Back to Journals » International Medical Case Reports Journal » Volume 16
A Rare Case of Cervical Vagal Nerve Schwannoma in a 30-Year-Old Ethiopian Man
Authors Aregawi AB
Received 17 December 2022
Accepted for publication 4 March 2023
Published 11 March 2023 Volume 2023:16 Pages 141—151
DOI https://doi.org/10.2147/IMCRJ.S401858
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ronald Prineas
Alazar Berhe Aregawi
Department of Surgery, Hawassa University Comprehensive Specialized Hospital, Hawassa University, Hawassa, Sidama, Ethiopia
Correspondence: Alazar Berhe Aregawi, Email [email protected]
Abstract: Schwannoma is a slowly growing benign tumor that arises from Schwann cells. Schwannomas affect both genders equally. It occurs in any age group, but most cases are seen between the third and fifth decade. About one-fourth to one-third of extracranial schwannomas cases originate in the head and neck region. The vagus nerve, followed by the cervical sympathetic chain, is the leading site of origin in the neck region. The majority of patients with schwannomas are asymptomatic. Patients with vagal nerve schwannomas in the neck primarily present with hoarseness of voice due to paralysis of the vocal cords. Because of their rarity and the lack of a neurologic deficit as a presenting symptom, preoperative consideration of schwannomas is tough, and several differential diagnoses may be entertained.The mainstay of treatment for vagal nerve schwannoma is complete surgical excision. Here we present a rare case of cervical vagal nerve schwannoma in a 30-year-old male farmer from Ethiopia. The patient presented with a gradually increasing neck swelling of 10 years duration. He started to have hoarseness in his voice five months prior to his presentation. On examination, he had a huge anterior neck swelling. He had two FNAC results, which were inconclusive, and a neck CT. With the consideration of multinodular goiter versus spindle cell neoplasm, the neck was explored, and complete excision of the mass was done. The excisional biopsy turned out to be a classical cervical schwannoma. So this report aims to make physicians aware of the rare case of schwannomas, particularly vagal nerve schwannomas. Clinicians should consider schwannomas in the differential diagnosis of a patient presenting with a cervical mass. Furthermore, they need to be well aware of the diagnostic workup, mainly the imaging modalities, which are essential for proper preoperative planning, surgical treatment, and postoperative complications of cervical schwannomas.
Keywords: schwannoma, neurilemmoma, vagal nerve schwannoma, neck mass, cervical mass
Introduction
Schwannoma is a slowly growing benign tumor that arises from Schwann cells.1–6 It is also referred to as a neurilemmoma.1,7 Josay Verocay, a pathologist from Prague, described schwannoma as a pathological condition for the first time in 1908.1 The majority are benign, and malignant schwannomas account for less than 5% of all soft tissue sarcomas.8,9
Schwannomas affect both genders equally. It also occurs in any age group, but most cases are seen between the third and fifth decade. They have a very minimal risk of malignant transformation.1,4–7,10,11 Extracranial schwannomas account for 1/4th to 1/3rd of the cases. The vagus nerve, followed by the cervical sympathetic chain, is the main site of origin in the neck region. Together, they constitute about 25% of all head and neck extracranial schwannomas.12,13
Cervical schwannomas are grouped as medial or lateral. The medial groups are those arising from the cervical sympathetic chain, or the last four cranial nerves. The lateral groups are those arising from the trunk of the cervical or brachial plexuses.1,8
Extracranial cervical vagal nerve schwannoma (VNS) is a rare tumor that commonly occurs between the third and fifth decades of life. So far, there are only 133 cases documented in the literature as of 2016.3,11 And there are only 50 cases of cervical sympathetic schwannoma reported in the literature.8
Patients with VNS in the neck primarily present with hoarseness of voice due to paralysis of the vocal cords. Patients can also have an involuntary cough upon palpation of the mass, which is strongly suggestive of vagal nerve origin.3,4,6,7,10,11,14 The other possible symptoms are dysphonia and dysphagia, particularly when the tumor is large.11
