Back to Journals » International Journal of Women's Health » Volume 9
A randomized crossover trial on the effect of compression stockings on nausea and vomiting in early pregnancy
Received 27 August 2016
Accepted for publication 12 December 2016
Published 22 February 2017 Volume 2017:9 Pages 89—99
DOI https://doi.org/10.2147/IJWH.S120809
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Elie Al-Chaer
Erika Mendoza,1 Felix Amsler2
1Venenpraxis, Wunstorf, Germany; 2Amsler Consulting, Basel, Switzerland
Introduction: This study aimed to assess the impact of wearing compression stockings on women’s quality of life (QoL) associated with nausea and vomiting in early pregnancy (NVP).
Methods: In this randomized, open, single-center, crossover study, 74 women were assigned 1:1 to 2 weeks with compression stockings followed by 2 weeks without or vice versa. The main outcomes were NVP-associated QoL, leg-related QoL, and dizziness, as assessed by the Nausea and Vomiting in Pregnancy Quality of Life (NVPQOL) questionnaire, Chronic Venous Disease Quality of Life (CIVIQ) questionnaire, and questions on dizziness at baseline and after each 2-week period, respectively. Daily NVP was assessed using the modified Pregnancy-Unique Quantification of Emesis and Nausea (PUQE) questionnaire. Data were analyzed using Pearson’s chi-square and independent t-tests.
Results: Fifty-eight women completed the study. QoL scores improved with compression treatment; changes in mean total scores were as follows: NVPQOL, -36.7 with compression vs -21.7 without (P<0.0001); and CIVIQ, -4.5 with compression vs +1.4 without (P=0.001). Mean dizziness scores were -3.2 with compression vs -0.4 without (P<0.0001). PUQE mean total score (standard deviation) was 4.9 (2.3) with compression vs 5.5 (2.2) without (P=0.042).
Discussion: Wearing of compression stockings in early pregnancy may improve nausea and vomiting-associated symptoms in addition to improving QoL factors.
Keywords: compression stockings, pregnancy, nausea, quality of life, dizziness, circulatory disturbances
Introduction
Approximately 50%–80% of women experience nausea and vomiting in early pregnancy (NVP), that is, during the first and early second trimesters. Onset of symptoms is usually at about the 5th week after the last menstrual period, with a peak at 8–12 weeks and resolution by 16–20 weeks.1,2 NVP may be categorized as emesis gravidarum, which is mild, or hyperemesis gravidarum, which requires treatment or even hospitalization. A systematic review of studies that used quantification instruments to measure NVP, excluding women with hyperemesis gravidarum, emphasized the detrimental impact of NVP on women’s quality of life (QoL), especially their ability to work.2
Nausea and vomiting have multiple causes. In pregnancy, physical examination and routine laboratory investigations should be performed to rule out known and treatable reasons for the condition.3 NVP is diagnosed only after other causes have been ruled out; furthermore, as it is limited to early pregnancy, it is often diagnosed retrospectively.3
Although the cause of NVP is still unknown, several possible contributing factors have been identified, including human chorionic gonadotropin (hCG). Levels of this hormone are highest in the first trimester, coincident with the peak of vomiting and nausea, and women with NVP have been shown to have higher hCG concentrations in serum and urine than asymptomatic pregnant women.4 Female sex hormones impair the function of smooth muscle, affecting esophageal, gastric, and small bowel motility, and this dysmotility may be another contributing factor to NVP.5,6 The same effect has been shown in nonpregnant women taking oral contraceptives.7 Psychological reasons have been postulated but never demonstrated.3 The factors possibly contributing to NVP have been reviewed by Lee and Saha.8 It has also been suggested that, in evolutionary terms,
NVP could have a protective effect in early pregnancy, realized by nutritional change, increased social support, more passive and careful behavior, earlier recognition of pregnancy and a positive influence on fetal development.9
It may be no coincidence, therefore, that NVP is associated with fewer fetal deaths and higher birth weight.10,11
Successful treatment of NVP would improve the QoL of pregnant women; however, therapies used in early pregnancy must be demonstrably safe and not increase the risk of spontaneous abortion or birth defects. A number of therapeutic interventions ranging from pharmaceutical products to natural therapies are available to help alleviate symptoms.1,12 However, women remain reluctant to take medications during pregnancy,13 and a safe, effective, and acceptable treatment for NVP is still to be found.
