifiliogr. 2018;109(8):677–686. doi:10.1016/j.ad.2018.05.005
48. Weinstein B, Henderson-Jackson E, Cruse CW, Brohl AS. PIK3CA-Related Overgrowth Syndrome (PROS) and angiosarcoma: a case report. Eplasty. 2020;20:ic6.
49. Venot Q, Blanc T, Rabia SH, et al. Targeted therapy in patients with PIK3CA-related overgrowth syndrome. Nature. 2018;558(7711):540–546. doi:10.1038/s41586-018-0217-9
50. Madsen RR, Vanhaesebroeck B, Semple RK. Cancer-Associated PIK3CA Mutations in Overgrowth Disorders. Trends Mol Med. 2018;24(10):856–870. doi:10.1016/j.molmed.2018.08.003
51. Steiner JE, Cottrell CE, Streicher JL, et al. Scarring in patients with PIK3CA-related overgrowth syndromes. JAMA Dermatol. 2018;154(4):452–455. doi:10.1001/jamadermatol.2017.6189
52. Bertino F, Chaudry G. Overgrowth syndromes associated with vascular anomalies. Semin Roentgenol. 2019;54(4):349–358. doi:10.1053/j.ro.2019.06.005
53. Mirzaa G, Timms AE, Conti V, et al. PIK3CA-associated developmental disorders exhibit distinct classes of mutations with variable expression and tissue distribution. JCI Insight. 2016;1(9):e87623. doi:10.1172/jci.insight.87623
54. Gucev ZS, Tasic V, Jancevska A, et al. Congenital lipomatous overgrowth, vascular malformations, and epidermal nevi (CLOVE) syndrome: CNS malformations and seizures may be a component of this disorder. Am J Med Genet A. 2008;146A(20):2688–2690. doi:10.1002/ajmg.a.32515
55. Alomari AI, Orbach DB, Mulliken JB, et al. Klippel-trenaunay syndrome and spinal arteriovenous malformations: an erroneous association. AJNR Am J Neuroradiol. 2010;31(9):1608–1612. doi:10.3174/ajnr.A2167
56. Adams DM, Trenor CC, Hammill AM
57. Kinsler VA, Boccara O, Fraitag S, Torrelo A, Vabres P, Diociaiuti A. Mosaic abnormalities of the skin: review and guidelines from the European Reference Network for rare skin diseases. Br J Dermatol. 2020;182(3):552–563. doi:10.1111/bjd.17924
58. Adashek JJ, Kato S, Lippman SM, Kurzrock R. The paradox of cancer genes in non-malignant conditions: implications for precision medicine. Genome Med. 2020;12(1):16. doi:10.1186/s13073-020-0714-y
59. Venot Q, Canaud G. PIK3CA-related overgrowth spectrum: animal model and drug discovery. C R Biol. 2021;344(2):189–201. doi:10.5802/crbiol.50
60. Yan W, Zhang B, Wang H, et al. Somatic frameshift mutation in PIK3CA causes CLOVES syndrome by provoking PI3K/AKT/mTOR pathway. Hereditas. 2021;158(1):18. doi:10.1186/s41065-021-00184-y
61. Canaud G, Hammill AM, Adams D, Vikkula M, Keppler-Noreuil KM. A review of mechanisms of disease across PIK3CA-related disorders with vascular manifestations. Orphanet J Rare Dis. 2021;16(1):306. doi:10.1186/s13023-021-01929-8
62. Luu M, Vabres P, Devilliers H, et al. Safety and efficacy of low-dose PI3K inhibitor taselisib in adult patients with CLOVES and Klippel-Trenaunay syndrome (KTS): the TOTEM trial, a Phase 1/2 multicenter, open-label, single-arm study. Genet Med. 2021;23(12):2433–2442. doi:10.1038/s41436-021-01290-y
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.