Back to Journals » Journal of Pain Research » Volume 12
A randomized, placebo- and active-controlled, multi-country, multi-center parallel group trial to evaluate the efficacy and safety of a fixed-dose combination of 400 mg ibuprofen and 100 mg caffeine compared with ibuprofen 400 mg and placebo in patients with acute lower back or neck pain
Authors Predel HG, Ebel-Bitoun C , Lange R, Weiser T
Received 24 May 2019
Accepted for publication 17 August 2019
Published 23 September 2019 Volume 2019:12 Pages 2771—2783
DOI https://doi.org/10.2147/JPR.S217045
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor E Alfonso Romero-Sandoval
Hans-Georg Predel,1 Caty Ebel-Bitoun,2 Robert Lange,3 Thomas Weiser4
1Institute of Cardiology and Sports Medicine, Department of Preventive and Rehabilitative Sports Medicine, German Sport University Cologne, Cologne, Germany; 2Consumer Health Care, Global Medical Head, Sanofi-Aventis, Paris, France; 3Consumer Health Care, Global Medical Affairs, Sanofi-Aventis Deutschland GmbH, Frankfurt am Main, Germany; 4Consumer Health Care, Medical Affairs, Sanofi-Aventis Deutschland GmbH, Frankfurt am Main, Germany
Correspondence: Thomas Weiser
Consumer Health Care, Medical Affairs, Sanofi-Aventis Deutschland GmbH, Industriepark Höchst, Frankfurt am Main 65926 Germany
Tel +49 693 053 9966
Email [email protected]
Background: Ibuprofen is a well-established analgesic for acute pain symptoms. In several acute pain models, caffeine has demonstrated an analgesic adjuvant effect. This randomized trial (NCT03003000) was designed to compare the efficacy of a fixed-dose combination of ibuprofen and caffeine with ibuprofen or placebo for the treatment of acute lower back/neck pain.
Methods: Patients with acute lower back/neck pain resulting in pain on movement (POM) ≥5 on a 10-point numerical rating scale were randomized 2:2:1 to receive orally, three times daily for 6 days, 400 mg ibuprofen+100 mg caffeine, 400 mg ibuprofen or placebo, respectively. The primary endpoint was change in POMWP (POM triggering highest pain score at baseline [worst procedure]) between baseline and the morning of day 2. Key secondary endpoints included POMWP area under curve (AUC) between baseline and the morning of day 4 (POMWPAUC72h) and day 6 (POMWPAUC120h).
Results: In total, 635 patients were randomized (256 ibuprofen + caffeine: 253 ibuprofen: 126 placebo). Active treatments exhibited similar reductions in POMWP, with an adjusted mean reduction of 1.998 (standard error [SE]: 0.1042) between baseline and day 2 for ibuprofen, 1.869 (SE: 0.1030) for ibuprofen + caffeine and 1.712 (SE: 0.1422) for placebo. Similar results were observed for POMWPAUC72h and POMWPAUC120h. Safety and tolerability was as expected.
Conclusion: A decrease in lower back/neck pain, indicated by reduced POMWP, was shown in all active treatment arms; however, treatment effects were small versus placebo. Ibuprofen plus caffeine was not superior to ibuprofen alone or placebo for the treatment of acute lower back/neck pain in this setting.
Keywords: ibuprofen, caffeine, acute back pain, acute neck pain
Introduction
Lower back pain is a common disorder; the estimated global point prevalence of activity limiting lower back pain lasting more than 1 day was 11.9±2.0% between 1980 and 2009.1 Lower back pain can be broadly categorized as nonspecific or specific. Nonspecific refers to lower back pain for which there is no clear causal relationship between the symptoms, physical findings and imaging findings whereas, for specific lower back pain, there is a pathoanatomical relationship between the pain and one or more pathological process.2 A review by Deyo and Weinstein found that among patients with specific lower back pain, 4% were diagnosed with disc herniation, 3% with spinal stenosis and 2% with spondylolisthesis.3 However, most cases of lower back pain (80–90%) are nonspecific and are often caused by non-pathological functional disturbances, such as sacroiliac joint syndrome.2 The causes of lower back pain can be mechanical, neuropathic or secondary to another cause.4 Approximately 90% of patients suffering from back pain achieve remission within 6 weeks.2
Neck pain is also becoming an increasingly common disorder; 1-year prevalence rates range from 4.8–79.5%, with most studies reporting an increased risk of neck pain in women.5 There is some consistency between the causes of lower back pain and those of neck pain, including compressive causes, such as disc herniation, and degenerative changes of the vertebrae and/or intervertebral discs.6 As with lower back pain, there is often uncertainty around the pathophysiology of neck pain and specific causes are rarely identified.
