Back to Journals » Clinical Ophthalmology » Volume 17
A Retrospective Study of Risk Factors Affecting Long-Term Outcomes Following Ab Interno Trabeculotomy and Goniotomy Concomitant with Phacoemulsification
Authors Okada N , Hirooka K, Onoe H, Tokumo K, Okumichi H , Kiuchi Y
Received 22 August 2023
Accepted for publication 14 November 2023
Published 22 November 2023 Volume 2023:17 Pages 3563—3568
DOI https://doi.org/10.2147/OPTH.S436594
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
Naoki Okada,1,2 Kazuyuki Hirooka,1,2 Hiromitsu Onoe,1,2 Kana Tokumo,1,2 Hideaki Okumichi,1,2 Yoshiaki Kiuchi1,2
1Department of Ophthalmology and Visual Science, Hiroshima University, Hiroshima, Japan; 2Hiroshima Eye Clinic, Hiroshima, Japan
Correspondence: Kazuyuki Hirooka, Department of Ophthalmology and Visual Science, Hiroshima University, 1-2-3 Kasumi, Minami-ku, Hiroshima, 734-8551, Japan, Tel +81-82-257-5247, Fax +81-82-257-5249, Email [email protected]
Purpose: To examine the potential risk factors affecting the long-term outcomes following a combination of phacoemulsification with ab interno trabeculotomy with the microhook (μLOT-Phaco) and goniotomy with the Kahook Dual Blade (KDB-Phaco).
Methods: For 12 months, we retrospectively examined a total of 100 eyes of 100 patients with primary open-angle glaucoma (POAG) and exfoliation glaucoma (EG) who had previously undergone surgery between December 2016 and December 2020. Patients with a preoperative intraocular pressure (IOP) ˂12 mmHg were excluded. Probability of success was calculated using the Kaplan–Meier method, with surgical failure defined as an IOP > 18 mmHg, < 20% IOP reduction or additional glaucoma surgery. The Cox proportional hazards model was used to examine the potential risk factors for failure, which included age, gender, type of glaucoma, surgical techniques, preoperative IOP, number and type of preoperative IOP-lowering medications, preoperative visual field mean deviation (MD) value, and axial length.
Results: For the 51 males and 49 females, mean preoperative age was 74.4 ± 9.0 years, with μLOT-Phaco performed in 44 and KDB-Phaco in 56 subjects. The type of glaucoma was POAG in 68 and EG in 32 eyes. Preoperative IOP was 20.5 ± 6.7 mmHg, while postoperative IOPs were 14.4 ± 4.2 mmHg, 13.7 ± 2.8 mmHg, and 14.6 ± 3.9 mmHg, respectively (P < 0.001). Significant decreases from the preoperative number of IOP-lowering medications (3.1 ± 1.2) were observed at 12, 24, and 36 months, respectively (1.2 ± 1.3, 1.6 ± 1.3, and 2.1 ± 1.4 (P < 0.001)). Probability of success at 12, 24, and 36 months postoperatively was 52.0%, 49.6%, and 47.7%, respectively. Lower preoperative IOP was shown to be a potential risk factor for surgical failure.
Conclusion: Long-term follow-ups showed IOP decreased in μLOT-Phaco and KDB-Phaco patients. Results suggest that patients with higher preoperative IOP may have better postoperative outcomes.
Keywords: MIGS, Kahook Dual Blade, intraocular pressure, glaucoma
Introduction
Glaucoma is one of the worldwide leading causes of blindness, with the number of glaucoma patients expected to continue to grow.1,2 Lowering intraocular pressure (IOP) is the only treatment proven to decrease the speed of the progression of the visual field defects.3 While conventional trabeculotomy requires complicated processes, trabeculotomy using the Tanito microhook (μLOT; Inami & Co., Ltd., Tokyo, Japan) and goniotomy using the Kahook Dual Blade (KDB; New World Medical, Rancho, Cucamonga, CA, USA) can be conducted via a corneal incision. This approach helps to eliminate both the complicated processes and scar formation. Many previous studies have reported on the efficacy of both μLOT and KDB.4–14 Furthermore, several previous studies have also reported finding no significant difference for both the short-term and long-term postoperative outcomes between μLOT-Phaco and KDB-Phaco.6,10,11
Esfandiari et al15 conducted a 5-year follow-up study on the postoperative outcomes of trabectome surgery and reported the potential risk factors for failure included lower baseline IOP, younger age, and higher central corneal thickness. Some studies have examined the potential risk factors that can affect the postoperative outcomes of μLOT and KDB, respectively.7,12 Tanito et al7 reported that older age, steroid glaucoma, developmental glaucoma, and absence of postoperative complications were associated with lower final IOPs. Iwasaki et al8 showed that lower preoperative IOP was associated with poor surgical outcomes. However, none of these studies identified the potential risk factors for surgical failure in conjunction with long-term follow-ups. As it is important to know the potential risk factors for surgical failure that can be found during long-term follow-ups, our present study examined the potential risk factors that can affect the long-term outcomes following the use of combination phacoemulsification with μLOT (μLOT-Phaco) and KDB (KDB-Phaco).
