Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 15
A Retrospective Analysis of the Relationship Between 25-OH-Vitamin D and Diabetic Foot Ulcer
Authors Wang F, Zhou L, Zhu D, Yang C
Received 19 January 2022
Accepted for publication 27 April 2022
Published 3 May 2022 Volume 2022:15 Pages 1347—1355
DOI https://doi.org/10.2147/DMSO.S358170
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Prof. Dr. Muthuswamy Balasubramanyam
Fenglin Wang,1,2 Luyao Zhou,1 Di Zhu,2 Caizhe Yang2
1Hebei North University, Zhangjiakou, Hebei, 075000, People’s Republic of China; 2Department of Endocrinology, Air Force Medical Center, Beijing, 100142, People’s Republic of China
Correspondence: Caizhe Yang; Di Zhu, Department of Endocrinology, Air Force Medical Center, Beijing, 100142, People’s Republic of China, Tel +86-1066928242 ; +86-1066926242, Email [email protected]; [email protected]
Background: The fat-soluble molecule vitamin D has attracted much attention since its pleiotropism was discovered. Its effectiveness can be attributed to the presence of vitamin D receptors in most of the body’s tissues. Based on the classical role of vitamin D in regulating calcium and phosphorus metabolism and maintaining bone health, the role of vitamin D in immunity, type 2 diabetes mellitus (T2DM), tumor and cardiovascular diseases has been further discovered. Some experiments have shown that vitamin D can restore the production of antimicrobial peptides (AMP) in primary diabetic foot ulcer (DFU) cells, which can improve in vitro wound healing, indicating its potential therapeutic use in DFU therapy. In addition, vitamin D can also inhibit the secretion of T-helper type 1 (Th1) cytokines IFN-Y and IL-2 while stimulating the production of Th2 cytokines, thereby promoting wound healing.
Objective: To investigate the relationship between 25-OH-vitamin D level and DFU in diabetes mellitus (DM) patients, and to provide a theoretical basis for the early prevention and treatment of DFU.
Methods: The clinical data of 429 hospitalized patients with DM were retrospectively analyzed in this case–control study. The patients were divided into the DFU group (n = 242) and non-DFU group (n = 187). Fasting venous blood was drawn from all subjects to detect serum 25-OH-vitamin D levels and blood biochemical parameters, the difference of parameters between DFU group and non-DFU group were analyzed, and the risk factors of DFU were analyzed by logistic regression.
Results: The difference between the two groups in age, DM duration, gender, diastolic blood pressure, serum creatinine, total cholesterol, triglyceride, high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, alanine aminotransferase, aspartate aminotransferase, albumin, white blood cell count, hemoglobin, hematocrit, 25-OH-vitamin D was statistically significant (p < 0.05). Multivariate logistic regression analysis showed that 25-OH-vitamin D is an independent protective factor for DFU [OR 95%, CI 0.984 (0.969, 0.998), p < 0.05]. 25-OH-vitamin D nutrition status distribution was different between non-DFU group and DFU group (P < 0.05). Vitamin D deficiency (< 50 nmol/L) accounted for 86.78% of all DFU patients, which was only 74.33% in non-DFU patients. The 25-OH-vitamin D levels of DFU patients from Wagner Grades 1 to 5 showed a downward trend (p < 0.01).
Conclusion: In conclusion, our study confirms that 25-OH-vitamin D is closely correlated with DFU and that 25-OH-vitamin D is an independent protective factor for DFU. Therefore, vitamin D screening or supplementation might be beneficial to prevent DFU and improve the prognosis of DM patients.