Clinical guidelines recommend that pregnant women wear compression stockings to prevent and/or treat venous edema, venous thromboembolism and varicose veins.14–16 A recent study investigated treatment compliance in women who were offered the option of wearing compression stockings between weeks 4 and 28 of pregnancy.17 Of 98 women, 69.4% agreed to wear compression stockings, of whom 58.8% wore them daily. The study reported that use of compression stockings significantly reduced leg pain and increased QoL, as assessed by the Chronic Venous Disease Quality of Life (CIVIQ) questionnaire. The largest positive effects were observed in the group of women who wore compression stockings every day.17
This study was undertaken following the first author’s personal experience with and without stockings in early pregnancy. The aim was to evaluate the effect of compression stockings on symptoms of nausea, vomiting, and dizziness between weeks 8 and 16 of pregnancy, and the impact on QoL, using validated measures.
Methods
Patients and study design
This randomized, single-center, open, crossover study was conducted in Wunstorf, Germany, from November 2013 to March 2015 (trial registration no DRKS00009679 in the German Clinical Trials Register). Pregnant women aged >18 years and with mild to moderate NVP were eligible to participate. Exclusion criteria included severe hyperemesis; a history of thrombosis, edema, or skin changes typical of chronic venous insufficiency; no palpable arterial pulses, deep, or superficial reflux on ultrasound; requirement for custom-made stockings; regular prior use of compression stockings; and inability to read and complete a questionnaire in German. Women who reported leg pain were referred for specialist medical advice to rule out venous disorders. The study was approved by the Ethics Committee of the Medical Chamber of Lower Saxony (BO/28/2013), and all participants provided written informed consent.
Eight local gynecologists were sent details of the planned research project, and asked to inform their patients about the study and pass on an information sheet. After reading the information, pregnant women who were willing to participate were requested to confirm their interest with the author’s office. The planned investigation was also reported in the local press, asking eligible patients to contact their gynecologist or the author’s office. The eligibility of potential study participants was assessed over the phone and eligible women were scheduled for a first visit during weeks 8–14 (inclusive) of their pregnancy. Staff explained to patients that the purpose of the study was to investigate the possible influence of stockings on symptoms in a manner that was intended to avoid influencing patients’ subsequent perceptions.
During the first visit, a detailed physical examination was conducted, including photoplethysmography (Elcat Vasoquant®, Elcat GmbH, Wolfratshausen, Germany) and duplex ultrasound (Fuji Fazone®, Fujifilm, Tokyo, Japan; fitted with an 8 MHz linear probe), to assess blood flow in deep and superficial leg veins. Medical history was assessed to ensure that women satisfied the inclusion and exclusion criteria. All women who were eligible were randomized 1:1, in three blocks of 20 participants, to 2 weeks with compression stockings followed by 2 weeks without (compression first) or the reverse sequence (compression second). Three blocks of 20 unmarked envelopes were prepared and sealed, containing 10 for each group, all identical in appearance; each woman chose an envelope at random. To achieve groups of equal size, women who dropped out were replaced after each block of participants by adding the corresponding number of identical-appearing unmarked envelopes. Due to the nature of the intervention, participants and investigators were not blinded to the treatment sequence.
During the compression period, participants were supplied with two pairs of appropriately sized medical calf compression stockings (23–32 mmHg at the ankle; Cotton Quality, SIGVARIS Management AG, Winterthur, Switzerland) and instructed to wear them during waking hours for a minimum of 5 hours every day and to document the number of hours worn. Participants were shown how to wear the stockings and given opportunities to practice putting them on.
Assessment of endpoints
The primary endpoint was the change in the Nausea and Vomiting in Pregnancy Quality of Life (NVPQOL) score between baseline and the end of each period (with or without compression). Secondary endpoints were the changes in dizziness, CIVIQ, and Pregnancy-Unique Quantification of Emesis and Nausea (PUQE) scores. Safety was assessed by the CIVIQ questionnaire and by monitoring leg-associated symptoms.
The NVPQOL questionnaire is a validated NVP-specific QoL instrument that contains 30 items and covers four general domains consisting of physical symptoms and aggravating factors, fatigue, emotions, and limitations (Table S1).18,19 The total score is between 30 and 210, with lower scores corresponding to better QoL.
