Back to Journals » Infection and Drug Resistance » Volume 13
A Real-World Study Comparing Various Antimicrobial Regimens for Bloodstream Infections Caused by Carbapenem-Resistant Gram-Negative Bacilli in a Tertiary Hospital, Shanghai, China, from 2010 to 2017
Authors Tan J, Yu W, Wu G, Shen J, Fang Y, Zhu H, Xiao Q, Peng W, Lan Y, Gong Y
Received 27 January 2020
Accepted for publication 16 June 2020
Published 21 July 2020 Volume 2020:13 Pages 2453—2463
DOI https://doi.org/10.2147/IDR.S247378
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Suresh Antony
Jiaying Tan,1,* Wenjin Yu,2,* Gang Wu,1 Jun Shen,1 Yong Fang,1 Hechen Zhu,1 Qianyi Xiao,3 Weixia Peng,3 Yukun Lan,3 Ye Gong1
1Department of Critical Care Medicine, Huashan Hospital, Fudan University, Shanghai 200040, People’s Republic of China; 2Department of Pharmacy , Huashan Hospital, Fudan University, Shanghai 200040, People’s Republic of China; 3Department of Preventive Medicine and Health Education, School of Public Health, Fudan University, Shanghai 200032, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Ye Gong Email [email protected]
Background: We conducted a real-world analysis of the effectiveness of different antibiotic regimens for bloodstream infections (BSIs) caused by carbapenem-resistant gram-negative bacilli (CR-GNB) in a Chinese population.
Methods: A retrospective observational study was conducted between January 2010 and December 2017. Patients with BSIs caused by CR-GNB confirmed by in vitro susceptibility tests were enrolled, and patient medical record data on antimicrobial agents and microbiological and clinical outcomes were extracted.
Results: A total of 175 individuals were included; 127 individuals (72.6%) received combination therapy (two or more antibiotics), while 48 individuals (27.4%) received monotherapy (single antibiotic). The all-cause 28-day mortality was 20.0%. Treatment success or presumed success rates were very similar between the monotherapy and combination therapy groups (58.3% versus 59.1%; P = 0.931). Combination therapy had a higher success rate trend than monotherapy in septic shock patients (40.7% versus 18.2%; P = 0.268). Improved therapeutic effects were observed in the active agent-containing group, although the differences were not significant.
Conclusion: Combination therapy likely has better therapeutic effects on critical BSIs caused by CR-GNB than monotherapy. Choosing a proper active agent in an antimicrobial regime is relatively crucial to the ultimate treatment outcome.
Keywords: bloodstream infection, carbapenem-resistant gram-negative bacilli, antimicrobial regimen, treatment success rate, real-world, Chinese population
Background
Carbapenems are a category of antibiotics belonging to beta-lactams; they have a broad antimicrobial spectrum and effective antibacterial activity, especially for gram-negative bacilli (GNB). However, the steadily increasing rate of carbapenem-resistant bacteria has become a significant public health problem in recent years,1,2 resulting in increased mortality, lengths of hospital stay and medical expenditures. Enterobacteriaceae, Acinetobacter spp. and Pseudomonas aeruginosa (P. aeruginosa) are the most common gram-negative bacterial species obtained from worldwide clinical samples.
Bloodstream infections (BSIs) are the most severe types of nosocomial infections and lead to poor prognoses, particularly in patients with advanced age, immunosuppression, poor functional status and comorbid conditions. BSIs caused by carbapenem-resistant GNB (CR-GNB) have become a major challenge for clinicians in selecting an effective treatment regimen. Currently, there is still a dearth of therapeutic options even based on in vitro susceptibilities, since the vast majority cases of CR-GNB infection are extensively drug-resistant (XDR) or even pandrug-resistant (PDR). It has been reported that specific combinations of antibiotics (some of which include carbapenems, sulbactam, tigecycline or colistin) may benefit patients who are infected by CR-GNB.3–8 Combined therapeutic regimens (two- or three-drug combinations) were recommended in a Chinese consensus statement based on limited clinical studies.9 In addition to one retrospective study conducted in Taiwan,10 no relevant clinical study concerning CR-GNB regimens has been conducted in mainland China. Thus, the issue of antibiotic options for CR-GNB infections remains unresolved.
Herein, we conducted an analysis on the effectiveness of different antibiotic regimens in a retrospective cohort of 175 individuals with BSIs caused by CR-GNB. More clinical evidence will guide the options for therapeutic regimens in the Chinese population.
