Back to Journals » International Medical Case Reports Journal » Volume 15
A Rarely Occurring Spinal Fracture Precipitated by Generalized Spasms of Tetanus Patient with Spondylitis Tuberculosis
Authors Huda F , Ong PA, Wibisono Y, Dian S , Ganiem AR
Received 5 July 2022
Accepted for publication 7 October 2022
Published 18 October 2022 Volume 2022:15 Pages 599—603
DOI https://doi.org/10.2147/IMCRJ.S367615
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Ronald Prineas
Fathul Huda,1,2 Paulus Anam Ong,2 Yusuf Wibisono,2 Sofiati Dian,2 Ahmad Rizal Ganiem2
1Department of Biomedical Sciences, Faculty of Medicine, Universitas Padjadjaran, Bandung, West Java, Indonesia; 2Department of Neurology, Faculty of Medicine, Universitas Padjadjaran/Dr. Hasan Sadikin Central General Hospital, Bandung, West Java, Indonesia
Correspondence: Fathul Huda, Email [email protected]
Background: The occurrence of spinal fracture due to tetanus nowadays is extremely rare, as compared to the 1950s, since the widely available anti-tetanus and antispasmodic therapy. The spinal fracture in tetanus patients is usually reported in higher thoracic vertebrae, previously with a rate as high as 57.5%. Spondylitis is the most common form of skeletal tuberculosis (TB) and can cause a spinal fracture. In Indonesia, tetanus is still reported, while tuberculosis is still endemic; however, co-infection of both diseases is rarely reported.
Case Presentation: A 36-year-old male was brought to our hospital with jaw stiffness, accompanied by fever. A history of dental cavities was present, and 5 days prior, he experienced a fishing hook wound on his right index finger. There was no history of TB. Physical examination showed meningismus, 2 cm trismus, abdominal spasm, opisthotonus, and spontaneous muscle spasms, without dysautonomia. In the third week of hospitalization, while his tetanus condition improved, he complained of weakness in both legs. A thorough history taking revealed a history of backache for 3 years. A wedge-shaped fracture on his 11th and 12th thoracic vertebrae was observed on radiographic examination. A spinal TB diagnosis was made, and treatment was started. He refused to get spinal surgery, then went home with 4 out of 5 motor strength scale. After three months, he returned to his routine activity as a food hawker with no motor deficits.
Conclusion: Tetanus spinal fracture is extremely rare nowadays; a thorough history of spinal problems/medication is compulsory for anticipation. This patient’s spinal fracture was deemed due to a preexisting TB spinal infection that was precipitated by prolonged continuous tetanic spasm due to general tetanus.
Keywords: infection, medulla spinalis, paraparesis inferior, spinal fracture, spondylitis tuberculosis, tetanus
Case Presentation
A 36-year-old male came to our emergency room with a chief complaint of jaw stiffness that made him difficult to open his mouth. He also complained of having difficulty swallowing. No complaint of abdominal muscle stiffness or whole body spasms. He also did not complain about having palpitation, difficulty in breathing, excessive salivation, nor sweating. He admitted that he had a fever for 2 days and a fishing hook wound on his index right fingertip around 5 days prior. He had dental cavities and did not have history of TB in any form. The patient could not recall his tetanus immunization status.
Upon examination in the emergency room, a slight fever was observed. Other vital signs were within normal limits. On neurological examination, we found meningismus, 2 cm trismus, abdominal spasm, opisthotonus posturing, and spontaneous muscle spasms without dysautonomia. No other neurological findings nor spinal deformity was observed. Laboratory findings showed a slight increase in leukocyte count (12,700 cell/μL), normal electrocardiogram (ECG), normal blood electrolyte and renal function, and no pneumonia in his chest x-ray.
The diagnosis was general tetanus Grade 3 Patel Joag, and he received intravenous metronidazol, human tetanus immunoglobulin, and tetanus toxoid. Benzodiazepine was used to control the spasms in titrated doses. He underwent wound care to eliminate the source of infection, but treatment for dental cavities was postponed due to his locked jaw condition. A tracheostomy was planned as part of the standard procedure, but the patient refused to get one.
During hospitalization, spontaneous and stimulated spasms worsened, and he developed dysautonomia with tachycardia, hyperthermia, hyperhydration, and hypersalivation. On observation, no signs of myocarditis or prolonged Qtc in ECG were found.
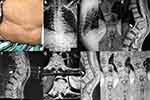
In the third week of treatment, while his tetanus condition improved, the patient started to complain about motor weakness in both legs. On examination, we observed 4 out of 5 motor scales, in the British Medical Research Council’s (MRC) motor scale, and increased knee-jerk reflexes. Inspection showed painful bulging/gibbus on the patient’s back with no pathological reflexes (Figure 1). The findings did not fit features of vertebral compression fracture due to tetanus which is usually mid-thoracic and painless.