Keywords: 25-OH-vitamin D, diabetic foot ulcer, vitamin D, diabetes mellitus
Introduction
Vitamin D deficiency is common worldwide. Vitamin D is a fat-soluble vitamin that is essential for numerous physiological functions, such as calcium/phosphorus homeostasis, bone metabolism, and stimulating insulin secretion1 and increasing insulin sensitivity.2 Serum 25-OH-vitamin D levels are the most common type of vitamin D in the body and are currently used as a gold index to evaluate vitamin D nutritional status.3 Vitamin D deficiency is defined as a 25-OH-vitamin D level of less than 20 ng/mL (50 nmol/L), whereas relative vitamin D insufficiency is defined as a 25-OH-vitamin D level ranging from 20 to 30 ng/mL (50–75 nmol/L). Vitamin D sufficiency was defined as a 25-OH-vitamin D level >30 ng/mL (75 nmol/L).4
Diabetes Mellitus (DM) has emerged as a global health issue, with a high prevalence globally. DM has a global incidence of 8.8% in 2017 and is expected to increase to 9.9% by 2045.5 Diabetic foot ulcer (DFU) is one of the most common and serious complications of DM, affecting 10% to 15% of DM patients during their lifetime.6,7 DFU is characterized by a long course of disease, high medical cost and a high risk of disability. Recent studies have shown that 25-OH-vitamin D deficiency is closely related to DM.8–10 More and more evidence has confirmed that vitamin D is not only involved in calcium and phosphorus metabolism but also in the occurrence and development of DM and its complications, such as diabetic retinopathy11,12 and diabetic peripheral neuropathy.13 The extraosseous advantage of vitamin D supplementation in preventing the development of early diabetic nephropathy was demonstrated in the research by Mahapatra.14 However, whether vitamin D is involved in the occurrence and development of DFU remains controversial. Razzaghi et al15 demonstrated that vitamin D supplementation for 12 weeks resulted in a significant improvement in DFU wound evolution, including ulcer length, width, depth, and erythema rate. Afarideh et al16 reported no difference in vitamin D levels between Iranian DFU patients and diabetic patients without DFU.
There are few studies on the relationship between vitamin D levels and DFU in the Chinese population. The main purpose of this study was to investigate the prevalence of vitamin D deficiency in Chinese inpatients with DM, and the relationship between serum 25-OH-vitamin D levels and DFU, in order to provide clinical evidence for the prevention and treatment of DFU.
Methods
Patients and Methods
A retrospective analysis was conducted on 429 DM inpatients, including 242 patients with DFU (DFU group) and 187 patients without DFU (non-DFU group), from January 2019 to October 2021 at the endocrinology department of the Air Force Medical Center, PLA. This study was conducted with the approval from the Ethics Committee of Air Force Medical Center, PLA (No.2021-100-PJ01), written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements. The diagnostic criteria of DM and DFU were based on the American Diabetes Association classification and the World Health Organization.17 DM is defined as a group of metabolic diseases characterized by hyperglycemia resulting from defects in insulin secretion, insulin action, or both, according to the American Diabetes Association.18 DFU is defined as “ulceration of the foot (distally from the ankle and including the ankle) associated with neuropathy and different grades of ischemia and infection” according to the World Health Organization.17 All patients included in the study were required to meet the following conditions: (1) older than 18 years old; (2) diagnosed with DM. Patients were divided into DFU group or non-DFU group according to whether they had DFU. The exclusion criteria were as follows: (1) severe impairment of consciousness, or poor general condition, (2) complicated by malignant tumor, serious heart, liver and kidney failure, (3) a history of diseases that affect serum 25-OH-vitamin D levels, such as thyroid and parathyroid disease, osteoporosis, and bone fractures, and (4) history of taking medications that affect serum 25-OH-vitamin D levels, such as calcium, vitamin D, oral contraceptives, glucocorticoids, and so on.
Data Collection
The following clinical data of the patients were retrospectively analyzed: gender, age, body mass index (BMI), smoking history, alcohol consumption history, hypertension history, coronary heart disease history, systolic blood pressure (SBP), diastolic blood pressure (DBP), duration of DM, glycerogelatin hemoglobin (HbA1c), fasting plasma glucose (FPG), total cholesterol (TC), triglyceride (TG), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), alanine aminotransferase (ALT), aspartate aminotransferase (AST), blood urea nitrogen (BUN), serum creatinine (Scr), serum uric acid (SUA), albumin (ALB), white blood cell count (WBC), hemoglobin (Hb), hematocrit (HCT) and 25-OH-vitamin D. The severity of DFU was assessed by Wagner classification.