Methods
Study Design and Patients
A retrospective observational study was conducted between January 2010 and December 2017 in Huashan Hospital, Fudan University, which is a large academic tertiary hospital in Shanghai, China. The enrolled subjects had at least one blood culture positive for GNB. In vitro susceptibility tests were used to confirm carbapenem resistance. Only the first CR-GNB BSI episode was considered, and polymicrobial bacteremia was included in the analysis. Furthermore, the initial targeted antibiotic regimen that was administered for ≥72 hrs was selected.
Patient medical records were reviewed, and the following clinical information was extracted: demographic data, underlying diseases, medical devices, length of hospital stay, length of intensive care unit (ICU) stay, Acute Physiology and Chronic Health Evaluation (APACHE) II score at BSI onset,11 extra-bloodstream infections, antimicrobial agents, and microbiological and clinical outcomes.
Microbiology and Antimicrobial Susceptibility Testing
Species identification and susceptibility testing were performed in the hospital’s microbiology laboratory using the disk diffusion method according to the Clinical and Laboratory Standards Institute (CLSI) criteria12 or with a Vitek 2 compact automated system (bioMérieux) following the instrument specifications, and the results were interpreted according to the CLSI criteria.12 For tigecycline, the US Food and Drug Administration interpretive criteria was applied.13 No minimum inhibitory concentration (MIC) data were available.
Definitions
The following terms were defined prior to the data analysis. The length of ICU stay was defined as the number of days in the ICU, including the central ICU, neurological care unit, neurosurgical care unit, trauma emergency care unit, liver transplantation care unit, severe infection care unit, etc.
The onset of BSI was defined as the collection date of the blood culture specimen that first yielded the study isolate. Polymicrobial BSI was defined as two or more study organisms present at the same time in a patient’s blood according to their culture results. Septic shock was defined as a clinical construct of sepsis within the first 48 hrs of BSI onset with persisting hypotension requiring vasopressors to maintain mean arterial pressure (MAP) ≥65 mmHg and a serum lactate level >2 mmol/L (or lactate data default) despite adequate volume resuscitation.14
Immunosuppression was defined as the condition of a patient who received immunosuppressants, chemotherapy, radiation, long-term or recent high-dose steroids, or who had a disease, such as leukemia, lymphoma or acquired immune deficiency syndrome (AIDS), that was sufficiently advanced to suppress resistance to infection.11 Neutropenia was defined in accordance with the Infectious Diseases Society of America (IDSA) guidelines as an absolute neutrophil count (ANC) of <500 cells/mm3 at the onset of BSI.15 Fever was defined as a temperature of ≥38°C that was sustained for more than 1 hr.15 Defervescence was defined as a temperature of ≤37.2°C that was sustained for more than 72 hrs.
The recorded extra-bloodstream infections were diagnosed before the onset of the studied BSI or occurred simultaneously. Intra-abdominal infections (IAIs) were separated into uncomplicated and complicated IAIs. Infection limited to a hollow viscus was considered an uncomplicated IAI, whereas those that extended into a normally sterile area of the abdomen, such as the peritoneal cavity, mesentery, retroperitoneum, another abdominal organ, or the abdominal wall, were defined as complicated IAIs.16 Solid organ abscess included the abscesses in the brain, eye, lung, liver, vertebra, etc. Infectious endocarditis was confirmed by echocardiography.
Carbapenem resistance was defined as drug resistance to any carbapenem according to in vitro susceptibility testing. Only strains confined to Stenotrophomonas maltophilia were excluded because of their natural resistance to carbapenems. Antimicrobial treatment for non-gram-negative agents or treatment for less than 72 hrs was considered invalid or inadequate. Antibiotics with known gram-negative activity administered either empirically but consistently or after obtaining the results of the susceptibility testing were defined as “targeted” treatments. Regimens were classified as monotherapy or combination therapy depending on the number of drugs included.
Treatment success was defined as the eradication of the study organisms according to repeated negative blood cultures; presumed success was defined as clinical cure according to the improvement in symptoms and signs associated with the underlying BSIs if subsequent blood cultures were not performed. Treatment failure was defined by repeated positive blood cultures, clinical deterioration or death attributed to the underlying BSI. The above evaluations were conducted 72 hrs after the initiation of the “targeted” therapy.