Materials and Methods
This retrospective study evaluated subjects that underwent μLOT-Phaco or KDB-Phaco at Hiroshima University Hospital, Hiroshima Eye Clinic, and Kagawa University Hospital from December 2016 to December 2020. Subjects that had been diagnosed as primary open-angle glaucoma (POAG) or exfoliation glaucoma (EG) were included, with all enrolled subjects having a postoperative observation period of at least 12 months. KDB with or without cataract surgery was more effective for eyes with EG as compared to that for POAG.6,16 Therefore, we included both EG and POAG patients. If bilateral surgery was performed, preoperative and follow-up data were collected only from the initial eye that underwent the surgery. All enrolled subjects were at least 20 years of age or older. The study protocol was approved by the Ethics Review Committee of Hiroshima University (E-2436). All subjects were given opt-out disclosure of research information in accordance with the Declaration of Helsinki.
Patients with a preoperative IOP ˂12 mmHg were excluded. Furthermore, patients with a history of glaucoma surgery and a history of ocular disease other than glaucoma and cataract were also excluded. The visual field mean deviation (MD) value was calculated by the Humphrey Field Analyzer (HFA; Carl Zeiss Meditec AG, Jena, Germany) using the 24–2 SITA standard program.
Surgical Techniques
Surgery was performed using the standard phacoemulsification technique with a 2.8-mm main port created on the temporal side of the cornea. The cataract surgical instrument used was the Whitestar Signature Pro (Johnson & Johnson; New Brunswick, NJ, USA). After removing the nucleus and cortex, the intraocular lens (IOL; DCB00V, Johnson & Johnson; XY1, HOYA, Tokyo, Japan) was inserted into the capsular bag. The trabecular meshwork was observed using a Hill surgical gonioprism (Ocular Instruments, Bellevue, WA, USA), with the microhook or KDB then inserted through the corneal wound and used to incise the Schlemm’s canal at the 120-degree nasal quadrant or 180-degree nasal and inferior quadrant. After removal of the remaining intraocular ophthalmic viscosurgical device, the procedure was completed by hydrating the port using a 27-gauge cannula.
Several surgeons performed the procedures, with each administering postoperative antibacterial therapy (1.5% levofloxacin), anti-inflammatory therapy (0.1% fluorometholone or 0.1% betamethasone, and nepafenac), and miosis therapy (2% pilocarpine) depending on the individual situation. Antibacterial therapy and steroid medications were continued for 1 month, while miosis therapy was continued for 1 to 3 months at the discretion of the surgeons. Most of the patients used nepafenac for 3 months. IOP-lowering medications were resumed in accordance with the postoperative IOP.
Statistical Analysis
All statistical analyses were performed using JMP software version 16 (SAS Inc., Cary, NC, USA). Corresponding t-tests were used to evaluate the preoperative IOP and the IOP at each of the 6-month time points that ranged from 6 to 48 months postoperatively. A Wilcoxon’s signed rank sum test was also used to evaluate the number of preoperative IOP-lowering medications from 6 to 48 months postoperatively. Preoperative IOP was defined as the IOP on the day of the examination when the decision was made to perform the surgery. Probability of success was calculated using the Kaplan–Meier method, with the criteria for surgical failure defined as an IOP >18 mmHg, <20% reduction in the IOP preoperatively or additional glaucoma surgery. A Cox proportional hazards model was utilized to evaluate potential risk factors for surgical failure, which included age, gender, type of glaucoma, surgical techniques, preoperative IOP, number and type of preoperative IOP-lowering medications including prostanoid receptor-related drugs (FP and EP2 prostanoid receptor agonist drugs), beta adrenergic blocker (β-blocker), carbonic anhydrase inhibitor (CAI), alfa-2 adrenergic agonist (α2 agonist), and rho-kinase inhibitor (ROCK inhibitor), preoperative visual field MD value, axial length, hyphema with niveau, and transient IOP elevation. Continuous variables are expressed as the mean ± standard deviation. A P value <0.05 was considered to be a statistically significant difference.