Despite the lack of efficacy data, acute back and neck pain are often treated with over-the-counter analgesics; the most common treatment being ibuprofen. Surprisingly, considering the widespread use of ibuprofen, we identified only one published study in which ibuprofen was investigated for the treatment of acute back pain, published by Dreiser et al.7 Although ibuprofen and diclofenac potassium were both found to be superior to placebo in this study, limited efficacy was reported.7 However, other studies have reported that 400 mg ibuprofen is a more effective analgesic than 1000 mg paracetamol or 325/650 mg aspirin for the treatment of other forms of acute pain (including dental pain, episodic tension-type headache, muscle contraction headache, postoperative pain after caesarean section).8–12 In spite of this, some patients still reported insufficient pain relief with 400 mg ibuprofen,9–11 which led to investigations into the analgesic adjuvant effect of caffeine.13,14 It must be noted that the definitions of “acute pain” differ greatly for back/neck pain and pain resulting from postpartum uterine cramping, tension headache, migraine or dental extraction, where the superiority of caffeine-containing analgesics has previously been demonstrated.15–20
Caffeine has demonstrated an analgesic adjuvant effect in several pain models. In a Cochrane review of 20 randomized double-blind studies involving 4,262 patients, it was concluded that adding ≥100 mg caffeine to standard doses of commonly used analgesics increased the proportion of patients reporting a good level of pain relief by a small but important amount.15 The ibuprofen + caffeine combination investigated here has shown superior efficacy to ibuprofen alone in a dental extraction pain model.20
Due to the available evidence supporting caffeine as an analgesic adjuvant in combination with nonsteroidal anti-inflammatory drugs (NSAIDs),15,16,18–20 it was thought that a combination of ibuprofen and caffeine could be more effective and provide faster pain relief than single-agent ibuprofen for the treatment of lower back or neck pain. The aim of this trial (NCT03003000; EudraCT 2016-000902-12) was to compare the efficacy of a fixed-dose combination of 400 mg ibuprofen and 100 mg caffeine with that of ibuprofen alone or placebo for the treatment of acute lower back or neck pain.
Methods
Trial design
This Phase III randomized, placebo- and active-controlled, double-blind, multi-centre, multi-country, 3-arm, parallel group trial was designed to evaluate the efficacy and safety of a fixed-dose combination of 400 mg ibuprofen and 100 mg caffeine, compared with 400 mg ibuprofen alone or placebo.
Patients were randomized using an interactive response technology (IRT) system provided by Quintiles Limited (Bloemfontein, South Africa) and Almac Clinical Services (Craigavon, United Kingdom). The randomization list was generated using a validated system, which involved a pseudo-random number generator to ensure that the resulting treatment was both reproducible and non-predictable. Patients were randomized in blocks (block size 5) to the treatment groups in a 2:2:1 ratio (ibuprofen + caffeine: ibuprofen: placebo). Randomization was stratified by country (Germany/Russia) and worst procedure site (back/neck). Patients, investigators and those involved in analyzing the data remained blinded with regard to assigned treatment until after database lock.
Standard protocol approvals, registration and patient consents
The IEC (Ärztekammer Nordrhein, Ethikkommission, Düsseldorf, Germany) of the Coordinating Investigator of the trial (H-G Predel) gave a favourable opinion for the trial on 13 Dec 2016.
Prior to start of the trial, the clinical trial protocol (CTP) 1335.5, dated 28 Jul 2016, the patient information leaflet, the informed consent form, and other locally required documents were reviewed by the IECs of the participating centres (Germany: Ärztekammer Nordrhein, Ethikkommission, Düsseldorf, Germany; Russia: Local Ethics Committees at “Alliance Biomedical-Russian Group”, “St. Petersburg State Budgeted Healthcare Institution (City Out-patient hospital #109)”, and “Medical Center (Reavita Med SPb), all St. Petersburg). The competent authority (Bundesinstitut für Arzneimittel und Medizinprodukte, Bonn, Germany) approved the trial on 31 Oct 2016. This trial was carried out in compliance with the clinical trial protocol, in accordance with the principles of the Declaration of Helsinki and the international conference of harmonisation good clinical practice guidelines, and in accordance with applicable regulatory requirements. Each patient signed and dated an informed consent form according to the local regulatory and legal requirements.
Participants
Eligible patients were adults aged ≥18 years with acute back or neck pain resulting in pain on movement (POM) ≥5 on a 10-point numerical rating scale (NRS) for at least one of five standardized POM procedures.21 Patients had acute back or neck pain for at least 24 hrs but less than 21 days. Patients were excluded if they had a history of three or more episodes of back or neck pain in the last 6 months, or if they had chronic back or neck pain (defined as pain for three weeks or longer). Additional exclusion criteria included back or neck pain due to an identifiable cause, surgery or rehabilitation due to back or neck pain in the last 12 months, or use of prohibited medication (including any anti-inflammatory drugs, heparinoids or muscle relaxants) within 3 days prior to Visit 1.
Treatment
Eligible patients were randomly assigned to receive 400 mg ibuprofen +100 mg caffeine, 400 mg ibuprofen or placebo orally every 6–8 hrs, three times per day, over each 24 hr period from day 1 until the morning of day 6. For the active treatments, total daily dosages were 1200 mg ibuprofen +300 mg caffeine, and 1200 mg ibuprofen.
Paracetamol was permitted as the only rescue medication at a maximum dose of 2 g per day. If required, following the first dose of trial medication, one to two tablets of 500 mg paracetamol were permitted up to twice daily to treat intolerable back or neck pain. Patients were instructed to record the number of tablets, along with the day and time that a dose of paracetamol was taken, but were encouraged not to take the rescue medication before the primary endpoint was assessed on day 2. Additionally, patients were advised to refrain from ingesting any caffeine-containing beverages, chocolate or alcohol during the trial.