Because of their rarity and the lack of a neurologic deficit as a presenting symptom, preoperative consideration of schwannomas is very difficult. Several differential diagnoses may be entertained for neck tumors, including carotid body tumors, inflammatory cervical lymphadenopathy, submandibular salivary gland tumors, neurofibroma, metastatic cervical lymphadenopathy, thyroid cysts or nodules, teratoma, brachial cysts, or lipomas.1,3,6,7,10,11,13,14 Even though complete surgical resection is the mainstay of treatment, it may be technically challenging because of its proximity to the vagus nerve, from which it arises, and the carotid artery.3,4 The approach depends on the size of the tumor, its location, and its relation to adjacent vessels. Hoarseness of voice is the primary complication following the excision of VNS.4
The case is rare and very interesting. We did not find any similar case reports in our country, Ethiopia, or in Africa as a whole. So this report aims to make physicians aware of the rare case of schwannomas, particularly cervical VNS. Clinicians should consider schwannomas in the differential diagnosis of a patient presenting with a cervical mass. Additionally, they must be fully informed about the diagnostic process, particularly the imaging modalities, which are crucial for accurate preoperative planning, surgical treatment, and the management of postoperative complications related to cervical schwannomas.
Case Presentation
We describe the case of a 30-year-old male farmer from Ethiopia who presented with an anterior neck swelling at the Hawassa University Comprehensive Specialized Hospital in Hawassa, Sidama, Ethiopia. He is married and illiterate. He came to our hospital with a referral paper from a general hospital in the city. The swelling gradually grew to its current size. In the five months leading up to his presentation to the hospital, his voice began to change. The swelling was painless. The hands did not experience any unusual sensations, numbness, or weakness. He had no unusual medical history. He had never undergone surgery. He was not taking any medications, and he had never had a drug allergy. There were no obvious clinical signs of thyroid dysfunction.
There was no history of dyspnea or dysphagia. The patient had no history of radiation exposure.
On physical examination
GA (General appearance): the patient looked comfortable.
vital signs
Pulse rate: 88 beats per minute. Respiratory rate: 20 breaths per minute. Temperature: afebrile to touch.
on head examination: There was no ptosis
A lymphoglandular examination revealed an anterior neck mass that was more prominent on the right side and measured about 28x20x16 cm (see Figures 1 and 2). There were no signs of inflammation in the skin above. The trachea significantly deviated to the left, the mass was firm, not pulsatile or tender, there was no evidence of local infiltration, it was only slightly mobile, there was no retrosternal extension, and there were no palpable cervical lymph nodes. The rest of his systemic examination was completed normally.
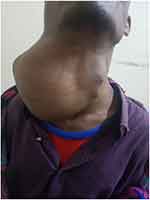 |
Figure 1 The patient in the surgical OPD. |
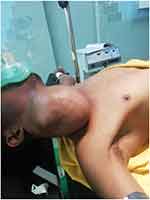 |
Figure 2 The patient on the OR table. |
On Investigations
Complete blood count—all within the normal limits.
on biochemical analysis: Thyroid function tests were all in the normal range.
An FNAC result from the hospital that referred the patient suggested spindle cell neoplasm with degenerative changes, while another result from Hawassa University Comprehensive Specialized Hospital suggested nodular colloid goitre.
On neck CT, there was a right thyroid gland ill-defined heterogeneous mass measuring 13x15x17 cm with post-contrast heterogeneous enhancement and significant mass effect on the trachea, cervical oesophagus, and larynx with no sign of invasion. There were no cervical lymph nodes detected. There was no retrosternal extension seen.
The initial differential diagnosis considered was multinodular goiter (see Figures 3 and 4) due to the appearance of a heterogeneous right thyroid mass.
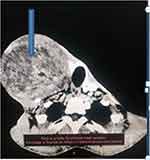 |
Figure 3 CT scan of the patient; the arrow shows the mass. |
 |
Figure 4 The same CT scan, more proximal cross-section, the arrow shows the mass. |
We had difficulty determining the precise diagnosis prior to surgery. The FNAC reports were contradictory. However, we decided to explore the neck because the patient’s neck CT result from the referring hospital showed a mass emerging from the thyroid gland with multinodular goiter as a possible differential. We did not consider cervical schwannoma, and we did not do a neck MRI.