To assess dizziness, the following four questions were added to the NVPQOL questionnaire:
- How often in the last week have you fainted or felt close to fainting after standing or straightening up quickly?
- How often in the past week have you felt dizzy during the day?
- How often in the past week have you had a sudden feeling of light-headedness?
- How often during the last week have you been restricted in your normal daily activity by circulatory problems (eg, faintness, dizziness, or light-headedness)?
The total score was obtained by summing the scores for each question.
Participants were also asked to complete a CIVIQ questionnaire, which is a validated tool developed to evaluate the impact of venous disease on QoL20 and is available at http://www.civiq-20.com/getting-copy/linguistic-versions-civiq-20/ (this questionnaire was developed by Professor Launois with an educational grant from Servier). Patients evaluate 20 items related to their QoL (pain [4 items] and physical [4 items], social [3 items], and psychological well-being [9 items]) on 5-point Likert scales. Answers are summed for each dimension and rescaled to a score ranging from 0 (best score, no impairment) to 100 (worst score, maximal impairment).
Participants were also given a modified PUQE questionnaire, which is a validated questionnaire that covers symptoms of nausea and vomiting during the preceding 12 hours (Table S2),21 to fill in daily at home until the next clinic visit (scheduled 2 weeks after the first visit). A question on the use of any drugs each day was added to the questionnaire. Higher scores reflect worse NVP. A question on compliance was added to the PUQE questionnaire to document whether the stockings were worn daily and the duration for which they were worn.
During the second study visit, the completed PUQE questionnaires were collected, the physical examination and QoL questionnaires were repeated, and new PUQE questionnaires issued. Used compression stockings were collected from the compression first group to ensure that no compression stockings were worn during the following 2 weeks. Participants in the compression second group were given stockings and introduced to their use, and were also given a new PUQE questionnaire to be completed at home. At the final visit 2 weeks later, the completed PUQE questionnaires were collected and the physical examinations repeated. The study participants completed a final set of the CIVIQ and NVPQOL and dizziness questionnaires. The questionnaires took ~15 minutes to complete, and all three were used in order to differentiate changes in edema-related complaints from those related to nausea and vomiting. Participants were also required to grade their experience based on four further questions that were compiled for this study (Table S3).
Statistical analysis
In response to advice from the Ethics Committee of the Medical Chamber of Lower Saxony in February 2011, a pilot study was performed on 20 patients. The results of this first group (20 patients enrolled, 17 finishing the pilot study) showed a significant reduction in most aspects of NVP as measured using both the NVPQOL and PUQE, allowing a power analysis to be conducted. The estimated number needed to achieve significant results was 60 (Table S4). Data were analyzed using Statistical Package for the Social Sciences (SPSS) version 13.0 (SPSS Inc, Chicago, IL, USA). Comparisons between the two groups were evaluated using Pearson’s chi-square and independent t-tests. A P-value of <0.05 was considered significant.
Results
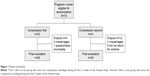
A total of 74 women were enrolled in the study. Sixteen of these patients dropped out of the study, as shown in Figure 1. Fifty-eight women completed the study and were included in the analysis set. Baseline data are shown in Table 1. Mean age was 31.7 years and mean gestational week was 9 weeks and 3 days. There were no statistically significant between-group differences in any baseline characteristics. Patients were allowed to use medications when considered necessary by their gynecologist, and the number and types of drugs taken did not differ between the phases with and without stockings. In the phases with/without stockings, respectively, 13/10 patients used a drug at least once, and on 10.9%/10.1% of all recorded days, a drug was taken.
Patient-reported compliance with compression therapy was high: 100% of participants reported wearing their compression stockings daily. Participants indicated that they wore the compression stockings for >8 hours on 7 days and for 5–8 hours on the other days during the 2-week compression period. The majority of patients completed their questionnaires: one CIVIQ questionnaire and one NVP plus dizziness questionnaire were not filled in at all; for all other questionnaires, >97% of individual questions were complete.