Blood samples were collected from the patients after 8 hours of fasting. FPG, TC, TG, HDL-C, LDL-C, ALT, AST and SUA were measured by Hitachi 7600 automatic biochemical analyzer. HbA1c was detected by HIGH performance liquid chromatography (HPLC) with bio-RAD glycosylated hemoglobin detector and original reagent. The level of 25-OH-vitamin D in serum was detected by electrochemiluminescence immunoassay (Roche E601 analyzer). All test indicators are completed by the Laboratory of Air Force Medical Center, and the instruments and equipment of the laboratory are regularly calibrated and maintained by professionals. All test operations are standardized, test reagents are within the shelf life, and the test results are accurate and reliable.
Statistical Analysis
All analyses were conducted using Statistical Product and Service Solutions 23.0 (SPSS 23.0) or GraphPad Prism 8. Data for categorical variables were presented as numbers (%). Shapiro–Wilk test was used to determine the normality of continuous data, and mean ± standard deviation (x±s) was used to describe normally distributed data, while median and interquartile range (IQR, 25–75%) were used to describe non-normally distributed data. The biochemical and hematological parameters between the two groups were compared using the Student’s t-test (for normally distributed data), Mann–Whitney test (for non-normally distributed data) or X2 test (for categorical variables). Comparison of 25-OH-vitamin D levels among five Wagner Grades was performed by nonparametric analysis using a Kruskal–Wallis test with post-hoc Dunn multiple comparison tests. Multivariate logistic regression was used to analyze the risk factors of DFU, and the OR value and 95% CI were calculated. The correlation between 25-OH-vitamin D and other clinical parameters was examined using Spearman correlation analysis. A P-value of less than 0.05 was considered statistically significant.
Results
Characteristics of Study Participants in the Two Groups
A total of 429 diabetic patients (187 non-DFU patients and 242 DFU patients) were included in this study. Baseline biochemical and hematological parameters between the two groups were compared. Age, DM duration, male percentage, Scr and WBC of the DFU group were higher than the non-DFU group (p < 0.05). The levels of DBP, TC, TG, HDL-C, LDL-C, ALT, AST, ALB, Hb, HCT, 25-OH-vitamin D in DFU group were lower than the non-DFU group (p < 0.05). No significant differences in other indices were detected (P > 0.05, Table 1).
 |
Table 1 Baseline Characteristics of Participants in the Two Groups |
Logistic Regression Analysis for the Risk of DFU
To further determine the relationship between serum 25-OH-vitamin D levels and DFU, multivariate logistic regression analysis was performed. Considering the effects of parameters that were different between non-DFU group and DUF group on diabetic foot, they were taken into account in multivariate logistic regression analysis. For logistic regression analysis, age, DM duration, gender, DBP, Scr, TC, TG, HDL-C, LDL-C, ALT, AST, WBC, ALB, Hb, HCT and 25-OH-vitamin D were used as independent variables, while DFU (absent = 0, present = 1) was utilized as the dependent variable. Multivariate logistic regression analysis showed that WBC (odds ratio [OR] 1.381 [95% CI 1.168–1.632], p = 0.000) and DM duration (odds ratio [OR] 1.052 [95% CI 1.016–1.090], p = 0.005) were significant risk factors for DFU (p < 0.05), whereas 25-OH-vitamin D (odds ratio [OR] 0.984 [95% CI 0.970–0.999], p = 0.035), ALB (odds ratio [OR] 0.907 [95% CI 0.836–0.985], p = 0.021), TG ([OR] 0.046 [95% CI 0.277–0.764], p = 0.003) and HDL-C ([OR] 0.042 [95% CI 0.009–0.199], p = 0.000) were protective factors of DFU, as shown in Table 2.