Assessments
The trial involved five visits: Visit 1 on day 1 (screening, randomization and initial dosing), Visit 2 on day 2 (assessment of the primary endpoint), Visit 3 on day 4 (assessment of efficacy and safety parameters), Visit 4 on day 6 (end-of-treatment visit) and Visit 5 on day 8 to day 10 (follow up by telephone interview). Patients were asked to return all unused trial medication and their diaries at each visit.
POM was assessed by the patient on performing one standardized, muscle group-specific movement, using a validated 10-point NRS that ranged from 0 (no pain) to 10 (worst pain possible for the condition). POM worst procedure (POMWP) was defined as the procedure or movement that resulted in the highest pain score at baseline. Additionally, pressure algometry (PA) was assessed by the investigator to determine the pressure (N/cm2 (1 N/cm2=10 kilopascal (kPA))) that elicited a pain reaction at a defined trigger point located in the area of POMWP.
A global assessment of efficacy was also performed. This was based on a 4-point verbal rating scale (0= poor; 1= fair; 2= good; 3= very good) in response to the question: “How would you rate the overall effect of the trial medication for relieving back or neck pain?” In addition, an assessment of average daily pain at rest was performed by the patients each evening before going to bed and was recorded in their diaries. Average pain at rest during the last 24 hrs was assessed using a 10-point NRS that ranged from 0 (no pain) to 10 (worst pain possible). Furthermore, patients completed the Oswestry Disability Questionnaire on days 2 and 6 of treatment, which comprised 10 topics concerning intensity of pain, lifting, ability to care for oneself, ability to walk, ability to sit, sexual function, ability to stand, social life, sleep quality, and ability to travel. The Oswestry Disability Index was used to assess the disability of a patient using a scale that ranged from 0 (no disability) to 100% (maximum disability possible). Safety was assessed through adverse event (AE) and serious AE (SAE) reporting.
Endpoints
The primary endpoint of this trial was change in POMWP between baseline (morning of day 1, before first dose) and the morning of day 2 (2 hrs after drug intake). Key secondary endpoints were POMWP area under curve (AUC) between baseline and the morning of day 4 (POMWPAUC72h) and POMWP area under curve between baseline and the morning of day 6 (POMWPAUC120h). Other secondary endpoints included: change in PA between baseline and the morning of day 2 (2 hrs after drug intake), global assessment of efficacy by the patient at the end of treatment (morning of day 6), number of patients with a decrease in POMWP of ≥30% and ≥50% between baseline and the morning of day 2 (2 hrs after drug intake), and time to first meaningful POMWP relief within 2 hrs of the first dose of trial medication. Further secondary endpoints included average daily pain at rest (assessed by the patient each evening before going to bed) and the Oswestry Disability Index (assessed at days 2 and 6 of treatment). Safety was assessed by the frequency of AEs and SAEs.
Statistical methods
The sample size used in this trial was based on an anticipated treatment difference of 1.2 on a 10-point NRS and a common standard deviation of 3, yielding a standardized treatment difference of 0.4 for the primary endpoint. A sample size of 300 patients per stratum of worst procedure site was required to achieve 86% power to detect a treatment difference of 1.2 for the primary endpoint between the treatment effects of ibuprofen + caffeine and ibuprofen, and 71% power between ibuprofen + caffeine and placebo.
All secondary endpoints were analyzed using the treated set (TS), which comprised all randomized patients who took at least one dose of trial medication. The full analysis set (FAS), which included all patients in the TS who provided a baseline value for POMWP at Visit 1 (before drug intake) and at least 1 POMWP-value post treatment at Visit 1 (morning of day 1, 2 hrs after drug intake) and at Visit 2 (morning of day 2, 2 hrs after drug intake), was used for the analysis of the primary endpoint.
The trial was analyzed using generalized linear models, which included terms for country (Germany or Russia), worst procedure site (back or neck) and disease severity as covariates. The primary endpoint was analyzed using a restricted maximum likelihood (REML) based repeated measures approach, based on a mixed-effect model for repeated measures analysis (MMRM), using all available POMWP data. The key secondary endpoints were analyzed using an analysis of covariance (ANCOVA), including treatment, country and worst procedure site as fixed effects and baseline POMWP as a continuous covariate. The change in PA and average daily pain at rest were analyzed analogously to the primary endpoint using all available longitudinal observations. The Oswestry Disability Index was also analyzed analogously to the primary endpoint. Other secondary endpoints were analyzed by logistical regression models, adjusted for country and worst procedure site. For analyses that utilized a likelihood-based repeated measures model, no imputation of missing values was performed. For the calculation of POMWPAUC72h and POMWPAUC120h, imputation rules for missing POMWP assessments were applied. Finally, analysis of safety and tolerability was done descriptively.