Results
Table 1 presents the clinical characteristics of the 100 eyes of the 100 patients enrolled in the study. The mean postoperative observation period was 34.1 ± 16.0 (12–63) months.
 |
Table 1 Clinical Backgrounds of Subjects |
While the mean preoperative IOP was 20.5 ± 6.7 mmHg, there was a significant decrease in the postoperative IOP to 14.6 ± 3.5 mmHg at 6 months, 14.4 ± 4.2 mmHg at 12 months, 13.7 ± 2.8 mmHg at 24 months, and 14.6 ± 3.9 mmHg at 36 months after surgery (P < 0.0001 at each point) (Table 2). While the mean number of preoperative IOP-lowering medications was 3.1 ± 1.2, the number of medications decreased to 1.0 ± 1.2 at 6 months, 1.2 ± 1.3 at 12 months, 1.6 ± 1.3 at 24 months, and 2.1 ± 1.4 at 36 months postoperatively (P < 0.0001 at each point) (Table 2).
 |
Table 2 Changes in IOP and Number of Preoperative and Postoperative IOP-Lowering Medication |
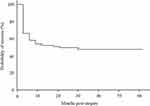
Probability of success was 52.0%, 49.6%, and 47.7% at 12, 24, and 36 months postoperatively (Figure 1). Postoperative complications included hyphema with niveau in 17.0% of cases, and transient high IOP exceeding 30 mmHg in 18.0% of the cases.
Table 3 shows the results of the univariate and multivariate analysis of risk factors using the Cox proportional hazards model. As for the univariate analysis, there were no significant differences detected for the age, gender, surgical technique, number and type of IOP-lowering medications, MD value, and axial length. However, there were significant differences detected for the type of glaucoma and the preoperative IOP. The risk ratio was 2.59 for POAG (P = 0.01), while the preoperative IOP (as the IOP increased by 1 mmHg) had a risk ratio of 0.90 (P < 0.001). For the multivariate analysis, there were no significant differences in the type of glaucoma detected. However, there were significant differences detected for the preoperative IOP. The risk ratio was 0.91 (P = 0.005) for the preoperative IOP (as the IOP increased by 1 mmHg).
 |
Table 3 Outcomes of Univariate and Multivariate Analysis |
Discussion
The usefulness of minimally invasive glaucoma surgery (MIGS) has recently been reported in numerous references.4–14 The present study analyzed data for two devices, the microhook and the KDB. Although previous studies have compared the microhook and KDB, they did not find any difference in the postoperative outcomes between the two devices.6,10–12 Therefore, we combined the outcomes of the two surgical methods in the current study. Furthermore, few reports have identified the risk factors that can affect the postoperative IOP, which is an important parameter for postoperative outcomes.7,12,15 Iwasaki et al12 reported on the postoperative long-term results and risk factors for KDB-Phaco. This previous study evaluated POAG and EG and concluded that the preoperative IOP was a risk factor. They defined surgical failure as an IOP >18 mmHg and <20% IOP reduction, which was the same as in the present study, and found a comparable risk ratio (0.89). Tanito et al7 reported on the mid-term results for 560 patients after μLOT, and found that the higher the preoperative IOP, the greater the rate of IOP reduction. Esfandiari et al15 previously evaluated the five-year outcomes of patients that underwent combined phacoemulsification and trabectome surgery and reported that a lower baseline IOP was associated with surgical failure. However, no other previous reports have analyzed the long-term risk factors for both KDB and μLOT. The risk factor derived from the present study was a low preoperative IOP, which is consistent with that reported in previous studies.12,15 A possible explanation for this is that a higher preoperative IOP may have resulted in a greater rate of IOP reduction, which would have made it easier to meet the definition of success.