 |
Table 2 Multivariate Logistic Regression Analysis for the Risk of DFU |
Comparison of Vitamin D Nutrition Status Distribution Between the Two Groups
In the non-DFU group, 139 cases (74.33%) were vitamin D deficiency, 41 cases (21.93%) were vitamin D insufficiency, and 7 cases (3.74%) were vitamin D sufficiency. However, in the DFU group, 210 cases (86.78%) were vitamin D deficiency, 24 cases (9.91%) were vitamin D insufficiency, and 8 cases (3.31%) were vitamin D sufficiency. The distribution of vitamin D nutrition status was significantly different between the non-DFU group and the DFU group (p < 0.05), as shown in Table 3.
 |
Table 3 Comparison of 25-OH-Vitamin D Nutrition Status Between Non-DFU Group and DFU Group |
Wagner Classification and 25-OH-Vitamin D Levels
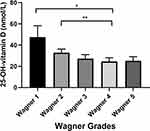
DFU patients were divided into 5 subgroups according to Wagner grading system. As shown in Figure 1, there was a significant difference of 25-OH-vitamin D levels among the 5 subgroups (p < 0.01). Multiple comparisons revealed that 25-OH-vitamin D levels of Wager Grades 1 and 2 were significantly higher than Wager Grade 4. There was a downward trend of 25-OH-vitamin D levels with deterioration of DFU.
Correlation Analysis Between 25-OH-Vitamin D and Other Parameters
Spearman correlation analysis revealed that 25-OH-vitamin D level was adversely related to hypertension history, HbA1c, TC, WBC and DFU (r = −0.098, −0.146, −0.115, −0.004, −0.217, p < 0.05), and positively correlated with ALT, ALB, Hb, and HCT (r value = 0.210, 0.216, 0.171, 0.171, p < 0.05). However, 25-OH-vitamin D level was not related to gender, age, DM duration, smoking history, drinking history, BMI, FPG, BUN, SCr, SUA, TG, HDL-C, LDL-C, AST and coronary heart disease history (p > 0.05), as shown in Table 4.
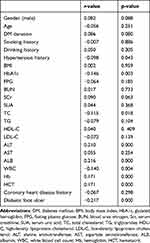 |
Table 4 The Correlation Between 25-OH-Vitamin D and Clinical Parameters in DM Patients |
Discussion
Vitamin D is an essential substance for the human body, and skin synthesis (90%) and intestinal absorption (10%) are the two main sources of vitamin D.19 Vitamin D must be hydroxylated into 25-OH-vitamin D in the liver before being converted into 25(OH)2D in the kidneys.3 Clinical routine measurement of serum 25-OH-vitamin D levels is used to assess vitamin D levels in the body, partly because the level of 25-OH-vitamin D in the blood is much higher than that of 1,25 (OH)2D. On the other hand, the detection period of 1.25 (OH)2D is long and the detection method is complex. The concentration of human serum 25-OH-vitamin D in the winter is lower than that in the summer due to geographical location, physiological factors, lifestyle, and a number of other factors. People who live farther from the equator have lower levels of vitamin D than those who live closer to the equator. However, studies on postmenopausal women and other populations have shown that vitamin D deficiency (25-OH-vitamin D < 30 ng/mL) is prevalent in Southeast Asia, and the average serum 25-OH-vitamin D level is low in most areas of China with relatively less sunshine in the winter and spring.20
The observed correlation between vitamin D deficiency and DM has received a lot of attention in recent years.21 Tang et al22 found that the vitamin D levels in the DF group were significantly lower than that in the non-DF group. Dai et al23 involving 51 patients investigated the relationship between 25-OH-vitamin D and DFU, and the results showed that 25-OH-vitamin D levels in DFU group were significantly lower than non-DFU group. A meta-analysis by Dai et al24 to evaluate the association between vitamin D deficiency and DFU reported a significant reduction in vitamin D levels in DFU group when compared with non-DFU group. Nevertheless, Afarideh et al16 came to contrary conclusions in Iranian patients. In a cross-sectional study reported in India in 2019, they found no significant difference in serum vitamin D levels in diabetic patients with and without foot infection.25 In this study, we investigated the relationship between serum 25-OH-vitamin D and DFU, and found 25-OH-vitamin D level is an independent protective factor for DFU. DFU is one of the most severe and debilitating complications of DM, being the leading cause of lower extremity amputations, reducing the quality of life26 and increasing mortality risk by 2.5-fold.27 It is often characterized by severe infection, high morbidity and mortality, with enormous social, psychological