Results
Demographics and baseline characteristics
A total of 635 patients, randomized across 19 sites in Germany and Russia, were included in the TS and FAS populations. In total, 519 (81.7%) patients were from sites in Germany and 116 (18.3%) were from sites in Russia. Overall, 289 (55.7%) patients from Germany had back pain and 230 (44.3%) patients had neck pain, whilst 56 (48.3%) patients from Russia presented with back pain and 60 (51.7%) patients had neck pain. Baseline assessments of pain were similar for each treatment group. Patient demographics and baseline disease characteristics can be found in Table 1.
 |
Table 1 Baseline and disease characteristics (Treated set) |
Following randomization, patients were assigned to ibuprofen + caffeine (n=256), ibuprofen (n=253) or placebo (n=126), as seen in Figure 1. The first patient was screened on the January 5, 2017 and the date of last contact with any patient was the September 28, 2017.
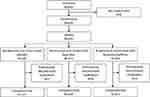 |
Figure 1 Disposition of patients. |
Treatment
In line with the treatment schedule, the overall mean duration of treatment was 6.2 days (6.2 days, ibuprofen + caffeine; 6.2 days, ibuprofen; 6.1 days, placebo) and the mean number of tablets taken per patient was 16.6 (16.6 tablets, ibuprofen + caffeine; 16.6 tablets, ibuprofen; 16.4 tablets, placebo), with nearly identical values in each treatment group. Similar proportions of patients assigned to ibuprofen + caffeine (12.1%), ibuprofen (8.7%) and placebo (12.7%) took rescue medication.
Efficacy
The superiority of ibuprofen + caffeine versus ibuprofen or placebo was not demonstrated with regard to reduction in POMWP. Adjusted mean reductions in POMWP from baseline to the morning of day 2 (2 hrs after drug intake) are shown in Table 2. The reduction in POMWP from baseline to day 2 was 1.998 (SE: 0.1042), 1.869 (SE: 0.1030) and 1.712 (SE: 0.1422) for the ibuprofen, ibuprofen + caffeine and placebo groups, which corresponds to pain reductions of 29.4%, 28.3% and 24.8% compared to baseline, respectively. The treatment difference between ibuprofen + caffeine, ibuprofen alone (P=0.3358) or placebo (P=0.3446) was not significant. With regard to the subgroup analyses for this endpoint, some heterogeneity was evident between the German and Russian subgroups. In Germany, patients assigned to placebo had the smallest mean change in POMWP (least reduction in pain score) on day 2 (1.581 [SE: 0.1599]) compared with ibuprofen + caffeine (1.883 [SE: 0.1126]) and ibuprofen alone (1.987 [SE: 0.1128]), corresponding to pain reductions of 22.9%, 29.0% and 29.2% compared to baseline. P-values were 0.5160 for the comparison between ibuprofen + caffeine and ibuprofen, and 0.1233 for ibuprofen + caffeine versus placebo. In contrast, in Russia, a strong placebo effect was seen, with reductions in POMWP on day 2 being the greatest for placebo (2.234 [SE: 0.1793]), compared with ibuprofen alone (1.970 [SE: 0.1240]) and ibuprofen + caffeine (1.769 [SE: 0.1217]), corresponding to pain reductions of 32.4%, 28.6% and 24.9% compared to baseline. Here, P-values were 0.2520 for the comparison between ibuprofen + caffeine and ibuprofen, and 0.0344 for ibuprofen + caffeine versus placebo. In terms of worst procedure site, patients with back pain tended to report a larger treatment difference between the active treatments and placebo than those with neck pain, who showed a tendency towards similar reductions in POMWP for each treatment group (Table 2).
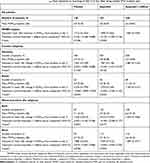 |
Table 2 Adjusted mean reduction in POMWP from baseline to morning of day 2 (2 hrs after drug intake) (Full analysis set) |
POMWPAUC72h was similar between patients receiving ibuprofen and those receiving ibuprofen + caffeine. The treatment difference between ibuprofen + caffeine and placebo was in favor of ibuprofen + caffeine (−0.288; 95% CI −0.572, −0.003; P=0.0474). In all treatment groups, the mean values for POMWPAUC120h were lower compared with those for POMWPAUC72h, indicating a small reduction in pain over time. Consistent with POMWPAUC72h (until day 4), the results of POMWPAUC120h (until the morning of day 6) suggested that patients assigned to placebo reported higher average pain scores than patients assigned to ibuprofen + caffeine or ibuprofen alone. The treatment difference between ibuprofen + caffeine and placebo was, again, in favor of ibuprofen + caffeine (−0.399; 95% CI −0.698, −0.100; P=0.0091). As observed for the primary endpoint, patients in Russia showed a stronger placebo effect for POMWPAUC72h and POMWPAUC120h than patients in Germany; however, the large difference in the number of patients enrolled from Russia (n=116) and those enrolled from Germany (n=519) should be noted. As seen for the primary endpoint, patients with back pain tended to report a larger treatment difference between the active treatments and placebo than those with neck pain. These data can be found in Tables S1 and S2.
Change in PA between baseline and the morning of day 2 (2 hrs after drug intake) is shown in Table S3. On day 2, the pressure on the trigger point in the area of the worst procedure site could be increased by at least 3 N/cm2 in all treatment groups. Only small differences in baseline and day 2 values were observed between the placebo (−3.734 [SE: 0.6107]), ibuprofen (−3.331 [SE: 0.4396]) and ibuprofen + caffeine (−3.175 [SE: 0.4366]) groups.