Mean scores for all NVPQOL domains decreased from baseline over time, indicating an improvement, and these decreases were greater for the period with compression (Table 2). Changes in NVPQOL are shown in Figure 2. Mean change from baseline in NVPQOL total score was significantly greater after the period with compression (−36.67) vs that without compression (−21.68; P<0.001). The detrimental impact of nausea and vomiting on QoL was therefore significantly lower during compression treatment. Similarly, participants experienced fewer periods of dizziness in the period with compression (mean change −3.22) vs that without compression (−0.44; P<0.001; Table 2). In the period without compression, episodes of dizziness increased over time (Figure S1).
  | Figure 2 Changes in NVPQOL score over time. |
Small, nonsignificant increases were observed in some CIVIQ scores from baseline to the end of the period without compression (Table 2). Mean change in total CIVIQ score from baseline differed significantly after the period with compression (−4.46) vs that without compression (+1.39).
Participants reported less pain in their legs, and fewer physical and psychological limitations after the period with compression than after the period without compression.
Baseline scores were not collected for the PUQE questionnaire. The daily evaluation of each parameter changed throughout the first week and plateaued in the second week (Figure S2). Therefore, the mean scores from the second week with and without compression were compared (Table 3). Compression significantly reduced the mean scores for nausea, retching, circulatory disturbance, and total PUQE. To provide a comparison with the more severe scores reported in a study that used medication to control NVP,22 patients were also divided into those with total PUQE scores of ≤6 (no or mild NVP) or >6 (moderate or severe NVP) without compression; mean PUQE total scores for these two categories are shown in Table 4.
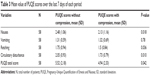  | Table 3 Mean value of PUQE scores over the last 7 days of each period |
Based on the questions, participants were asked at the end of the 4-week study, 50% felt that compression helped against nausea, 67% confirmed that compression helped them against heavy legs, 62% would go on wearing compression stockings or would wear them again, and over 80% would recommend wearing compression stockings during the first weeks of pregnancy.
Neither skin lesions nor alterations were reported, as assessed by physical examination, nor were any concerns expressed that caused participants to stop wearing the stockings.
Discussion
The results of this study indicate that wearing compression stockings for a 2-week period in early pregnancy significantly improved self-reported NVPQOL and all NVP-related parameters; dizziness; and leg-related QoL, pain and physical and psychological parameters. To our knowledge, this is the first study to evaluate the role of compression stockings in nausea and vomiting-associated symptoms and dizziness in early pregnancy. The first author was prompted to conduct this study following her own personal experience and consultations with previous patients.
Previous assessments of normal values for NVP and PUQE scores reported that the most severe NVP symptoms were observed between weeks 12–1619 or 10–1423 of pregnancy. As this study recruited women between gestational weeks 8 and 14, most participants would have experienced peak symptoms during the 4-week study. Participants were randomized into two groups with different treatment sequences in order to make allowance for the effect of a natural reduction in NVP symptoms as pregnancy progresses.
NVP-related QoL, as assessed by the NVPQOL questionnaire, improved significantly during both study periods (with and without compression). The natural evolution of symptoms over time explains the improvement from baseline following no compression. However, the crossover design of this study compensates for the effect of this natural evolution, and the results show that compression resulted in a greater improvement than no compression. When participants were categorized as above or below median with respect to their baseline NVPQOL score, the effect of compression was the same (data not shown), confirming that even women with mild emesis did benefit from compression. Although the degree of change in NVPQOL score that corresponds to “clinically significant improvement” is not yet established, it is clear from the results that compression gave rise to greater improvements than no compression.
Our study excluded women without any symptoms and those with very severe symptoms. The PUQE mean value calculated by Lacasse et al was based on a nonselected population, which may have included women with NVP symptoms at either end of the scale.21 In contrast, our study focused on a clinically important group of women, whose experience of NVP affects their QoL, but whose symptoms do not require acute medical intervention.
Pharmacologic intervention studies using the PUQE questionnaire start with higher PUQE scores, as medication should only be given in hyperemesis gravidarum. In one study, median PUQE score at baseline was 9.0 points in the group that received a delayed-release combination of doxylamine succinate 10 mg and pyridoxine hydrochloride 10 mg, and 8.8 points in the placebo group.22 Mean reductions were 4.8 points with intervention and 3.9 points with placebo (a difference of 0.9 points between the two groups; the total PUQE scores were not reported). To report results in a comparable population, we considered only the 22 patients with a PUQE score >6 points (ie, moderate or severe NVP) at baseline. Mean total PUQE score of these patients was 7.8 points without compression and 6.2 points with compression (a difference of 1.6 points; P=0.014), indicating that the difference between compression and placebo in these patients was at least equal to that observed following the use of medication.