In the univariate analysis, disease type was also detected as being a risk factor, as was the preoperative IOP. Furthermore, the presence of POAG resulted in having an increased risk ratio. Even so, there was a significantly higher preoperative IOP in the EG group (22.9 ± 1.1 mmHg) as compared to the POAG group (18.2 ± 0.8 mmHg) (P = 0.001). The 20% IOP reduction rate, which is part of the definition of surgical success in this study, may have contributed to the higher risk ratio in the POAG group with the lower mean preoperative IOP. There has also been a report that IOP-lowering medications may have some effect on the IOP, although this was not detected as a risk factor in the present study. Okuda et al17 reported that patients treated preoperatively with ripasudil hydrochloride hydrate (Kowa, Nagoya, Japan), which is the only rho-kinase (ROCK) inhibitor available in Japan, led to significantly better 1-year postoperative outcomes after μLOT as compared to patients who were not treated with the ROCK inhibitor. Thus, it is likely that ripasudil was not identified as a risk factor in the present study due to the inclusion of both μLOT and KDB data and the different backgrounds of the patients, all of whom had previously undergone concomitant cataract surgery. Goda et al18 have also suggested that the IOP-lowering effect of ripasudil may be useful in predicting the post-LOT outcomes. However, while the present study used ripasudil eye drops in 28 eyes preoperatively, it did not investigate the IOP-lowering effect before and after the addition of ripasudil eye drops, so this remains unclear. Okuda et al19 also reported that prolonged use of IOP-lowering medications before μLOT had a negative effect on postoperative outcomes. Since we did not investigate the duration of the use of preoperative IOP-lowering medications, this point also remains unclear.
Regarding the technique, KDB-Phaco was performed by one surgeon, while the μLOT-Phaco procedure was performed by multiple surgeons. One of the effects of having multiple surgeons involves the extent of the Schlemm’s canal incision. In the present study, the incision was set at 120 degrees or 180 degrees in accordance with the judgment of the surgeon. In our previous papers, we reported that the incision range affects corneal higher-order aberrations at 3 months postoperatively20 but does not affect the postoperative outcomes.13 Thus, it can be considered that the incision range had no effect in the present study.
The present study had some limitations. First, only POAG and EG were used as the disease types. In routine practice, the type of glaucoma surgery is determined based on the patient’s background, glaucoma stage, and IOP, with μLOT and KDB additionally performed for disease types other than POAG and EG when the conditions are met. Therefore, it is possible that the present results may not reflect those observed in actual clinical practice. Second, the duration of the preoperative eye drop use is unknown. The facilities where this study was conducted basically treated patients who were being treated at local clinics up until the time they were referred for surgery. We did not examine the duration of the use of the IOP-lowering medications, as it was not practical to evaluate all of the medical data from the local ophthalmology clinics, as this could lead to missing data during the data collection, thereby affecting the accuracy of the analysis.
In conclusion, the results of the present study showed that the use of μLOT-Phaco and KDB-Phaco reduced long-term IOP. These findings suggest that subjects with a higher preoperative IOP may have better postoperative outcomes.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Quigley HA, Broman AT. The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol. 2006;90(3):262–267. doi:10.1136/bjo.2005.081224
2. Tham YC, Li X, Wong TY, Quigley HA, Aung T, Cheng CY. Global prevalence of glaucoma and projections of glaucoma burden through 2040: a systematic review and meta-analysis. Ophthalmology. 2014;121(11):2081–2090. doi:10.1016/j.ophtha.2014.05.013