With regard to the global assessment of efficacy at the end of treatment (Figure 2), most patients rated the trial medication as “good” (46 [36.5%] placebo; 115 [45.5%] ibuprofen; 116 [45.3%] ibuprofen + caffeine) or “fair” (35 [27.8%] placebo; 57 [22.5%] ibuprofen; 66 [25.8%] ibuprofen + caffeine). Overall, 22.2% of patients assigned to placebo rated the trial medication as “poor” compared with only 12.5% of patients assigned to ibuprofen + caffeine. The difference seen between the global assessment of efficacy by the patient for placebo and that for ibuprofen + caffeine was significant (P=0.0045) and in favor of ibuprofen + caffeine.
 |
Figure 2 Global assessment of efficacy by the patient at the end of treatment (treated set). P=0.9603 for ibuprofen + caffeine vs ibuprofen, and P=0.0045 vs placebo, respectively. |
Decreases in POMWP of ≥30% or ≥50% between baseline and the morning of day 2 (2 hrs after drug intake) are shown in Table S4. The number of patients who reported a clinically meaningful decrease in POMWP of ≥30% by day 2 was similar between all groups (39.5%, 43.9% and 38.9% for the ibuprofen + caffeine, ibuprofen and placebo groups, respectively), with no significant difference between ibuprofen + caffeine and ibuprofen (P=0.3129), as well as placebo (P=0.9022)). Decreases in POMWP of ≥50% between baseline and day 2 were much lower than the decreases in POMWP of ≥30% (17.2%, 23.3% and 13.5% for the ibuprofen + caffeine, ibuprofen and placebo groups, respectively; ibuprofen + caffeine vs ibuprofen (P=0.0864); ibuprofen + caffeine vs placebo (P=0.3301)).
Time to first meaningful POMWP relief within 2 hrs after the first dose of trial medication is shown in Table S5. Across all treatment groups, less than 25% of patients reported meaningful POMWP relief within 2 hrs of the first dose of trial medication. Analyses showed no major differences over time between the three treatment groups (ibuprofen + caffeine vs ibuprofen (P=0.3534); ibuprofen + caffeine vs placebo (P=0.9384)).
Average daily pain scores decreased from baseline to day 6 for all treatment arms (Figure 3). On days 2–4 of treatment, a significant treatment difference was seen on average daily pain scores at rest for the ibuprofen + caffeine group compared with the placebo group (P=0.0046, P=0.0333 and P=0.0020 on days 2, 3 and 4, respectively). Reductions in average daily pain scores at rest were generally similar between the ibuprofen + caffeine and the ibuprofen alone groups from baseline to day 6; however, a significant treatment effect was seen between the active treatments on day 2 (P=0.0420). A similar pattern for each treatment group was also observed in the subgroup analysis of average daily pain scores at rest by worst procedure site for lower back pain and neck pain (with P=0.0380 for treatment difference between ibuprofen + caffeine and ibuprofen on day 2; Figure 3B), whereas effects of active treatments on neck pain appeared to be smaller (Figure 3C).
In addition, on days 2 and 6, Oswestry Disability Index scores22 were higher for patients receiving placebo (24.1% and 16.3% on days 2 and 6, respectively) compared with those receiving ibuprofen alone (22.3% and 13.5% on days 2 and 6, respectively) or ibuprofen + caffeine (22.1% and 13.4% on days 2 and 6, respectively), indicating a greater disability at these time points in this patient group (Figure 4). When compared with placebo, a significant treatment effect was observed on Oswestry Disability Index scores following ibuprofen + caffeine treatment on both day 2 (P=0.0136) and day 6 (P=0.0102). No significant treatment difference was seen on Oswestry Disability Index scores on days 2 and 6 when comparing the two active treatments (P=0.7629 and P=0.8351, respectively). On day 6, neck pain treatment effects for ibuprofen + caffeine versus placebo were statistically different (P=0.0123; Figure 4C).
Safety
The overall proportion of patients with treatment-emergent AEs was low, with no patients reporting severe AEs during the treatment period. Patients who received placebo reported the lowest frequency of all-grade AEs (5.6%) compared with those who received ibuprofen + caffeine (7.8%) and ibuprofen alone (7.1%). The most commonly reported AEs were headache (1.9%) and upper abdominal pain (1.1%). In total, five patients had AEs that led to treatment discontinuation (ibuprofen + caffeine, n=2; ibuprofen, n=2; placebo, n=1). An overall summary of AEs and the most frequently occurring AEs is shown in Table 3.
 |
Table 3 Incidence of adverse events and most frequent adverse events (Treated set) |
Discussion
This trial was designed to evaluate the superiority of efficacy and safety of the fixed-dose combination of 400 mg ibuprofen plus 100 mg caffeine compared with 400 mg ibuprofen alone or placebo for the treatment of acute lower back or neck pain. The trial was the first to assess the analgesic adjuvant effect of caffeine for the treatment of acute lower back or neck pain.