Inclusion and Exclusion Criteria
Inclusion criteria: individuals with at least one blood culture positive for GNB. Exclusion criteria: individuals without carbapenem resistance; not the first CR-GNB BSI episode; not treated for ≥72 hrs in the initial targeted antibiotic regimen; without antibiotic therapy or treatment of Gram-negative covered antimicrobials; strains confined to Stenotrophomonas maltophilia.
Statistical Analysis
The outcomes measured were the all-cause mortality within 28 days after BSI onset, as well as the treatment success or presumed success rates. Patients discharged with an improved condition before day 28 were considered survivors, while those requesting discharge with symptom exacerbation and vital sign instability were considered nonsurvivors. The results are expressed as the mean±standard deviation (SD) or median (interquartile range; IQR) (continuous variables) or as percentages of the group from which they were derived (categorical variables). Student’s t-test and the Mann–Whitney U-test were used to compare normally and non-normally distributed continuous variables, respectively. Categorical variables were evaluated with the χ2 or two-tailed Fisher’s exact tests. A Cox proportional hazards model was used to identify the factors independently associated with mortality. The sets of variables that were identified from the univariate analysis as statistically significant were entered into the model, and their contribution was assessed using the likelihood ratio test. Two-tailed tests were used to determine statistical significance; a P value of <0.05 was considered significant. Data were processed and analyzed using STATA statistical software (StataCorp LP, version 15).
Results
Figure 1 shows the flowchart of the participant selection process. Among the 893 GNB isolates of BSIs from 2010 to 2017, a total of 245 (27.4%) isolates were carbapenem-resistant. Among the 43 individuals who received inadequate treatment, 25 (58.1%) isolates were Klebsiella pneumoniae (K. pneumoniae), 15 (34.9%) were Acinetobacter baumannii (A. baumannii), and 2 (4.7%) were P. aeruginosa. Rapid death after the onset of BSI occurred in the 36 excluded individuals with less than 72 hrs of treatment. There were 4 patients with both K. pneumoniae and A. baumannii polymicrobial infection during the first CR-GNB BSI episode. The carbapenem-resistant rate among blood GNB isolates significantly increased from 20.0% in 2013 to 38.6% in 2017 (P = 0.005) (Figure 2). The characteristics of the 175 individuals (171 patients) who met the inclusion criteria of our study are shown in Table 1. One hundred and twenty-nine individuals (73.7%) were male, and 46 (26.3%) were female. The mean age was 54.6 years (SD, 17.5 years) (median, 57 years; range, 14 to 102 years). Regarding the species of isolates, 85 (48.6%) were K. pneumoniae, 8 (4.6%) were Escherichia coli (E. coli), 54 (30.9%) were A. baumannii, and 10 (5.7%) were P. aeruginosa (Supplementary Table S1). The probable source of BSI is shown in Supplementary Table S1. In 122 of the individuals, the portal of entry could not be deduced through retrospective observation. Overall, the median length of hospital stay was 32 days (IQR, 22 to 57 days), and the median length of ICU stay was 18 days (IQR, 0 to 40 days). Moreover, the median duration of mechanical ventilation was 4 days (IQR, 0 to 17 days).
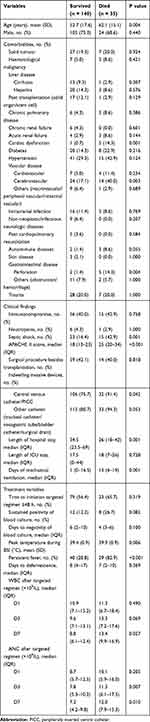 |
Table 1 Characteristics of Individuals with Bloodstream Infections Caused by CR-GNB According to All-Cause 28-Day Mortality |
 |
Figure 1 Flowchart of the study selection process. |
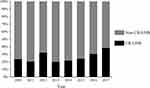 |
Figure 2 Percentages of resistance to carbapenems among GNB isolates in BSIs throughout the study period. |
Treatment
Antibiotic therapy was selected at the discretion of the attending clinician, and drugs were administered with current standard doses that were adjusted according to creatinine clearance when indicated.17 Most of the isolates lacked in vitro susceptibility data for tigecycline, colistin and fosfomycin. Regarding the targeted treatments, 18 individuals (10.3%) received at least one active drug, while 84 (48.0%) received no active drug within 48 hrs after the onset of bacteremia. Moreover, 37 individuals (21.1%) received at least one active drug, and 36 (20.6%) received therapy with no active drug after the first 48 hrs of the BSI. Altogether, 127 individuals (72.6%) received two or more drugs to eliminate the CR-GNB isolate. The remaining 48 individuals (27.4%) received single-drug regimens. The antibiotic regimens are presented in detail in Supplementary Table S2. The individuals in the monotherapy and combination therapy groups were comparable in terms of age, sex, severity of BSI, presence of immunosuppression and neutropenia, severity of sepsis, medical intervention, lengths of hospital and ICU stay, and probable source of BSI (Table 2, Supplementary Table S3). Except for the presence of diabetes, which was higher in the combination therapy group than in the monotherapy group (19.7% versus 6.3%; P = 0.031), all the other comorbidities were comparable between these two groups. Regarding extra-bloodstream infection, both skin and soft tissue infection and solid organ abscess had higher prevalence rates in the combination therapy group than in the monotherapy group.