and economic consequences.27 The pathological mechanism of vitamin deficiency in DFU patients is as follows: one of the risk factors of neurotic foot ulcer in DM is serum vitamin D deficiency. Vitamin D deficiency or insufficiency can aggravate islet size through humoral and cellular immune mechanisms cell apoptosis, resulting in blood glucose level control instability. Vitamin D deficiency causes nerve growth factor (NGF) decrease and nutritional disorders, triggering the nervous system inflammatory response and accelerating the occurrence and development of neuropathy. Patients with DFU have a corresponding reduction in activity and spend less time in the sun, which leads to vitamin D deficiency or insufficiency. Vitamin D can inhibit the secretion of the T helper type 1 cytokines IFN-γ and IL-2 while stimulating the production of Th2 cytokines, which may promote wound healing.28 Kurian et al29 have shown that vitamin D influences multiple phases of wound healing and thereby accelerates the process. It modulates various cells involved in proliferation and remodeling phases. Vitamin D also enhances the expression of antimicrobial peptides that help eliminate the microbes, as well as suppresses the proinflammatory responses while enhancing the anti-inflammatory responses.
A meta-analysis by Pei et al30 assessed the effects of lipids and lipoproteins on diabetic foot development in patients with DM. The authors conclude that diabetic foot is associated with reduced HDL cholesterol levels. Our investigation yielded similar results. We also found that the DFU group had lower HDL-C and TG levels than the non-DFU group (p < 0.05). A multivariate logistic regression analysis showed that HDL-C, TG and albumin were independent protective factors of DFU (p < 0.05). Ansell et al31 reported the anti-inflammatory effects of HDL-C with a decrease in inflammatory cytokines and vascular leukocyte adhesion molecules, participation in innate immunity and prevention of LDL oxidation. In recent years, there has been an increase in the number of researches on the impact of nutrition on DFU development and healing.32,33 Brooke’s study34 found that among DFU patients, albumin levels were significantly different between those requiring amputation and those without amputation, and the results showed that DFU amputees had significantly lower albumin levels than non-amputees. In clinical practices, DFU patients should not only emphasize the limited control of dietary intake but should also pay attention to the treatment of individual medical nutrition, control blood glucose within a reasonable and effective range while strengthening nutritional support treatment, especially the need to eat high-quality protein diet to promote DFU healing.
The results of this study show that vitamin D deficiency is prevalent in DFU patients. Vitamin D deficiency (< 50 nmol/L) accounted for 86.78% of all DFU patients. DFU patients had lower vitamin D levels than DM patients, and the difference was statistically significant (p < 0.05). Monitoring vitamin D level is critical for foot and ankle surgeons for several reasons, including the fact that serum vitamin D levels have been proven to enhance glycemic control in diabetic patients.35,36 Serum vitamin D levels have been shown to improve glycemic control, and vitamin D supplementation was found to decrease HbA1c level in DM patients.35,37 Activated vitamin D is required for normal insulin receptor function and is involved in the development of DM. A randomized, double-blind, placebo-controlled trial by Razzaghi et al showed a more significant improvement in wound parameters with vitamin D supplementation compared to placebo.15 Tiwari et al38 emphasized that in addition to hyperglycemia, vitamin D deficiency may increase the risk of infection in DFU patients by decreasing immune response cells that respond to infection. Zubair et al39 found that lower 25-OH-vitamin D level plays an important role in the pathogenesis of DFU, and this process involves a variety of mechanisms. Hyperglycemia in diabetics impedes the normal production of cytokines, which slows wound healing.40 In rats, topical vitamin D administration enhanced wound healing in a dose-dependent manner. Another study discovered that calcitriol increased endothelial cell and keratinocyte migration in DFU models.41 Therefore, vitamin D supplementation can assist in blood sugar management, diabetic foot healing, and infection control.