The patient was kept NPO, given maintenance fluids overnight, and taken to the operating room the following day. Under general anaesthesia, the patient was placed supine (in a semi-fowler position), the neck was cleaned, and it was draped. The mass was removed via a large neck collar incision. The right external carotid artery was anteriorly displaced and entered the mass, necessitating its sacrifice. The internal carotid artery had been preserved and resembled a cord. It appeared to have been substantially compressed by the mass. The thyroid gland appeared healthy. The mass was well encapsulated. It was completely removed, and there was no rupture. There were no lymph nodes in the cervical region that were enlarged. We cleaned the wound with salaine and closed it with a nasogastric tube as a drain. Figures 5 and 6 depict intraoperative images. We did not have a magnifying loop to help with the surgery, just like we did not have a lot of other high-tech tools. We did not identify the vagus nerve or the cervical sympathetic trunk specifically. In reality, because schwannoma was never taken into consideration, special care was not taken to determine the nerve of origin.
 |
Figure 5 An intraoperative view of the mass. |
 |
Figure 6 The completely excised mass. |
Following surgery, the follow-up went smoothly. The drain was taken out 48 hours later, and the patient was discharged on the fifth postoperative day. In spite of his hoarseness, he was doing well when he twice visited the follow-up clinic in a single month. There were no recurrence indicators at the one-year follow-up, but the hoarseness persisted. We did not offer him speech therapy because neither we nor the rest of the country had a facility for it. The patient was unable to attend our follow-up clinic despite our repeated attempts to reach him.
Excisional Biopsy Result
Multiple sections show capsulated tissue containing proliferation of spindle cells exhibiting nuclear waving and kinking with scanty eosinophilic cytoplasm, hypo- and hypercellular areas forming Verocay bodies, hyalinized blood vessels, and cystic areas full of macrophages. No signs of malignancy were observed, including mitosis, necrosis, nuclear pleomorphism, or vascular or capsular invasion. A lateral neck mass 2* to a classic cervical schwannoma with hemorrhagic and cystic degenerative changes was the diagnosis. See Figures 7–11.
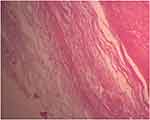 |
Figure 7 High power field photomicrograph of the histology of the tumor showing capsule (using hematoxylin and eosin stain). |
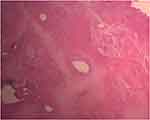 |
Figure 8 High power field photomicrograph of the histology of the tumor showing hyalinized blood vessels (using hematoxylin and eosin stain). |
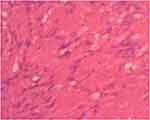 |
Figure 9 High power field photomicrograph of the histology of the tumor showing nuclear palisading forming a verocay body (Hematoxylin and eosin stain). |
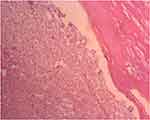 |
Figure 10 High power field photomicrograph of the histology of the tumor showing cyclic degeneration with lots of macrophages (using hematoxylin and eosin stain). |
 |
Figure 11 High power field photomicrograph of the histology of the tumor showing hypocellular or myxoid areas (using hematoxylin and eosin stain). |
Discussion
About one-fourth to one-third of extracranial schwannomas cases originate in the head and neck area.1,7,8,11–14 The majority of patients with schwannomas are asymptomatic. If symptoms develop, it depends on the location and size of the tumor.1,7,15 Patients can complain of pain, breathing difficulty, difficulty swallowing, nasal bleeding, or voice change. Others claim to experience tingling and numbness near the affected nerve’s distribution area. In patients with schwannomas, if they develop pain and neurologic deficits, it is an ominous sign that it is a malignancy.8 In our case, a persistent neck enlargement was the main complaint, which was followed by a change in voice that started happening five months prior to his presentation. The main complaint of cervical VNS patients who are experiencing symptoms is a change in voice. There have also been reports of cervical VNSs causing asymptomatic internal carotid artery compression, which could lead to brain ischemia.3 Both were noted in our case.