 |
Table 2 Characteristics of Individuals with Bloodstream Infections Caused by CR-GNB According to Treatment Regimen |
Outcomes
The all-cause 28-day mortality was 20.0% (35 of 175 individuals died). The effects of host-, infection-, and treatment-related factors on 28-day mortality were assessed with a univariate analysis (Table 1, Supplementary Table S1). Adverse outcomes appeared to be more likely among patients with advanced age, pulmonary infection other than bacteremia, a relatively high APACHE II score, septic shock, an indwelling intravascular catheter, increased time on mechanical ventilation, and comorbidities of cardiac dysfunction, cerebrovascular disease and gastrointestinal perforation than in patients without such conditions. The timing of targeted antimicrobial administration did not have an apparent predictive value for the outcome. Moreover, increased mortality was observed in those with a relatively high peak temperature, persistent fever, and a high level of leukocyte maintenance after therapy was administered for a week. The hospital stay was longer in survivors than in non-survivors because of the 28-day mortality grouping. Surprisingly, mortality seemed to be higher in those treated with combination schemes than in those who received monotherapy (13.4% versus 8.3% for 14-day mortality, 22.0% versus 14.6% for 28-day mortality, and 30.7% versus 25.0% for mortality due to infection), but the difference was not statistically significant (Table 3).
By entering the host-, infection-, and treatment-related variables with potential effects on mortality in the Cox proportional hazards model, the factors of cardiac dysfunction (hazard ratio [HR] of death compared to none, 6.02; 95% confidence interval [CI], 2.10 to 17.25; P = 0.001), gastrointestinal perforation (HR of death compared to none, 3.28; 95% CI, 1.49 to 7.20; P = 0.003), APACHE II score at the onset of BSI (HR of death per 1-score increase, 1.03; 95% CI, 1.00 to 1.06; P = 0.034), and persistent fever (HR of death compared to none, 1.47; 95% CI, 1.07 to 2.03; P = 0.018) were identified as independent predictors of adverse outcomes.
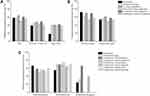
The treatment success or presumed success rates were very similar between the monotherapy and combination therapy groups (58.3% versus 59.1%; P = 0.931). Among the different treatment groups, the highest success rate (61.0%) was observed in individuals treated with carbapenem-containing combinations, as shown in Supplementary Table S2. The effect of various treatments was further assessed in different subsets of individuals (Figure 3). The treatment success rate steadily declined from the highest in all study individuals to the lowest in the most critically ill individuals (Figure 3A). The protective effects of combination therapy were better than that of monotherapy in those suffering from septic shock, but this advantage was not significant (40.7% versus 18.2%; P = 0.268). There was a therapeutic benefit provided by carbapenems, but the difference was not significant among the different severities of BSIs. Triple or more drug combinations were not superior to double combinations in all the subgroups.
Further analysis was conducted by classifying treatment regimens as active agent containing or lacking according to the in vitro susceptibility. Increased therapeutic effects could be obtained from the group with at least one active agent, although no significant differences were observed (Table 3). Unexpectedly, when the therapeutic regimen contained an active drug, monotherapy was not inferior to combination therapy (Figure 3B), despite the slightly lower active agent containing rate in monotherapy than in combination therapy (22.9% versus 34.6%; P = 0.136).
A higher proportion of individuals were administered a targeted monotherapy regimen than combination therapy within 48 hrs (72.9% versus 52.8%; P = 0.016), as shown in Table 2. For the subset of regimens with at least one active agent, the outcome of mortality was not improved, and the success rate was even decreased when treatment began in the first 48 hrs of BSI (Table 3).