3. Heijl A, Leske MC, Bengtsson B, Hyman L, Bengtsson B, Hussein M; Early Manifest Glaucoma Trial Group. Reduction of intraocular pressure and glaucoma progression: results from the Early Manifest Glaucoma Trial. Arch Ophthalmol. 2002;120(10):1268–1279. doi:10.1001/archopht.120.10.1268
4. Tanito M, Sano I, Ikeda Y, Fujihara E. Short-term results of microhook ab interno trabeculotomy, a novel minimally invasive glaucoma surgery in Japanese eyes: initial case series. Acta Ophthalmol. 2017;95(5):e354–e360. doi:10.1111/aos.13288
5. Tanito M, Ikeda Y, Fujihara E. Effectiveness and safety of combined cataract surgery and microhook ab interno trabeculotomy in Japanese eyes with glaucoma: report of an initial case series. Jpn J Ophthalmol. 2017;61(6):457–464. doi:10.1007/s10384-017-0531-z
6. Omoto T, Fujishiro T, Asano-Shimizu K, et al. Comparison of 12-month surgical outcomes of ab interno trabeculotomy with phacoemulsification between spatula-shaped and dual-blade microhooks. Jpn J Ophthalmol. 2021;65(3):402–408. doi:10.1007/s10384-020-00806-4
7. Tanito M, Sugihara K, Tsutsui A, Hara K, Manabe K, Matsuoka Y. Midterm results of microhook ab interno trabeculotomy in initial 560 eyes with glaucoma. J Clin Med. 2021;10(4):814. doi:10.3390/jcm10040814
8. Seibold LK, Soohoo JR, Ammar DA, Kahook MY. Preclinical investigation of ab interno trabeculectomy using a novel dual-blade device. Am J Ophthalmol. 2013;155(3):524–529. doi:10.1016/j.ajo.2012.09.023
9. Sieck EG, Epstein RS, Kennedy JB, et al. Outcomes of Kahook Dual Blade goniotomy with and without phacoemulsification cataract extraction. Ophthalmol Glaucoma. 2018;1(1):75–81. doi:10.1016/j.ogla.2018.06.006
10. Aoki R, Hirooka K, Goda E, et al. Comparison of surgical outcomes between microhook ab interno trabeculotomy and goniotomy with the Kahook Dual Blade in combination with phacoemulsification: a retrospective comparative case series. Adv Ther. 2021;38(1):329–336. doi:10.1007/s12325-020-01543-3
11. Okada N, Hirooka K, Onoe H, Okumichi H, Kiuchi Y. Comparison of mid-term outcomes between microhook ab interno trabeculotomy and goniotomy with the Kahook Dual Blade. J Clin Med. 2023;12(2):558. doi:10.3390/jcm12020558
12. Iwasaki K, Kakimoto H, Orii Y, Arimura S, Takamura Y, Inatani M. Long-term outcomes of a Kahook Dual Blade procedure combined with phacoemulsification in Japanese patients with open-angle glaucoma. J Clin Med. 2022;11(5):1354. doi:10.3390/jcm11051354
13. Okada N, Hirooka K, Onoe H, Murakami Y, Okumichi H, Kiuchi Y. Comparison of efficacy between 120° and 180° schlemm’s canal incision microhook ab interno trabeculotomy. J Clin Med. 2021;10(14):3181.
14. Fliney GD, Kim E, Sarwana M, et al. Kahook Dual Blade versus trabectome (KVT): comparing outcomes in combination with cataract surgery. Clin Ophthalmol. 2023;17:145–154. doi:10.2147/OPTH.S391527
15. Esfandiari H, Shah P, Torkian P, et al. Five-years clinical outcomes of combined phacoemulsification and trabectome surgery at a single glaucoma center. Graefes Arch Clin Exp Ophthalmol. 2019;257(2):357–362. doi:10.1007/s00417-018-4146-y
16. Wakil SM, Birnbaum F, Vu DM, McBurney-Lin S, ElMallah MK, Tseng H. Efficacy and safety of a single-use dual blade goniotomy: 18-month results. J Cataract Refract Surg. 2020;46:1408–1415. doi:10.1097/j.jcrs.0000000000000263
17. Okuda M, Mori S, Ueda K, et al. Favorable effect of ripasudil use on surgical outcomes of microhook ab interno trabeculotomy. Graefes Arch Clin Exp Ophthalmol. 2023;261:2603–2610. doi:10.1007/s00417-023-06040-1
18. Goda E, Hirooka K, Mori K, Kiuchi Y. Intraocular pressure-lowering effects of Ripasudil: a potential outcome marker for Trabeculotomy. BMC Ophthalmol. 2019;19(1):243. doi:10.1186/s12886-019-1253-4
19. Okuda M, Mori S, Takano F, et al. Association of the prolonged use of anti-glaucoma medications with the surgical failure of ab interno microhook trabeculotomy. Acta Ophthalmol. 2022;100(6):e1209–e1215. doi:10.1111/aos.15090
20. Onoe H, Hirooka K, Okumichi H, Murakami Y, Kiuchi Y. Corneal higher-order aberrations after microhook ab interno trabeculotomy and goniotomy with the Kahook Dual Blade: preliminary early 3-month results. J Clin Med. 2021;10(18):4115. doi:10.3390/jcm10184115
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.