NSAIDs are often the first line treatment for lower back pain; however, clinical data are sparse. In a review by Machado et al, the treatment difference between NSAIDs and placebo was reported to be below the threshold for clinical importance for the treatment of spinal pain,23 suggesting that limitations exist for the use of NSAIDs in lower back or neck pain. Based on existing research in which caffeine has been used as an adjuvant in analgesic combination products with NSAIDs,15,16,18–20 it was anticipated that, upon the addition of caffeine to ibuprofen, an increase in efficacy (thus improved POMWP) would be observed. The mechanisms by which caffeine enhances the efficacy of analgesics are not yet well understood; however, proposed mechanisms of action include: increased drug absorption, decreased metabolic clearance, blockade of peripheral pro-nociceptive adenosine signaling and activation of the central noradenosine pathway, downregulation of cyclooxygenase-2 (COX-2), and changes in mood that lead to changes in pain perception.15 It must be noted however, that the definitions of “acute pain” are largely different for back/neck pain (acute lower back pain is commonly defined as pain lasting <6 weeks; in this trial, acute lower back pain was defined as pain lasting >24 hrs and <21 days) compared with pain resulting from postpartum uterine cramping, tension headache, migraine or dental extraction, where the superiority of caffeine-containing analgesics has previously been demonstrated.15–20
In the present trial, ibuprofen + caffeine was not superior to ibuprofen alone or placebo at relieving acute lower back or neck pain. On assessing change in POMWP from baseline to day 2, the primary endpoint of this trial, changes were similar between all treatment groups. In addition, the treatment difference observed for ibuprofen + caffeine compared with that for ibuprofen alone or placebo was not significant. Further analysis indicated that the reduction in POMWP from baseline to day 2 was not significant for ibuprofen alone compared with placebo. This may be a result of the unexpected findings in the Russian subgroup, in which a strong placebo effect was reported, and that may have influenced the overall findings of this trial. In Russia, reductions in POMWP on day 2 were largest for the placebo group compared with the ibuprofen + caffeine and ibuprofen groups, indicating a strong placebo effect. In contrast, in Germany, patients assigned to placebo had the smallest change in POMWP on day 2 compared with ibuprofen + caffeine and with ibuprofen alone. The findings in the German subgroup suggest that the methodology of this trial was sound as there was a placebo response in this subgroup and, although it was not significant, both active treatments demonstrated an analgesic effect greater than that seen with placebo (reduction in POMWP of 1.883, 1.987 and 1.581 following ibuprofen + caffeine, ibuprofen alone and placebo, respectively).
The findings for the primary endpoint were supported by the secondary endpoint analyses. Between baseline and the morning of day 4, patients receiving ibuprofen alone had the lowest AUC with regard to POMWP, followed by ibuprofen + caffeine and placebo. The treatment difference between ibuprofen + caffeine and placebo was significant for this endpoint (P=0.0474). The results indicate that patients receiving placebo reported higher average pain scores for POMWPAUC120h, than those receiving ibuprofen + caffeine or ibuprofen alone. The treatment difference between ibuprofen + caffeine and placebo was, again, significant (P=0.0091) for this endpoint. The difference seen between the global assessment of efficacy by patients in the placebo group and those assigned to ibuprofen + caffeine was also significant (P=0.0045) and in favor of ibuprofen + caffeine. As for change in PA, on day 2 the pressure on the trigger point could be increased by at least 3 N/cm2 for all treatment groups, with no significant treatment differences observed between groups. Furthermore, the number of patients who reported a clinically meaningful decrease in POMWP of ≥30% was similar between all treatment groups (39.5%, 43.9% and 38.9% for the ibuprofen + caffeine, ibuprofen and placebo groups, respectively).
Data collected for average daily pain at rest and the Oswestry Disability Index, suggested that ibuprofen + caffeine was superior to placebo for the treatment of acute lower back or neck pain. For average daily pain at rest, a significant treatment effect was observed on days 2–4 of treatment for the ibuprofen + caffeine group compared with the placebo group. Pain scores for average daily pain at rest were similar between the ibuprofen + caffeine and ibuprofen alone groups, with a treatment effect in favor of ibuprofen + caffeine seen on day 2. As for the Oswestry Disability Index scores, on days 2 and 6, patients receiving placebo had significantly higher scores (greater disability) than those receiving ibuprofen + caffeine. No significant treatment differences were observed on the Oswestry Disability Index scores when comparing the ibuprofen + caffeine and ibuprofen groups.