Several limitations were discovered throughout our study. First, the molecular mechanisms underlying the role of 25-OH-vitamin D in wound healing in diabetic patients are still poorly understood. Further research is needed in the future. Second, the influence of some individual factors on 25-OH-vitamin D levels, such as lifestyle, sun exposure and diet, was not taken into account.
Conclusions
In summary, this is a relatively large-scale research investigating the relationship between serum 25-OH-vitamin D levels and DFU in Chinese inpatients. We demonstrated the associations of 25-OH-vitamin D with DFU. After adjusting for numerous potential confounding factors including age, sex, duration of DM and biochemical parameters, 25-OH-vitamin D remained an independent protective factor for DFU. We demonstrate a decreasing trend of 25-OH-vitamin D levels in patients with Wagner Grade 1–5 DFU for the first time. Therefore, regular detection of serum 25-OH-vitamin D levels in Chinese diabetic population and supplementation of 25-OH-vitamin D levels in DM patients through diet or other methods might help prevent the occurrence of DFU or improve the prognosis of DFU.
Abbreviations
DFU, diabetic foot ulcer; BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; HbA1c, glycated hemoglobin; FPG, fasting plasma glucose; BUN, blood urea nitrogen; Scr, serum creatinine; SUA, serum uric acid; TC, total cholesterol; TG, triglycerides; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; ALT, alanine aminotransferase; AST, aspartate aminotransferase; ALB, albumin; WBC, white blood cell count; Hb, hemoglobin; HCT, hematocrit.
Consent to Publish
All authors read and approved the submitted manuscript.
Acknowledgments
This study was supported by Beijing Capital Clinical Characteristic Application Research Project (Grant No. Z181100001718025).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Wang W, Ye S, Qian L, et al. Sex-specific association of serum 25-hydroxyvitamin D(3) with insulin resistance in Chinese han patients with newly diagnosed type 2 diabetes mellitus. J Nutr Sci Vitaminol. 2018;64(3):173–178. doi:10.3177/jnsv.64.173
2. Zhao H, Zhen Y, Wang Z, et al. The relationship between vitamin D deficiency and glycated hemoglobin levels in patients with type 2 diabetes mellitus. Diabetes Metab Syndr Obes. 2020;13:3899–3907. doi:10.2147/dmso.S275673
3. DeLuca HF. Overview of general physiologic features and functions of vitamin D. Am J Clin Nutr. 2004;80(6 Suppl):1689s–1696s. doi:10.1093/ajcn/80.6.1689S
4. Holick MF. Resurrection of vitamin D deficiency and rickets. J Clin Invest. 2006;116(8):2062–2072. doi:10.1172/jci29449
5. Cho NH, Shaw JE, Karuranga S, et al. IDF diabetes atlas: global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res Clin Pract. 2018;138:271–281. doi:10.1016/j.diabres.2018.02.023
6. Frykberg RG, Zgonis T, Armstrong DG, et al. Diabetic foot disorders. A clinical practice guideline (2006 revision). J Foot Ankle Surg. 2006;45(5 Suppl):S1–66. doi:10.1016/s1067-2516(07)60001-5
7. Vas PRJ, Edmonds M, Kavarthapu V, et al. The diabetic foot attack: “‘Tis Too Late to Retreat!”. Int J Low Extrem Wounds. 2018;17(1):7–13. doi:10.1177/1534734618755582
8. Alcubierre N, Castelblanco E, Martínez-Alonso M, et al. Vitamin D deficiency is associated with poorer satisfaction with diabetes-related treatment and quality of life in patients with type 2 diabetes: a cross-sectional study. Health Qual Life Outcomes. 2018;16(1):44. doi:10.1186/s12955-018-0873-3
