Back to Journals » International Medical Case Reports Journal » Volume 16
A Rare Case of Apixaban-Induced Subdural Hematoma in Elderly Heart Failure Patient
Authors Abdirahman Ahmed S , Hassan MO , Abdi IA , Mohamud MA , Waberi MM, Elmi Abdi A, Hassan Fujeyra AM, Ali AA, Sheikh Hassan M
Received 28 July 2023
Accepted for publication 22 September 2023
Published 28 September 2023 Volume 2023:16 Pages 623—626
DOI https://doi.org/10.2147/IMCRJ.S432794
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Scott Fraser
Said Abdirahman Ahmed,1 Mohamed Omar Hassan,1 Ishak Ahmed Abdi,1 Mohamed Abdullahi Mohamud,1 Mohamud Mire Waberi,1 Ahmed Elmi Abdi,1 Abdullahi Mohamed Hassan Fujeyra,2 Abdijalil Abdullahi Ali,3 Mohamed Sheikh Hassan4
1Department of Cardiology at Mogadishu Somali-Turkish Training and Research Hospital, Mogadishu, Somalia; 2Dean College of Medicine and Health Science at Abrar University, Mogadishu, Somalia; 3Cardiovascular Surgery Department at Mogadishu Somali-Turkish Training and Research Hospital, Mogadishu, Somalia; 4 4 Neurology Department at Mogadishu Somali-Turkish Training and Research Hospital, Mogadishu, Somalia
Correspondence: Said Abdirahman Ahmed, Email [email protected]
Abstract: New oral anticoagulants (NOACs) have become more popular in the last few decades. Although apixaban has been proven to be safer than warfarin and causes less hemorrhage in comparison to other NOACs, it still poses a risk of spontaneous bleeding. We present here an 81-year-old male known case of heart failure with reduced ejection fraction (HFrEF) associated with an apical thrombus of 0.93× 1.29 cm who presents with cognitive decline, slurred speech, and right side weakness following apixaban use for his apical thrombus. On further evaluation of non-contrast brain computerized tomography (CT), there was a large extra-axial subacute subdural hematoma with thick septations in the left parietal region, measuring 2.6 cm in thickness, causing an a mass effect, and an a midline shift of 1 mm. Following neurosurgery, cardiology, and anesthesiology discussions, the surgery was deferred due to his age and coexisting conditions with regular follow-ups. The patient has now gained full consciousness and is currently undergoing physiotherapy. This case highlights an elderly patient with apixaban-induced subdural hemorrhage, which is a rare entity in the medical literature. Although apixaban is safer than other NOACs, it may cause subdural hemorrhage.
Keywords: new oral anticoagulants, NOACs, subdural hematoma, apical thrombus, heart failure
Introduction
New oral anticoagulants (NOACs) that either inhibit thrombin (dabigatran) or inhibit coagulation factors (F Xa), such as rivaroxaban, apixaban, edoxaban, and betrixaban, have become more popular in the last few decades.1 Apixaban, used to prevent systemic embolism and stroke in patients with atrial fibrillation and deep venous thrombosis (DVT), has been proven to be safer than warfarin.2 However, apixaban, like other anticoagulants, carries the risk of fatal bleeding issues, including gastrointestinal, subarachnoid, and potentially subdural bleeding.
A subdural hematoma is the collection of blood between the dura mater and the arachnoid mater. It can happen as a result of trauma or spontaneously in certain people. The most common cause of chronic subdural hematomas (cSDHs) is bridging vein tears as they cross the dural cell layer. Inertial brain injury, both severe and minor, is recorded in two-thirds of patients and can cause tears in those vulnerable arteries.3 Chronic subdural hematoma (cSDH) is more common in elderly people with a history of minor head injuries, whereas acute subdural hematoma (ASDH) is more common in patients who have suffered severe head trauma.4
Very few cases of apixaban-induced subdural hematomas have been reported in the literature, and mostly in these cases, the subdural hematomas are in the spine and not in the cranium. These cases highlight the importance of considering this potential complication in patients receiving apixaban therapy, especially elderly individuals who may be at a higher risk of bleeding. According to one study, a 60-year-old man with a history of hypertension and oligodendroglioma suffered cranial subdural hemorrhage when taking apixaban for deep venous thrombosis.5 We present an 81-year-old male patient with spontaneous subdural hemorrhage due to the use of apixaban for apical thrombus, and we also describe the related literature review.