Taken together, the results of this trial are in line with those of existing literature for ibuprofen alone, where inconsistent treatment effects have been reported.16–19,21,23 Although ibuprofen + caffeine did not demonstrate significant superiority over placebo for the primary endpoint of this trial, for multiple secondary endpoints (POMWPAUC72h and POMWPAUC120h, global assessment of efficacy, average daily pain at rest and Oswestry Disability Index) ibuprofen + caffeine was reported to have a superior treatment effect compared with placebo, indicating a larger reduction in pain. The analysis of the secondary and other endpoints of this trial supports the hypothesis that the combination of ibuprofen plus caffeine does have an effect on acute lower back and neck pain but that this was not revealed by the primary efficacy variable of the trial. The findings from the secondary and other analyses of this trial are in line with a study of ibuprofen alone by Dreiser et al which found that patients receiving ibuprofen recorded a significant improvement in pain intensity on a 100 mm visual analogue scale (approximately 11 mm) compared with placebo (P<0.001)7 They do not suggest however, that the combination of ibuprofen + caffeine is superior to ibuprofen alone, unlike a number of studies investigating the effects of ibuprofen + caffeine in other pain models (tension-type headache and dental extraction) which have shown the combination treatment to have a superior treatment effect than both placebo and ibuprofen alone.16,18–20
When taken collectively with previously published trial data, the results of this trial pose the question of what effective treatments are available for back pain, particularly as over-the-counter medications. Paracetamol, the recommended first-line treatment for acute non-specific lower back pain for a long time, did not reduce time to recovery compared with placebo in patients with lower back pain in a large clinical trial by Williams et al.24 Furthermore, ibuprofen and diclofenac resulted in only a borderline pain reduction (approximately 11 mm on a 100 mm visual analogue scale) in the study by Dreiser et al.7 It appears that one very effective treatment for back pain reported so far is topical cream containing a capsaicinoid and a nicotinic acid ester (nonivamide and nicoboxil).25 In a study by Blahova et al, lower back pain was significantly reduced (P=0.0001) following nonivamide/nicoboxil treatment compared with placebo, with 36.2% of patients reporting an onset of pain relief as fast as 30 mins after administration of nonivamide/nicoboxil.25
The present trial had several design strengths. Firstly, POM is recognized as a reliable endpoint for evaluating the efficacy of acute pain treatments.21,26,27 In addition, the scales used for assessing analgesic efficacy in this trial are standard and are used almost universally in controlled clinical trials of analgesic drugs. Furthermore, in this trial POM assessments were always supported by the same adequately trained person per individual patient to ensure consistency in scoring. This trial was designed prospectively to compare ibuprofen + caffeine versus placebo, as well as ibuprofen + caffeine versus ibuprofen, whereas it is more common practice for comparisons of active treatments versus placebo to be performed as post-hoc analyses.
Limitations of this trial included the large difference in patient numbers between the German and Russian subgroups. Additionally, a sample size of 300 patients per stratum concerning the worst procedure site was required to have 86% power to detect a difference of 1.2 on a 0–10 NRS for the primary endpoint. This was only met by the back pain subgroup which consisted of 345 patients, whereas the neck pain subgroup comprised only 290 enrolled patients.
Conclusion
For the overall trial population, a decrease in acute lower back and neck pain, as indicated by a reduction in POMWP between baseline and the morning of day 2, was shown in all treatment arms. However, there was an unexpectedly strong placebo response, with none of the active treatments demonstrating significant superiority over placebo for the treatment of acute lower back or neck pain. This trial therefore failed to demonstrate that the fixed-dose combination of 400 mg ibuprofen plus 100 mg caffeine is superior to 400 mg ibuprofen alone or placebo for relieving acute lower back or neck pain in this trial population or within the trial setting. Although ibuprofen has previously been shown to be significantly effective for the short-term symptomatic relief of acute and chronic lower back pain without sciatica, effect sizes were small in this trial. Thus, it remains open as to whether the combination of ibuprofen plus caffeine is superior to ibuprofen alone for the treatment of acute lower back or neck pain, as a superior benefit may be limited to a subpopulation of patients.
Data sharing statement
Qualified researchers may request access to patient level data and related study documents including the clinical study report, study protocol with any amendments, blank case report form, statistical analysis plan, and dataset specifications. Patient level data will be anonymized and study documents will be redacted to protect the privacy of trial participants. Further details on Sanofi’s data sharing criteria, eligible studies, and process for requesting access can be found at: https://www.clinicalstudydatarequest.com
Acknowledgment
The trial (NCT03003000) was sponsored by Boehringer Ingelheim Pharma GmbH & Co and the development of this manuscript supported by Sanofi-Aventis, the current Market Authorization Holder.
The authors would like to acknowledge Simon Hitier (Sanofi-Aventis) for his statistical support, and Evie Lambert of iMed Comms, an Ashfield Company, part of UDG Healthcare plc for medical writing support that was funded by Sanofi in accordance with Good Publications Practice (GPP3) guidelines (http://www.ismpp.org/gpp3).
Disclosure
Caty Ebel-Bitoun, Robert Lange and Thomas Weiser are employees at Sanofi Aventis. Hans-Georg Predel has received study grants, honoraria for speaking at congresses and consultancy fees from Sanofi-Aventis and personal fees from Reckitt Benckiser, during the conduct of the study. The authors report no other conflicts of interest in this work.
References
1. Hoy D, Bain C, Williams G, et al. A systematic review of the global prevalence of low back pain. Arthritis Rheum. 2012;64(6):2028–2037. doi:10.1002/art.34347
2. Casser HR, Seddigh S, Rauschmann M. Acute lumbar back pain. Dtsch Arztebl Int. 2016;113(13):223–234. doi:10.3238/arztebl.2016.0223
3. Deyo RA, Weinstein JN. Low back pain. N Engl J Med. 2001;344(5):363–370. doi:10.1056/NEJM200102013440508
4. Cohen SP, Argoff CE, Carragee EJ. Management of low back pain. BMJ. 2008;337:a2718. doi:10.1136/bmj.a2718
5. Hoy DG, Protani M, De R, Buchbinder R. The epidemiology of neck pain. Best Pract Res Clin Rheumatol. 2010;24(6):783–792. doi:10.1016/j.berh.2011.01.019
6. Evans G. Identifying and treating the causes of neck pain. Med Clin North Am. 2014;98(3):645–661. doi:10.1016/j.mcna.2014.01.015
7. Dreiser RL, Marty M, Ionescu E, Gold M, Liu JH. Relief of acute low back pain with diclofenac-K 12.5 mg tablets: a flexible dose, ibuprofen 200 mg and placebo-controlled clinical trial. Int J Clin Pharmacol Ther. 2003;41(9):375–385. doi:10.5414/cpp41375
8. Cooper SA, Schachtel BP, Goldman E, Gelb S, Cohn P. Ibuprofen and acetaminophen in the relief of acute pain: a randomized, double-blind, placebo-controlled study. J Clin Pharmacol. 1989;29(11):1026–1030.