Preoperative diagnosis lowers the risk of possible complications, such as Horner’s syndrome and paralysis of the vocal cords, and helps the surgeon plan better for the best possible outcome.8 However, a definitive diagnosis of schwannomas preoperatively is tough. Commonly performed diagnostic investigations for schwannomas are fine-needle aspiration cytology (FNAC), ultrasonography, computed tomography (CT), and magnetic resonance imaging (MRI). MRI is the preferred imaging technique for detailed evaluation, like identifying the nerve of origin and determining vascular relations, which play a pivotal role in preoperative planning.1,2,4,6–8 On CT or MRI, cervical VNS displaces the internal jugular vein laterally and the internal carotid artery medially, but in the case of cervical sympathetic chain schwannomas, both vessels are displaced together without being separated.3,6,11,13,14,16 This was not described in our CT report. In cases of cervical sympathetic chain schwannoma, the tumor is posterior to the internal jugular vein and internal carotid artery.17 A neck MRI would have been better than a CT scan, but only a few places in the country can do it according to the protocol. Schwannomas appear iso- to hypointense on T1-weighted images and appear hyperintense on T2-weighted images. Schwannomas show variable enhancement on contrast administration. Heterogeneous and hyperintense appearances can be noted on T2-weighted images, which are due to necrosis, cystic degeneration, or hemorrhage.18,19 Regarding biopsy, many authors do not recommend needle or open techniques as part of the preoperative workup.6,7,11,14 FNAC accuracy depends on the cytopathologist’s skill and the specimen’s quality.7 Our patient underwent a FNAC twice, and the results were contradictory.
Gross schwannomas are encapsulated. They are solid or cystic spindle cell mesenchymal tumors. Diagnosing Schwannomas can be confirmed by biopsy using electron microscopy and immunohistochemical analysis (S-100 and Leu-7). Common diagnostic features include the presence of fibrous capsules, Antoni A and Antoni B areas, cellular zones, and Verocay bodies. Antoni A is characterized by spindle-like Schwann cells with palisading nuclei, and Antoni B has hypocellularity with large myxoid tissue. Degenerative changes, vascular sclerosis, and hemorrhagic changes can also be demonstrated, particularly in larger and older schwannomas. Occasionally, microcyst formation is evident with the pseudoepithelial lining of the Schwann cells.1,2,7,8 Almost all of these features were demonstrated in our case.
According to an algorithm published in 2015 by Yafit et al, there are three options for treating extracranial schwannomas of the head and neck. This includes only observation and regular follow-up in asymptomatic patients, surgery in symptomatic cases, and palliative radiotherapy in patients not fit to undergo surgery. The experience of radiotherapy is primarily related to vestibular schwannomas.1,13 Ando et al, in their report of 23 cases of schwannomas diagnosed with MRI, recommended that close follow-up for size increment and any feature of nerve compression is sufficient for asymptomatic cases.1,7 Eventually, patients would require surgery unless they were poor surgical candidates.13
The mainstay of treatment for cervical VNS is complete surgical excision.7,11 Every effort should be made to preserve the nerve of origin by proper dissection, especially in benign cases. The fact that the tumor is avascular makes intracapsular dissection possible, and the tumor gets separated from the nerve of its origin. There have been reports of enucleation without the risk of recurrence and keeping a nerve that works well.2,6–8,10,20 If the surgeon fails to preserve the nerve, he has to do a primary repair or a nerve graft, using a microsurgical technique, with or without medialization of the vocal cord.6,7,11,20 Tumors with no obvious nerve of origin should be completely removed.16
In general, the main surgical options are complete excision with nerve grafting,intracapsular dissection and debulking. Debulking is associated with a higher risk of recurrence. Even with intracapsular dissection, it is often difficult to guarantee nerve integrity, so preoperatively, patients should be well counseled about the possibilities of neurologic deficits. Always, the main goal of surgery should be tumor excision with the least possible neurologic deficit. Whichever technique is employed, the nerve of origin is likely to be affected. The rate of nerve palsy following complete excision and intracapsular dissection is 100% and 67%, respectively.19,21,22