The patient underwent a vertebral x-ray and magnetic resonance imaging (MRI). X-ray images showed a wedge-shaped fracture at the 11th-12th thoracic vertebrae (Figure 1). MRI images showed a compression fracture of 11th-12th thoracic vertebrae with gibbus and change of body intensity that compressed the spinal canal and pressed the spinal cord. Inhomogeneous vertebral body intensity changes in the 11th-12th thoracic vertebrae and the intervertebral disc were also observed, which was in line with the diagnosis of spondylosis TB and spondylodiscitis TB (Figure 1).
A thorough history taking following the MRI revealed a history of backache for 3 years, presumably due to spinal TB. On a deeper anamnesis, it was revealed that he had the diagnosis of spinal TB and had received TB treatment for several months, but he did not realize that the present condition might be related to that.
Treatment for spinal TB was then started using a standard regimen. A consultation for spinal surgery was made, but the patient refused to get operated. After 27 days of hospitalization, the patient recovered from tetanus and went home with 4 on the MRC motor scale. At follow-up, 3 months later, the patient has returned to his routine activity as a food hawker with no motor deficits (Figure 2).
 |
Figure 2 Patient’s condition 3 months after discharge. The patient is able to tiptoe walking (A), continues his work as a food hawker (B), and sits (C) without any difficulty or pain. |
Discussion
Vertebral fracture as a complication of tetanus is extremely rare nowadays; as such, almost no current textbook mentions this.1 This kind of fracture was a norm complication in the middle of the last century. In a West African study in 1965, where general tetanus often happened, 57.5% of patients had 1 or more vertebrae fracture, especially in T2-T10.1,2 Mechanism of action of compression fracture of mid-thoracic vertebrae caused by tetanus is probably due to excessive and repeated muscle contraction, accompanied with the shape of the mid-thoracic vertebrae in which the body of the vertebrae is relatively longer but narrow, with a wider transverse diameter as compared to the higher vertebrae. These two conditions result in less arch support for compressive longitudinal flexion pressure, which is detrimental in tetanic spasms.2
Opisthotonus is a hyperextension of the postural paraspinal spasm of the body due to tetanic muscle spasm, but at the same time, the anterior part of the torso, which comprises the neck, and abdominal muscle, also experiences spasms. There is no thoracic flexor muscle. Spasmodic contraction of both extension and flexion of the torso that is long-standing and continuous happens as in this patient resulting in too much exertion on the vertebrae and fracturing the vertebral body.3
Vertebrae are the most frequent locations of skeletal TB, comprising more than 50%. This is due to a hematogenous spread of infection to the vertebral body near the disc. It developed into bony damage and a caseous process, and later the infection/abscess spread to disc space and the surrounding vertebrae. In complicated spinal TB, patients present with deformity, instability, and neurological deficit. Uncomplicated spinal TB is one in which diagnosis is made prior to the development of such complications.4 The paravertebral abscess could run para-discal, central, anterior, posterior, and on a lesser occasion, develops skipped lesions. When the vertebral body collapses, a sharp angulation or gibbus will form. Spinal cord damage might occur due to the direct compression of the abscess, tissue granulation, a burst bone component, or vertebral misalignment, and sometimes due to ischemia related to thrombosis of spinal arteries.5,6
Usually, the mid-thoracic vertebral compression fracture due to tetanus is not painful and does not develop into neurological deficits.2 Most often, it is identified by a radiologist when examining it for other purposes.3
However, in this patient, due to his hospitalization for diagnosis of tetanus, in the beginning, we have thought about vertebral compression fracture complications of tetanus. But then, after physical examination followed by imaging, another diagnosis was made. The location of the fracture, gibbus/vertebrae bulging (which was not readily noticeable at the beginning of hospitalization), and wedge-shaped appearance in imaging led us to the diagnosis of spinal TB. A wedge-shaped fracture in tropical and developing countries like Indonesia is most likely due to tuberculosis infection.5,6
In 1959, Colangelo reported a case of spinal compression fracture in multiple vertebrae (3rd-9th thoracic vertebrae) in a 16-year-old boy with tetanus. The patient was discharged with a spinal brace and could walk after 2 months of hospitalization.7 The latest report on spinal fracture due to tetanus was reported by Wilson et al and Nte and Job in 2012. A 51-year-old male patient from Michigan has cauda equina syndrome due to an L2 compression fracture that developed into a burst fracture. His vertebrae were stabilized using an expandable cage and spinal fusion. No infection or neoplastic sign was found from pathological examination.1 A 13-year-old girl presented with active tetanus and multiple collapses of the 3rd-6th thoracic vertebrae that were confirmed due to tetanus.8
Recent findings showed no significant difference in functional outcome between surgery and conservative treatment in uncomplicated case of spinal TB. However, surgery remains the preferred mode of treatment for this patient with complicated (vertebra deformity) spondylitis TB.9,10 Spinal surgery using kyphoplasty, vertebroplasty, or spinal fusion are treatments of choice. Both kyphoplasty and vertebroplasty are minimally invasive for vertebral augmentation surgery. While kyphoplasty is a minimally invasive surgery that reconstructs the shape of the vertebrae to its original shape using a balloon and special bony material as filler, vertebroplasty employs some form of cement to harden the fractured vertebrae. On the other hand, spinal fusion is a surgery to join two or more vertebrae into a single structure.11–13
Another approach is a conservative approach to fixate the spine using orthosis, in which several types and kinds of orthosis can be used depending on the location of vertebral fracture and patient condition. It could be totally prefabricated, custom mold, or hybrid.14,15
For this patient, spinal surgery was offered to the patient as the treatment of choice. However, the patient refused. Hence, we could not microbiologically (using nucleic acid amplification test/XpertMTB/RIF or other methods) and pathologically confirm the cause of the fracture and infections. He was then given oral antituberculous drugs and thoraco-lumbal spinal orthosis. The clinical condition improved, and at a 3-month follow-up, he could perform previous physical activity and chose to continue using the orthosis and not have the surgery.