The infective species were as follows: 101 cases caused by Enterobacteriaceae (57.7%), 55 caused by Acinetobacter spp. (31.4%), 10 caused by P. aeruginosa (5.7%) and 9 caused by other species (5.2%). As shown in Figure 3C, a slightly increased but nonsignificant success rate was discovered in the monotherapy group for Enterobacteriaceae isolates. Carbapenems provided a positive therapeutic effect on BSIs cause by Enterobacteriaceae and P. aeruginosa isolates. Sulbactam had a certain effect on Acinetobacter spp. isolates. In addition to the natural resistance to P. aeruginosa, tigecycline had a decreased or vain therapeutic effect on Enterobacteriaceae and Acinetobacter spp. isolates. All these treatment effects were not statistically significant.
Discussion
Our study confirmed the high mortality and low antimicrobial treatment success rates associated with CR-GNB BSIs, similar to the previous studies.18–21 The data presented here suggest that cardiac dysfunction, gastrointestinal perforation, the severity of underlying diseases, and response to antibiotic therapy were of paramount importance in determining survival or death. However, the antibiotic regimen did not have a significant impact on the outcome of death; compared to monotherapy, combination therapy was not associated with a decreased risk of mortality. This result is quite different from those in previous studies.22–24
We selected treatment success or presumed success rates as relatively comprehensive outcomes to evaluate various antimicrobial schemes. The therapeutic effect of combination therapy was not significantly superior to that of monotherapy, even in the septic shock subset, which apparently showed a positive trend for combination therapy. This result may be due to the small sample size in this study, particularly in the subgroups. For Enterobacteriaceae isolates, a slightly increased but nonsignificant success rate was associated with monotherapy. More than half of the excluded individuals with less than 72 hrs of treatment were infected with K. pneumoniae, resulting in the omission of those who experienced rapid death and were probably infected with hypervirulent K. pneumoniae. For the remaining patients with Enterobacteriaceae isolates, clinicians had more than 72 hrs to conduct targeted therapy administration. Since some hypervirulent isolates were very likely to be excluded from the study, monotherapy became relatively effective compared to combination therapy. Moreover, this result was also in line with treatment containing at least one active agent; these results are inconsistent with previous studies and can probably be attributed to the influence of the exclusion criteria as well.
To further assess the efficacy of different treatment schemes, we divided the individuals who received combination therapy into different two groups on the basis of treatment with carbapenems, sulbactam or tigecycline. The carbapenem-containing regimens did not produce remarkable therapeutic effects in any of the subgroups, even the Enterobacteriaceae or P. aeruginosa isolate groups. There was no significant protective effect of sulbactam-containing regimens on BSIs caused by Acinetobacter spp. isolates. For tigecycline, no treatment effect was observed in any individuals with BSIs caused by CR-GNB.
Successful treatment was observed when patients were administered treatment with no active agent in our research. This might be attributed to the difficulty of correlating in vitro susceptibility testing with in vivo clinical effectiveness.25 That is, despite in vitro resistance to a certain antibiotic, there may still be activity in vivo. Carbapenems are the most studied antimicrobial drugs that possess this characteristic.
However, the activity of some antibiotics, such as the positive “carbapenem effect”, is rather unlikely to occur against CR-GNB, such as carbapenemase-producing K. pneumoniae strains (CP-Kps), when their MICs are ≥16 μg/mL. However, in our study, there were 68.6% (120 of 175 individuals) PDR strains, and the MICs of the antibiotics used might have exceeded the upper limits, resulting in nonsignificance between the various antimicrobial regimens.
More individuals were administered the targeted regimen within 48 hrs of BSI onset in the monotherapy group than in the combination therapy group. This might be explained by the fact that only one antibiotic with gram-negative activity was often chosen first for empirical therapy. There was no correlation between the initiation time of therapy and treatment success.
After excluding the patients with rapid death that occurred within 72 hrs of BSI onset, the 48-hr time period to initiate treatment was no longer important. The regimen selection is relatively crucial, especially when choosing proper active agents, regardless of monotherapy or combination therapy.
BSIs caused by CR-GNB is a global challenge. Our research could be considered real-world evidence of the effects of various antimicrobial regimens in the Chinese population, especially in the situation of default MIC values. There were several limitations in this study. As previously mentioned, the sample size, particularly in the monotherapy group, was not large enough because this was a single-center study. Rapid deaths were inevitably eliminated due to inadequate treatment that could not be evaluated. This further led to a reduction in the number of research samples. More real-world, large-scale clinical trials are needed for further research.