9. Tohidi M, Bozorgmanesh M, Mohebi R, et al. Non-linear association between 25-hydroxyvitamin D and the incidence of type 2 diabetes: a community-based nested case-control study. Diabet Med. 2013;30(8):934–938. doi:10.1111/dme.12180
10. Hurskainen AR, Virtanen JK, Tuomainen TP, et al. Association of serum 25-hydroxyvitamin D with type 2 diabetes and markers of insulin resistance in a general older population in Finland. Diabetes Metab Res Rev. 2012;28(5):418–423. doi:10.1002/dmrr.2286
11. Alcubierre N, Valls J, Rubinat E, et al. Vitamin D deficiency is associated with the presence and severity of diabetic retinopathy in type 2 diabetes mellitus. J Diabetes Res. 2015;2015:374178. doi:10.1155/2015/374178
12. Reddy GB, Sivaprasad M, Shalini T, et al. Plasma vitamin D status in patients with type 2 diabetes with and without retinopathy. Nutrition. 2015;31(7–8):959–963. doi:10.1016/j.nut.2015.01.012
13. Lv WS, Zhao WJ, Gong SL, et al. Serum 25-hydroxyvitamin D levels and peripheral neuropathy in patients with type 2 diabetes: a systematic review and meta-analysis. J Endocrinol Invest. 2015;38(5):513–518. doi:10.1007/s40618-014-0210-6
14. Mahapatra HS, Kumar A, Kulshreshtha B, et al. Effect of vitamin D on urinary angiotensinogen level in early diabetic nephropathy. Indian J Nephrol. 2021;31(4):341–348. doi:10.4103/ijn.IJN_67_20
15. Razzaghi R, Pourbagheri H, Momen-Heravi M, et al. The effects of vitamin D supplementation on wound healing and metabolic status in patients with diabetic foot ulcer: a randomized, double-blind, placebo-controlled trial. J Diabetes Complications. 2017;31(4):766–772. doi:10.1016/j.jdiacomp.2016.06.017
16. Afarideh M, Ghanbari P, Noshad S, et al. Raised serum 25-hydroxyvitamin D levels in patients with active diabetic foot ulcers. Br J Nutr. 2016;115(11):1938–1946. doi:10.1017/s0007114516001094
17. Jeffcoate WJ, Macfarlane RM, Fletcher EM. The description and classification of diabetic foot lesions. Diabet Med. 1993;10(7):676–679. doi:10.1111/j.1464-5491.1993.tb00144.x
18. Gavin JR, Alberti KG, Davidson MB, DeFronzo RA. Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes Care. 2003;26(Suppl 1):S5–20. doi:10.2337/diacare.26.2007.s5
19. Piantanida E, Gallo D, Veronesi G, et al. Cardiometabolic healthy and unhealthy obesity: does vitamin D play a role? Endocr Connect. 2017;6(8):943–951. doi:10.1530/ec-17-0304
20. Qiao Z, Li-Xing S, Nian-Chun P, et al. Serum 25(OH)D level and parathyroid hormone in Chinese adult population: a cross-sectional study in Guiyang urban community from Southeast of China. Int J Endocrinol. 2013;2013:150461. doi:10.1155/2013/150461
21. Song Y, Wang L, Pittas AG, et al. Blood 25-hydroxy vitamin D levels and incident type 2 diabetes: a meta-analysis of prospective studies. Diabetes Care. 2013;36(5):1422–1428. doi:10.2337/dc12-0962
22. Tang W, Chen L, Ma W, et al. Association between vitamin D status and diabetic foot in patients with type 2 diabetes mellitus. J Diabetes Investig. 2022. doi:10.1111/jdi.13776
23. Dai J, Yu M, Chen H, et al. Association between serum 25-OH-vitamin D and diabetic foot ulcer in patients with type 2 diabetes. Front Nutr. 2020;7:109. doi:10.3389/fnut.2020.00109
24. Dai J, Jiang C, Chen H, et al. Vitamin D and diabetic foot ulcer: a systematic review and meta-analysis. Nutr Diabetes. 2019;9(1):8. doi:10.1038/s41387-019-0078-9