9. Hersh EV, Levin LM, Cooper SA, et al. Ibuprofen liquigel for oral surgery pain. Clin Ther. 2000;22(11):1306–1318.
10. Packman B, Packman E, Doyle G, et al. Solubilized ibuprofen: evaluation of onset, relief, and safety of a novel formulation in the treatment of episodic tension-type headache. Headache. 2000;40(7):561–567.
11. Schachtel BP, Furey SA, Thoden WR. Nonprescription ibuprofen and acetaminophen in the treatment of tension-type headache. J Clin Pharmacol. 1996;36(12):1120–1125.
12. Cooper SA, Needle SE, Kruger GO. Comparative analgesic potency of aspirin and ibuprofen. J Oral Surg. 1977;35(11):898–903.
13. Palmer H, Graham G, Williams K, Day R. A risk-benefit assessment of paracetamol (acetaminophen) combined with caffeine. Pain Med. 2010;11(6):951–965. doi:10.1111/j.1526-4637.2010.00867.x
14. Sawynok J. Caffeine and pain. Pain. 2011;152(4):726–729. doi:10.1016/j.pain.2010.10.011
15. Derry CJ, Derry S, Moore RA. Caffeine as an analgesic adjuvant for acute pain in adults. Cochrane Database Syst Rev. 2014;12:CD009281.
16. McQuay HJ, Angell K, Carroll D, Moore RA, Juniper RP. Ibuprofen compared with ibuprofen plus caffeine after third molar surgery. Pain. 1996;66(2–3):247–251. doi:10.1016/0304-3959(96)03043-6
17. Laska EM, Sunshine A, Mueller F, Elvers WB, Siegel C, Rubin A. Caffeine as an analgesic adjuvant. JAMA. 1984;251(13):1711–1718.
18. Forbes JA, Beaver WT, Jones KF, et al. Effect of caffeine on ibuprofen analgesia in postoperative oral surgery pain. Clin Pharmacol Ther. 1991;49(6):674–684. doi:10.1038/clpt.1991.85
19. Diamond S, Balm TK, Freitag FG. Ibuprofen plus caffeine in the treatment of tension-type headache. Clin Pharmacol Ther. 2000;68(3):312–319. doi:10.1067/mcp.2000.109353
20. Weiser T, Richter E, Hegewisch A, Muse DD, Lange R. Efficacy and safety of a fixed-dose combination of ibuprofen and caffeine in the management of moderate to severe dental pain after third molar extraction. Eur J Pain. 2018;22(1):28–38. doi:10.1002/ejp.1068
21. Predel HG, Giannetti B, Pabst H, Schaefer A, Hug AM, Burnett I. Efficacy and safety of diclofenac diethylamine 1.16% gel in acute neck pain: a randomized, double-blind, placebo-controlled study. BMC Musculoskelet Disord. 2013;14:250. doi:10.1186/1471-2474-14-250
22. Fairbank JC. Why are there different versions of the Oswestry disability index? J Neurosurg Spine. 2014;20(1):83–86. doi:10.3171/2013.9.SPINE13344
23. Machado GC, Maher CG, Ferreira PH, Day RO, Pinheiro MB, Ferreira ML. Non-steroidal anti-inflammatory drugs for spinal pain: a systematic review and meta-analysis. Ann Rheum Dis. 2017;76(7):1269–1278. doi:10.1136/annrheumdis-2016-210597
24. Williams CM, Maher CG, Latimer J, et al. Efficacy of paracetamol for acute low-back pain: a double-blind, randomised controlled trial. Lancet. 2014;384(9954):1586–1596. doi:10.1016/S0140-6736(14)60805-9
25. Blahova Z, Holm JC, Weiser T, Richter E, Trampisch M, Akarachkova E. Nicoboxil/nonivamide cream effectively and safely reduces acute nonspecific low back pain - a randomized, placebo-controlled trial. J Pain Res. 2016;9:1221–1230. doi:10.2147/JPR.S118329
26. Predel HG, Giannetti B, Connolly MP, Lewis F, Bhatt A. Efficacy and tolerability of a new ibuprofen 200mg plaster in patients with acute sports-related traumatic blunt soft tissue injury/contusion. Postgrad Med. 2018;130(1):24–31. doi:10.1080/00325481.2018.1401422
27. Predel HG, Connolly MP, Bhatt A, Giannetti B. Efficacy and safety assessment of acute sports-related traumatic soft tissue injuries using a new ibuprofen medicated plaster: results from a randomized controlled clinical trial. Phys Sportsmed. 2017;45(4):418–425. doi:10.1080/00913847.2017.1382305
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