Conclusions
The present study reconfirms the high mortality and low antimicrobial treatment success rates associated with CR-GNB BSIs. Combination therapy showed a treatment success rate similar to that of monotherapy, but it trended toward effectiveness in patients with severe morbid conditions, such as septic shock. Despite the lack of statistical significance, specific combinations of antibiotics seemed to be effective for BSIs caused by several specific kinds of isolates, such as those containing carbapenems for Enterobacteriaceae and P. aeruginosa isolates, as well as sulbactam for Acinetobacter spp. isolates. However, tigecycline did not show any therapeutic effect on BSIs caused by any isolate cultured from the bloodstream. Notably, the appropriate selection of an active agent is more vital to the anti-infection outcome than the treatment regimen alone.
Abbreviations
BSI, bloodstream infection; CR-GNB, carbapenem-resistant gram-negative bacilli; K. pneumoniae, Klebsiella pneumoniae; E. coli, Escherichia coli; A. baumannii, Acinetobacter baumannii; P. aeruginosa, Pseudomonas aeruginosa; XDR, extensively drug-resistant; PDR: pandrug-resistant; ICU, intensive care unit; APACHE, Acute Physiology and Chronic Health Evaluation; CLSI, Clinical and Laboratory Standards Institute; MIC, minimum inhibitory concentration; MAP, mean arterial pressure; AIDS, acquired immune deficiency syndrome; IDSA, Infectious Diseases Society of America; ANC, absolute neutrophil count; IAI, intra-abdominal infection; SD, standard deviation; IQR, interquartile range; HR, hazards ratio; CI, confidence interval; CP-Kp, carbapenemase-producing K. pneumoniae.
Data Sharing Statement
All data generated or analyzed during this study are included in this published article.
Ethics Approval and Consent to Participate
The present study was approved by the Institutional Review Board (IRB) of Huashan Hospital, Fudan University. Specific patient consent to review their medical records, which contained in their consent form for admission, was not required by the IRB. The raw data were accessed from the photocopy medical record browser, which was granted by the director of Medical Record Administration through routine application procedure. The IRB approval included the statement covering patient data confidentiality and compliance with the Declaration of Helsinki.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that they have no competing interests.
References
1. World Health Organization. Guidelines for the Prevention and Control of Carbapenem-Resistant Enterobacteriaceae, Acinetobacter Baumannii and Pseudomonas Aeruginosa in Health Care Facilities. Geneva: World Health Organization; 2017.
2. Hu FP, Guo Y, Zhu DM, et al. Resistance trends among clinical isolates in China reported from CHINET surveillance of bacterial resistance, 2005–2014. Clin Microbiol Infect. 2016;22(Suppl 1):S9–14. doi:10.1016/j.cmi.2016.01.001
3. Daikos GL, Markogiannakis A. Carbapenemase-producing Klebsiella pneumoniae: (when) might we still consider treating with carbapenems? Clin Microbiol Infect. 2011;17(8):1135–1141. doi:10.1111/j.1469-0691.2011.03553.x
4. Giannella M, Trecarichi EM, Giacobbe DR, et al. Effect of combination therapy containing a high-dose carbapenem on mortality in patients with carbapenem-resistant Klebsiella pneumoniae bloodstream infection. Int J Antimicrob Agents. 2018;51(2):244–248. doi:10.1016/j.ijantimicag.2017.08.019
5. Karageorgopoulos DE, Falagas ME. Current control and treatment of multidrug-resistant Acinetobacter baumannii infections. Lancet Infect Dis. 2008;8(12):751–762. doi:10.1016/S1473-3099(08)70279-2
6. Adnan S, Paterson DL, Lipman J, Roberts JA. Ampicillin/sulbactam: its potential use in treating infections in critically ill patients. Int J Antimicrob Agents. 2013;42(5):384–389. doi:10.1016/j.ijantimicag.2013.07.012
7. Hagihara M, Housman ST, Nicolau DP, Kuti JL. In vitro pharmacodynamics of polymyxin B and tigecycline alone and in combination against carbapenem-resistant Acinetobacter baumannii. Antimicrob Agents Chemother. 2014;58(2):874–879. doi:10.1128/AAC.01624-13
8. Kontopidou F, Giamarellou H, Katerelos P, et al. Infections caused by carbapenem-resistant Klebsiella pneumoniae among patients in intensive care units in Greece: a multi-centre study on clinical outcome and therapeutic options. Clin Microbiol Infect. 2014;20(2):O117–23. doi:10.1111/1469-0691.12341
9. Guan X, He L, Hu B, et al. Laboratory diagnosis, clinical management and infection control of the infections caused by extensively drug-resistant Gram-negative bacilli: a Chinese consensus statement. Clin Microbiol Infect. 2016;22(Suppl 1):S15–25. doi:10.1016/j.cmi.2015.11.004
10. Chang YY, Chuang YC, Siu LK, et al. Clinical features of patients with carbapenem nonsusceptible Klebsiella pneumoniae and Escherichia coli in intensive care units: a nationwide multicenter study in Taiwan. J Microbiol Immunol Infect. 2015;48(2):219–225. doi:10.1016/j.jmii.2014.05.010
11. Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med. 1985;13(10):818–829. doi:10.1097/00003246-198510000-00009