The results of this study show that the strongest impact of compression was on circulatory disturbances. The impact on QoL of venous leg symptoms, assessed using the CIVIQ questionnaire, increased slightly during the period without compression. In contrast, a significant reduction in total CIVIQ symptoms was reported in the group with compression. The results of the present study are consistent with those of Allegra et al,17 who measured the effect of wearing stockings on the functional symptoms of venous disease in the pregnant women over a period of 6 months and reported a reduction in the impact on QoL of leg symptoms. The women in the previous study had baseline global CIVIQ scores of 36.6±15.6 (those who refused to wear stockings) and 47.8±15.7 (those who agreed to wear them); therefore, at baseline the impact on their QoL was much greater than that reported by our patients (baseline score 16.7±14.8; this smaller baseline score should be viewed within the context that none of the patients were suffering from venous disease, as it was an exclusion criterion). Changes in the CIVIQ score after 6 months were −13.5±9.6 in women who wore the stockings every day, −10.7±11.3 in those who wore them at least once every 2 days, and +3.3±9.4 in those who did not wear them (P<0.0001),17 compared with −4.5 in our patients during compression and +1.4 in our patients during the period without compression after 2 weeks. The lower changes in our study should be viewed within the context of the absence of venous disease and short duration of compression.
Circulatory disturbance, recorded with four new questions relating specifically to dizziness, was the only item showing a clearly ascending course, indicating worsening symptoms, during the period without compression. Compression had a favorable effect on these symptoms.
The effect of pregnancy on the gastrointestinal tract is well established.5,6 Hypotheses regarding the cause of NVP have focused on the role of female pregnancy hormones on smooth muscles, as summarized in a review of the literature on hyperemesis gravidarum.24 Deep calf veins are a venous reservoir with walls of smooth muscle. Hormonal changes in early pregnancy also act on the smooth muscles of the vasculature and are, hence, linked with conditions, such as varicose veins and venous thromboembolism. The impact of compression stockings on heart rate following standing up from a lying position in the third trimester of gestation25 might help to explain the compression-related improvement in dizziness, particularly in the first trimester of pregnancy. Furthermore, compression reduces the volume of blood remaining in the calf veins,26 possibly improving brain perfusion.27 This finding might offer an explanation for the reported reduction in dizziness observed in the present study and might also indirectly influence the feeling of nausea. However, these explanations are speculative, and the mechanism whereby compression improves symptoms is currently unknown.
The majority of participants in the present study reported that wearing stockings helped to alleviate the symptoms of NVP or heavy legs, that they would continue wearing the stockings after the study, and that they would recommend the stockings to others.
Limitations
A potential limitation of the present study is the fact that it was not possible to blind either the participants or investigators to the wearing of stockings in this crossover trial. As with any open study, the potential for bias due to the placebo effect is considerable, particularly as the main endpoints were self-reported. QoL assessments are by nature subjective; the instruments chosen for assessing QoL, validated questionnaires, were the best available option. Finally, as the patient-reported compliance with compression therapy was 100%, in this study, the beneficial effects may not be fully realized in real-world use, where patients are less likely to be fully compliant. However, real-life compliance may well be very high if patients notice that wearing stockings improves their symptoms, and as the stockings are needed only for a limited period. The promising results of the present study warrant further investigation in a larger randomized trial, especially considering that compression stockings have no known adverse effect on the unborn child or the mother. No other factors, apart from the wearing of compression stockings, could be identified to explain the observed results.
Conclusion
In this study, compression stockings reduced symptoms of nausea and vomiting, and could be a nonpharmaceutical instrument to alleviate these symptoms if confirmed in further studies.
Acknowledgments
This was an investigator-initiated study for which SIGVARIS Management AG provided a restricted grant.
The authors would like to thank the gynecologists who referred patients, the office staff, and all the patients willing to participate in this study, as well as Dr Heiko Czerlinsky and Dr Gabriele Czerlinsky, Berlin, for their help in the pilot study.
Medical writing assistance was provided by Grace Townshend and submission assistance by Nicholas Crabb, both of Watermeadow Medical Ltd, an Ashfield company (funded by SIGVARIS Management AG).