Case Presentation
An 81-year-old male came to the cardiology outpatient clinic with shortness of breath, fatigue, and coughing at night. He had diabetes, for which he was on medication. His vital signs were normal. On examination, he had crackles in both lungs and S3 on the auscultation in the heart, as well as mild bilateral lower limb edema. His electrocardiography showed a complete right bundle branch block, while echocardiography demonstrated an ejection fraction of 30–35 associated with an apical thrombus of 0.93×1.29 cm and moderate mitral regurgitation (Figure 1A and B). The patient was managed with ramipril 2.5mg, carvedilol 6.25 mg, furosemide 40 mg, spironolactone 25 mg, and apixaban of 2.5mg BID. Unfortunately, the patient lost follow-up and presented again with a cognitive decline, slurred speech, and right hemiparesis. The patient did not have a recent history of head trauma, or seizure event. He was disoriented, dysarthric and had difficulty in obeying commands. Motor examination showed right side weakness (2/5 based on MRC). Pupils were isochoric, and reactive to light. There was no facial asymmetry. Likewise, other cranial examinations were normal. Extensive laboratory examinations, including full blood count, renal and liver function tests and coagulation profile were in the normal limits. A non-contrast brain CT showed chronic subdural hematoma of 2.6 cm thickness in the left parietal region (Figure 2A and B). The apixaban was discontinued. Following neurosurgery, cardiology, and anesthesiology discussions, the surgery was deferred due to the patient’s age and coexisting comorbidities and the risk of general anesthesia. The patient was managed conservatively. Up on the next follow-up, the patient’s condition improved massively with an improved cognitive state, normal speech and also had an improved motor status with power of 4/5 (based on MRC score) and as a result, he was referred for physiotherapy and rehabilitation center.
 |
Figure 1 (A) Electrocardiogram (ECG) with complete right bundle branch block (RBBB). (B) Echocardiogram showing apical thrombus 0.93×1.29cm. |
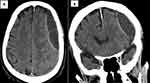 |
Figure 2 Brain computed tomography (CT) showing subdural hematoma of 2.6 cm in both axial (A) and coronal (B) views. |
Discussion
Apixaban, a novel oral anticoagulant, is a direct factor Xa inhibitor that has gained popularity due to its efficacy and safety profile when compared with traditional anticoagulants such as warfarin. But unfortunately, apixaban, like other anticoagulants, has a tendency to cause bleeding diathesis, including subdural hematoma. It is the only new oral anticoagulant (NOAC) approved by Food and Drug Administration (FDA) for hemodialysis (HD) patients.6 Gastrointestinal, cerebral, and soft tissue bleeding have all been well-documented events, but subdural bleeding was infrequently reported in the literature.7–9 An uncommon occurrence of spinal subdural hematoma caused by apixaban medication for non-rheumatic atrial fibrillation was described in a case study.10 Another case report by reported a spontaneous thoracic spinal subdural hematoma associated with apixaban therapy.11 These cases highlight the importance of considering subdural hematoma as a potential complication in patients receiving apixaban therapy.
It is important to note that subdural bleeding with apixaban is infrequent relative to other bleeding problems. Apixaban had the lowest prevalence of cerebral haemorrhage among a similar class of inhibitors, according to a literature study.12 This highlights that, while apixaban may cause subdural hematomas, the overall risk of bleeding with this medication is relatively uncommon.
The exact mechanism through which apixaban can cause subdural hematomas is unknown. However, it is suspected that apixaban’s anticoagulant activity may raise the risk of bleeding, particularly bleeding into the subdural region. Furthermore, older age, renal impairment, and concurrent use of other drugs like anticoagulant drugs, non-steroidal anti-inflammatory drugs (NSAIDs), or selective serotonin reuptake inhibitors (SSRIs) could raise the risk of subdural hematoma in individuals on apixaban therapy; therefore, care should be taken with those susceptible patients, which requires monitoring.13
The clinical manifestation of apixaban-induced subdural hemorrhage varies depending on its location and degree of associated mass effect. Headaches, neurological impairments, changes in mental status, and, in severe cases, loss of consciousness are common symptoms.5 Prompt diagnosis and care are critical for avoiding complications and improving patient outcomes.
In terms of therapy, apixaban-induced subdural hematoma is normally treated with a multidisciplinary approach. In cases of substantial mass impact or neurological impairment, neurosurgical intervention is required. A large craniotomy is the standard approach for acute subdural hematoma (ASDH); a decompressive craniotomy may also be performed if necessary to prevent subsequent brain injury.12,14 In less severe cases like this case, conservative therapy with constant monitoring and anticoagulation reversal may be sufficient.15 Due to his age and concomitant comorbidities, the patient was managed conservatively with follow-up.