At surgery, the tumor appears as a well-circumscribed yellowish to white mass.6,7 This was the case with our patient.Since we had not considered schwannoma in the first place, we did not pay special attention to identifying the nerve of origin during the patient’s complete excision. It appeared to be a vagal nerve schwannoma based on its anatomical location and the fact that it was discovered anterior to the internal carotid artery. Surgery done for VNS is highly associated with denervation of the vocal cords and paralysis. This denervation commonly occurs when the tumor is close to the jugular foramen.5 The incidence of postoperative vocal-cord paralysis has reached up to 85%.6 Reports of preoperative vocal cord paralysis were found to be 15%, but hoarseness is always present following surgery. So, it’s important to check the vocal cords before surgery and talk to the patient in detail about any problems that could arise.6,11,14 Some reports say that the recurrent laryngeal nerves should be checked by observing the vocal cords directly and using neurophysiologic monitoring during surgery.11,13,16 We did not check the vocal cords before surgery because we thought the change in the patient’s voice was caused by the pressure on the larynx and trachea. During extubation, we did not check the condition of the vocal cords postoperatively either. We wanted to do an indirect laryngoscopy, but neither the hospital nor the patient could pay for one to be done in a private hospital. We lack the necessary equipment for voice therapy, so we did not offer the service. In fact, we wanted him to have more follow-ups, but he refused, possibly due to the financial burden of living in a rural area far from our hospital.
Conclusion
Cervical schwannomas, specifically vagal nerve schwannoma, are a very rare entity, and clinicians should consider schwannomas when a patient presents with a cervical mass. In addition, they must have a thorough understanding of the diagnostic process, particularly the imaging modalities, which are essential for accurate preoperative planning, surgical treatment, and management of postoperative complications associated with cervical schwannomas.
Data Sharing Statement
The data used to support the findings of this study will be available from the corresponding author upon reasonable request.
Ethical Review
After obtaining permission from the Hawassa University Institutional Review Board, written informed consent was obtained from the patient for the publication of this case report, which includes images of the patient.
Acknowledgments
Dr. Endashaw Taye, a pathologist in the Department of Pathology at Hawassa University Comprehensive Specialized Hospital, did an excellent job preparing the pathologic slides, for which the author would like to express sincere gratitude. In addition, I would like to thank our patient for permitting us to write this case report.
Disclosure
The author declares no conflicts of interest for this work.
References
1. Elkarim MM, Khan A, Asiri M. Supraclavicular Cervical Schwannoma: a Case Report. Cureus. 2019;11:10.
2. Airlangga PA, Prijambodo B, Hidayat AR, Benedicta S. Schwannoma of the upper cervical spine: a case report. Chin J Traumatol English. 2019;22(6):368–372. doi:10.1016/j.cjtee.2019.07.005
3. Keshelava G, Robakidze Z. Cervical vagal schwannoma causing asymptomatic internal carotid artery compression. Ann Vasc Surg. 2020;63:460.e9–460.e11. doi:10.1016/j.avsg.2019.09.021
4. Ramdass AA, Yao M, Natarajan S, Bakshi PK. A rare case of vagus nerve schwannoma presenting as a neck mass. Am J Case Rep. 2017;18:908–911. doi:10.12659/AJCR.904084
5. Nakano CGY, Massarollo LCB, Volpi EM, Barbosa JG, Arias V, Ueda RYY. Schwannoma senil do nervo vago, resecço com monitorizaço contnua do nervo larngeo inferior. Braz J Otorhinolaryngol. 2008;74(2):316. doi:10.1016/S1808-8694(15)31108-3
6. Chiofalo MG, Longo F, Marone U, Franco R, Petrillo A, Pezzullo L. Cervical vagal schwannoma a case report. Acta Otorhinolaryngol Ital. 2009;29(1):33–35.