Conclusion
Tetanus spinal fracture is extremely rare nowadays, and a thorough history of spinal problems/medication is compulsory as anticipation for other possible causes, such as spinal TB infection. This patient’s spinal fracture was initially assumed due to prolonged continuous tetanic spasm due to general tetanus. However, after deeper history taking, examination, and radiology study, the cause of the spinal fracture was found due to be a different main cause, which was spinal TB infection precipitated by general tetanus. This co-infection of TB and tetanus is still a relevant issue in some parts of the globe, especially in places where both diseases are still prevalent.
Data Sharing Statement
The authors declare that they had full access to all of the data in this study, and the authors take complete responsibility for the integrity of the data. All original data are available in the Department of Neurology, Faculty of Medicine, Universitas Padjadjaran/Dr. Hasan Sadikin Central General Hospital. Data used to support the findings of this study are available from the corresponding author upon request.
Ethics Statement
Based on the regulations of the Universitas Padjadjaran and Dr. Hasan Sadikin Central General Hospital, institutional review board approval is not required for case reports.
Funding
The authors declare that this case report has received no financial support.
Disclosure
The authors report no conflicts of interest in relation to this work.
References
1. Wilson TJ, Orringer DA, Sullivan SE, Patil PG. An L-2 burst fracture and cauda equina syndrome due to tetanus. J Neurosurg Spine. 2012;16(1):82–85. doi:10.3171/2011.7.SPINE11335
2. Davis PR, Rowland HA. Vertebral fractures in west Africans suffering from tetanus: a clinical and osteological study. J Bone Joint Surg Br. 1965;47:61–71. doi:10.1302/0301-620X.47B1.61
3. Bohrer SP. Spinal fractures in tetanus. Radiology. 1965;85(6):1111–1116. doi:10.1148/85.6.1111
4. Rajasekaran S, Soundararajan DCR, Shetty AP, Kanna RM. Spinal tuberculosis: current concepts. Glob Spine J. 2018;8(4_suppl):96S–108S. doi:10.1177/2192568218769053
5. Garg RK, Somvanshi DS. Spinal tuberculosis: a review. J Spinal Cord Med. 2011;34(5):440–454. doi:10.1179/2045772311Y.0000000023
6. Tuli SM. Historical aspects of Pott’s disease (spinal tuberculosis) management. Eur Spine J. 2013;22(Suppl 4):529–538. doi:10.1007/s00586-012-2388-7
7. Colangelo C. Compression fractures of the thoracic vertebrae in a patient with tetanus. J Am Med Assoc. 1959;170(4):455–457. doi:10.1001/jama.1959.63010040001013
8. Nte A, Gabriel-Job N. Tetanus with multiple wedge vertebral collapses: a case report in a 13 year old girl. Niger J Paediatr. 2013;40:189–191.
9. Yong LN, Ahmedy F, Yin KN, Engkasan JP. Functional outcomes in spinal tuberculosis: a review of the literature. Asian Spine J. 2021;15(3):381–391. doi:10.31616/asj.2020.0086
10. Qu JT, Jiang YQ, Xu GH, et al. Clinical characteristics and neurologic recovery of patients with cervical spinal tuberculosis: should conservative treatment be preferred? A retrospective follow-up study of 115 cases. World Neurosurg. 2015;83(5):700–707. doi:10.1016/j.wneu.2015.01.015
11. Hoyt D, Urits I, Orhurhu V, et al. Current concepts in the management of vertebral compression fractures. Curr Pain Headache Rep. 2020;24(5):16. doi:10.1007/s11916-020-00849-9
12. McCarthy J, Davis A. Diagnosis and management of vertebral compression fractures. Am Fam Physician. 2016;94(1):44–50.
13. Musbahi O, Ali AM, Hassany H, Mobasheri R. Vertebral compression fractures. Br J Hosp Med. 2018;79(1):36–40. doi:10.12968/hmed.2018.79.1.36
14. Parreira PCS, Maher CG, Megale RZ, March L, Ferreira ML. An overview of clinical guidelines for the management of vertebral compression fracture: a systematic review. Spine J. 2017;17(12):1932–1938. doi:10.1016/j.spinee.2017.07.174
15. Chang V, Holly LT. Bracing for thoracolumbar fractures. Neurosurg Focus. 2014;37(1):E3. doi:10.3171/2014.4.FOCUS1477
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.