The US Food and Drug Administration (FDA) approved andexanet alfa as an antidote for patients using rivaroxaban or apixaban who require anticoagulation reversal due to life-threatening or uncontrolled bleeding. Andexanet alfa (also known as coagulation factor Xa) reverses factor Xa inhibition by acting as a decoy factor Xa with no procoagulant characteristics, thereby competing with naturally synthesized and circulating factor Xa.16
Conclusion
In conclusion, while apixaban is generally considered a safe and effective anticoagulant, it carries a risk of bleeding complications, including subdural hematoma. Frequency of apixaban-induced subdural hematoma is relatively low compared to other bleeding complications associated with this medication. Healthcare providers should be aware of this potential complication, especially in elderly patients or those with underlying risk factors. Close monitoring and prompt management are essential to ensure optimal patient outcomes.
Consent
Written informed consent was obtained from the patient’s family to have the case details and any accompanying images published.
Funding
There is no funding to report.
Disclosure
The authors declare no conflicts of interest in this work.
References
1. Crowther M, Cuker A. How can we reverse bleeding in patients on direct oral anticoagulants? Kardiol Pol. 2019;77(1):3–11. doi:10.5603/KP.a2018.0197
2. Deng Y, Tong Y, Deng Y, Zou L, Li S, Chen H. Non–vitamin K antagonist Oral anticoagulants versus warfarin in patients with cancer and atrial fibrillation: a systematic review and meta‐analysis. J Am Heart Assoc. 2019;8(14):e012540. doi:10.1161/JAHA.119.012540
3. Msheik A, Fares Y, Mohanna M, et al. Middle meningeal artery embolisation: the review of a new treatment for chronic subdural hematomas. Surg Neurol Int. 2023;14:14. doi:10.25259/SNI_1112_2022
4. Snopko P, Kolarovszki B, Opsenak R, Hanko M, Benco M. Chronic calcified subdural hematoma-case report of a rare diagnosis. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2020;164(2):209–212. doi:10.5507/bp.2019.041
5. Alayad E, Khairy S, Aloraidi A. Apixaban-induced subdural bleeding: case presentation and literature review. Case Rep. 2018;2018:bcr–2018.
6. Wendte J, Voss G, VanOverschelde B. Influence of apixaban on antifactor Xa levels in a patient with acute kidney injury. Am J Health Syst Pharm. 2016;73(8):563–567. doi:10.2146/ajhp150360
7. Bloom BJ, Filion KB, Atallah R, Eisenberg MJ. Meta-analysis of randomized controlled trials on the risk of bleeding with dabigatran. Am J Cardiol. 2014;113(6):1066–1074. doi:10.1016/j.amjcard.2013.11.049
8. Abu Hishmeh M, Srivastava P, Lougheide Q, Srinivasan M, Murthy S. Massive spontaneous hemothorax as a complication of apixaban treatment. Case Rep Pulmonol. 2018;2018:1–4. doi:10.1155/2018/8735036
9. Rinehart DR, Lockhart NR, Hamilton LA, Langdon JR, Rowe AS. Management of apixaban-associated subdural hematoma: a case report on the use of factor eight inhibitor bypassing activity. Crit Care Med. 2015;43(6):e203–e207. doi:10.1097/CCM.0000000000000909
10. Colell A, Arboix A, Caiazzo F, Grivé E. Iatrogenic spinal subdural hematoma due to apixaban: a case report and review of the literature. Case Rep Hematol. 2018;2018:1–5. doi:10.1155/2018/4507638
11. Ardebol J, Cahueque M, Lopez W, Azmitia E. Spontaneous thoracic spinal subdural hematoma associated with apixaban therapy. J Surg Case Rep. 2019;2019(4):rjz115. doi:10.1093/jscr/rjz115
12. Beynon C, Potzy A, Unterberg AW, Sakowitz OW. Emergency neurosurgical care in patients treated with apixaban: report of 2 cases. Am J Emerg Med. 2014;33(6):858–e5.
13. Lee MT, Park KY, Kim MS, You SH, Kang YJ, Jung SY. Concomitant use of NSAIDs or SSRIs with NOACs requires monitoring for bleeding. Yonsei Med J. 2020;61(9):741. doi:10.3349/ymj.2020.61.9.741
14. Kessler CM, Goldstein JN. A new strategy for uncontrollable bleeding after treatment with rivaroxaban or apixaban. Clin Adv Hematol Oncol. 2019;17(suppl 15):1–20.
15. Haas S, Camm AJ, Bassand JP, et al. Predictors of NOAC versus VKA use for stroke prevention in patients with newly diagnosed atrial fibrillation: results from GARFIELD-AF. Am Heart J. 2019;213:35–46. doi:10.1016/j.ahj.2019.03.013
16. Kaatz S, Bhansali H, Gibbs J, Lavender R, Mahan CE, Paje DG. Reversing factor Xa inhibitors–clinical utility of andexanet alfa. J Blood Med. 2017;8:141–149. doi:10.2147/JBM.S121550
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