12. Wayne PA. Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing.
13. Food and Drug Administration. Highlights of Prescribing Information Tygacil. Silver Spring, MD: Food and Drug Administration; 2015.
14. Singer M, Deutschman CS, Seymour CW, et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA. 2016;315(8):801–810. doi:10.1001/jama.2016.0287
15. Freifeld AG, Bow EJ, Sepkowitz KA, et al. Clinical practice guideline for the use of antimicrobial agents in neutropenic patients with cancer: 2010 update by the infectious diseases society of America. Clin Infect Dis. 2011;52(4):e56–93. doi:10.1093/cid/cir073
16. Mazuski JE, Tessier JM, May AK, et al. The surgical infection society revised guidelines on the management of intra-abdominal infection. Surg Infect. 2017;18(1):1–76. doi:10.1089/sur.2016.261
17. Gilbert DN, Moellering RC, Eliopoulos GM, Chambers HF, Saag MS. The Sanford Guide to Antimicrobial Therapy, Antimicrobial Therapy. Inc, Sperryville, VA; 2010.
18. Tzouvelekis LS, Markogiannakis A, Psichogiou M, Tassios PT, Daikos GL. Carbapenemases in Klebsiella pneumoniae and other Enterobacteriaceae: an evolving crisis of global dimensions. Clin Microbiol Rev. 2012;25(4):682–707. doi:10.1128/CMR.05035-11
19. Falagas ME, Tensarli GS, Karageorgopoulos DE, Vardakas KZ. Deaths attributable to carbapenem-resistant Enterobacteriaceae infections. Emerg Infect Dis. 2014;20(7):1170–1175. doi:10.3201/eid2007.121004
20. Zak-Doron Y, Benattar YD, Pfeffer I, et al. The association between empirical antibiotic treatment and mortality in severe infections caused by carbapenem-resistant Gram-negative bacteria: a prospective study. Clin Infect Dis. 2018;67(12):1815–1823. doi:10.1093/cid/ciy371
21. Buehrle DJ, Shields RK, Clarke LG, Potoski BA, Clancy CJ, Nguyen MH. Carbapenem-resistant Pseudomonas aeruginosa bacteremia: risk factors for mortality and microbiologic treatment failure. Antimicrob Agents Chemother. 2016;61(1):
22. Qureshi ZA, Paterson DL, Potoski BA, et al. Treatment outcome of bacteremia due to KPC-producing Klebsiella pneumoniae: superiority of combination antimicrobial regimens. Antimicrob Agents Chemother. 2012;56(4):2108–2113. doi:10.1128/AAC.06268-11
23. Daikos GL, Tsaousi S, Tzouvelekis LS, et al. Carbapenemase-producing Klebsiella pneumoniae bloodstream infections: lowering mortality by antibiotic combination schemes and the role of carbapenems. Antimicrob Agents Chemother. 2014;58(4):2322–2328. doi:10.1128/AAC.02166-13
24. Ng TM, Teng CB, Lye DC, Apisarnthanarak A. A multicenter case-case control study for risk factors and outcomes of extensively drug-resistant Acinetobacter baumannii bacteremia. Infect Control Hosp Epidemiol. 2014;35(1):49–55. doi:10.1086/674387
25. Stratton CW. In vitro susceptibility testing versus in vivo effectiveness. Med Clin North Am. 2006;90(6):1077–1088. doi:10.1016/j.mcna.2006.07.003
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.