25. Danny Darlington CJ, Suresh Kumar S, Jagdish S, et al. Evaluation of serum vitamin D levels in diabetic foot infections: a cross-sectional study in a tertiary care center in South India. Iran J Med Sci. 2019;44(6):474–482. doi:10.30476/ijms.2018.44951
26. Ribu L, Hanestad BR, Moum T, et al. A comparison of the health-related quality of life in patients with diabetic foot ulcers, with a diabetes group and a nondiabetes group from the general population. Qual Life Res. 2007;16(2):179–189. doi:10.1007/s11136-006-0031-y
27. Erdogan M, Solmaz S, Canataroglu A, et al. Plasma thrombin-activatable fibrinolysis inhibitor (TAFI) antigen levels in diabetic foot ulcers. Endocrine. 2010;37(3):449–454. doi:10.1007/s12020-010-9329-1
28. van Etten E, Mathieu C. Immunoregulation by 1,25-dihydroxyvitamin D3: basic concepts. J Steroid Biochem Mol Biol. 2005;97(1–2):93–101. doi:10.1016/j.jsbmb.2005.06.002
29. Kurian SJ, Miraj SS, Benson R, et al. Vitamin D supplementation in diabetic foot ulcers: a current perspective. Curr Diabetes Rev. 2021;17(4):512–521. doi:10.2174/1573399816999201012195735
30. Pei E, Li J, Lu C, et al. Effects of lipids and lipoproteins on diabetic foot in people with type 2 diabetes mellitus: a meta-analysis. J Diabetes Complications. 2014;28(4):559–564. doi:10.1016/j.jdiacomp.2014.04.002
31. Ansell BJ, Fonarow GC, Navab M, et al. Modifying the anti-inflammatory effects of high-density lipoprotein. Curr Atheroscler Rep. 2007;9(1):57–63. doi:10.1007/bf02693941
32. Molnar JA, Underdown MJ, Clark WA. Nutrition and chronic wounds. Adv Wound Care. 2014;3(11):663–681. doi:10.1089/wound.2014.0530
33. Haughey L, Barbul A. Nutrition and lower extremity ulcers: causality and/or treatment. Int J Low Extrem Wounds. 2017;16(4):238–243. doi:10.1177/1534734617737639
34. Brookes JDL, Jaya JS, Tran H, et al. Broad-ranging nutritional deficiencies predict amputation in diabetic foot ulcers. Int J Low Extrem Wounds. 2020;19(1):27–33. doi:10.1177/1534734619876779
35. Aljabri KS, Bokhari SA, Khan MJ. Glycemic changes after vitamin D supplementation in patients with type 1 diabetes mellitus and vitamin D deficiency. Ann Saudi Med. 2010;30(6):454–458. doi:10.4103/0256-4947.72265
36. Mirhosseini N, Vatanparast H, Mazidi M, et al. Vitamin D supplementation, glycemic control, and insulin resistance in prediabetics: a meta-analysis. J Endocr Soc. 2018;2(7):687–709. doi:10.1210/js.2017-00472
37. Lee CJ, Iyer G, Liu Y, et al. The effect of vitamin D supplementation on glucose metabolism in type 2 diabetes mellitus: a systematic review and meta-analysis of intervention studies. J Diabetes Complications. 2017;31(7):1115–1126. doi:10.1016/j.jdiacomp.2017.04.019
38. Tiwari S, Pratyush DD, Gupta B, et al. Prevalence and severity of vitamin D deficiency in patients with diabetic foot infection. Br J Nutr. 2013;109(1):99–102. doi:10.1017/s0007114512000578
39. Zubair M, Malik A, Meerza D, et al. 25-Hydroxyvitamin D [25(OH)D] levels and diabetic foot ulcer: is there any relationship? Diabetes Metab Syndr. 2013;7(3):148–153. doi:10.1016/j.dsx.2013.06.008
40. Tian XQ, Chen TC, Holick MF. 1,25-dihydroxyvitamin D3: a novel agent for enhancing wound healing. J Cell Biochem. 1995;59(1):53–56. doi:10.1002/jcb.240590107
41. Trujillo V, Marín-Luevano P, González-Curiel I, et al. Calcitriol promotes proangiogenic molecules in keratinocytes in a diabetic foot ulcer model. J Steroid Biochem Mol Biol. 2017;174:303–311. doi:10.1016/j.jsbmb.2017.10.013
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.