7. Lahoti BK, Kaushal M, Garge S, Aggarwal G. Extra vestibular schwannoma: a two year experience Indian. J Otolaryngol Head Neck Surg. 2011;63(4):305–309.
8. Colreavy MP, Lacy PD, Hughes J, et al. Head and neck schwannomas: a 10-year review. J Laryngol Otol. 2000;114(2):119–124. doi:10.1258/0022215001905058
9. Nazari I, Esfehani MH, Nouri M, Naeemi AR, Salimi J, Zafarghandi MR. Cervical sympathetic schwannoma: report of two cases and review of the literature. Acta Med Iran. 2014;52(7):569–574.
10. Cavallaro G, Pattaro G, Iorio O, Avallone M, Silecchia G. A literature review on surgery for cervical vagal schwannomas. World J Surg Oncol. 2015;13(1):15–18. doi:10.1186/s12957-015-0541-6
11. Cervical Vagal GD. Schwannoma review of all reported cases and our reports. Int J Neurol Brain Disord. 2016;3(2):1–6. doi:10.15436/2377-1348.16.729
12. Navaie M, Sharghi LH, Cho-Reyes S, Keefe MA, Howie BA, Setzen G. Diagnostic approach, treatment, and outcomes of cervical sympathetic chain schwannomas: a global narrative review. Otolaryngol Head Neck Surg. 2014;151(6):899–908. doi:10.1177/0194599814549550
13. Sandler ML, Sims JR, Sinclair C, et al. Vagal schwannomas of the head and neck: a comprehensive review and a novel approach to preserving vocal cord innervation and function. Head Neck. 2019;41(7):2450–2466. doi:10.1002/hed.25758
14. Behuria S, Rout TK, Pattanayak S. diagnosis and management of schwannomas originating from the cervical vagus nerve. Ann R Coll Surg Engl. 2015;97(2):92–97. doi:10.1308/003588414X14055925058355
15. Skolnik AD, Loevner LA, Sampathu DM, et al. Cranial nerve schwannomas: diagnostic imaging approach. Radiographics. 2016;36(5):1463–1477. doi:10.1148/rg.2016150199
16. Netterville JL, Groom K. Function-sparing intracapsular enucleation of cervical schwannomas. Curr Opin Otolaryngol Head Neck Surg. 2015;23(2):176–179. doi:10.1097/MOO.0000000000000147
17. Furukawa M, Furukawa MK, Katoh K, Tsukuda M. Differentiation between schwannoma of the vagus nerve and schwannoma of the cervical sympathetic chain by imaging diagnosis. Laryngoscope. 1996;106(12):1548–1552. doi:10.1097/00005537-199612000-00021
18. Ruj ZZ, Govindaraj J, Raghavan B. Cranial nerve schwannoma- A pictorial essay. Indian J Radiol Imaging. 2020;30(30):116–125. doi:10.4103/ijri.IJRI_17_20
19. liu HL, Yu SY, Li GKH, Wei WI. Extracranial head and neck schwannomas: a study of the nerve of origin. Eur Arch Oto. 2011;268(9):1343–1347. doi:10.1007/s00405-011-1491-4
20. Gibber MJ, Zevallos JP, Urken ML. Enucleation of vagal nerve schwannoma using intraoperative nerve monitoring. Laryngoscope. 2012;122(4):790–792. doi:10.1002/lary.22485
21. Shrikrishna BH, Jyothi AC, Kulkarni NH, Mazhar MS, Head E. Neck Schwannomas: our Experience. Indian J Otolaryngol Head Neck Surg. 2016;68(2):241–247. doi:10.1007/s12070-015-0925-5
22. Wang B, Yuan J, Chen X, Xu H, Zhou Y, Dong P. Extracranial non-vestibular head and neck schwannomas. Saudi Med J. 2015;36(11):1363–1366. doi:10.15537/smj.2015.11.12314
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