Author contributions
Dr Mendoza was involved in the conception and design of this work; the collection, analysis, and interpretation of data; the drafting and critical revision of the article; has approved the version to be published; and agrees to be accountable for all aspects of the article.
Mr Amsler was involved in the conception and design of this work; the analysis and interpretation of data; the drafting and critical revision of the article; has approved the version to be published; and agrees to be accountable for all aspects of the article.
Disclosure
Mr Amsler received payment from SIGVARIS Management AG for his statistical work and reports no other conflicts of interest in this work. Dr Mendoza reports no conflicts of interest in this work.
References
King TL, Murphy PA. Evidence-based approaches to managing nausea and vomiting in early pregnancy. J Midwifery Womens Health. 2009;54(6):430–444. | ||
Wood H, McKellar LV, Lightbody M. Nausea and vomiting in pregnancy: blooming or bloomin’ awful? A review of the literature. Women Birth. 2013;26(2):100–104. | ||
Chandra K. Development of a Health-Related Quality of Life (HRQL) Instrument for Nausea and Vomiting in Pregnancy (NVP) [master’s thesis]. Ottawa, Canada: University of Toronto; 2000. | ||
Masson GM, Anthony F, Chau E. Serum chorionic gonadotrophin (hCG), schwangerschaftsprotein 1 (SP1), progesterone and oestradiol levels in patients with nausea and vomiting in early pregnancy. Br J Obstet Gynaecol. 1985;92(3):211–215. | ||
Nagler R, Spiro HM. Heartburn in late pregnancy. Manometric studies of esophageal motor function. J Clin Invest. 1961;40:954–970. | ||
Soules MR, Hughes CL Jr, Garcia JA, Livengood CH, Prystowsky MR, Alexander E 3rd. Nausea and vomiting of pregnancy: role of human chorionic gonadotropin and 17-hydroxyprogesterone. Obstet Gynecol. 1980;55(6):696–700. | ||
Van Thiel DH, Gavaler JS, Stremple J. Lower esophageal sphincter pressure in women using sequential oral contraceptives. Gastroenterology. 1976;71(2):232–234. | ||
Lee NM, Saha S. Nausea and vomiting of pregnancy. Gastroenterol Clin North Am. 2011;40(2):309–334. | ||
Kohl S, Kainer F, Schiefenhovel W. Übelkeit und Erbrechen als evolutionäre Mechanismen der vielschichtigen Anpassung an die Schwangerschaft. Eine kulturenvergleichende Untersuchung an 565 Probandinnen [Nausea and vomiting as evolutionary mechanisms of the complex adaptation reaction to pregnancy. A comparative study of 565 subjects]. Z Geburtshilfe Neonatol. 2009;213(5):186–193. German. | ||
Tierson FD, Olsen CL, Hook EB. Nausea and vomiting of pregnancy and association with pregnancy outcome. Am J Obstet Gynecol. 1986;155(5):1017–1022. | ||
Czeizel AE, Puho E. Association between severe nausea and vomiting in pregnancy and lower rate of preterm births. Paediatr Perinat Epidemiol. 2004;18(4):253–259. | ||
Magee LA, Mazzotta P, Koren G. Evidence-based view of safety and effectiveness of pharmacologic therapy for nausea and vomiting of pregnancy (NVP). Am J Obstet Gynecol. 2002;186 (Suppl 5):S256–S261. | ||
Baggley A, Navioz Y, Maltepe C, Koren G, Einarson A. Determinants of women’s decision making on whether to treat nausea and vomiting of pregnancy pharmacologically. J Midwifery Womens Health. 2004;49(4):350–354. | ||
National Institute for Health and Care Excellence. Antenatal care for uncomplicated pregnancies. 2008. Available from: https://www.nice.org.uk/guidance/cg62. Accessed February 2, 2017. | ||
Queensland CG. Venous thromboembolism (VTE) prophylaxis in pregnancy and the puerperium – Queensland Clinical Guideline 2014. 2014. Available from: https://www.clinicalguidelines.gov.au/portal/2349/venous-thromboembolism-vte-prophylaxis-pregnancy-and-puerperium. Accessed February 2, 2017. | ||
Royal College of Obstetricians and Gynaecologists. Thrombosis and embolism during pregnancy and the puerperium, reducing the risk. 2015. Available from: https://www.rcog.org.uk/en/guidelines-research-services/guidelines/gtg37a/. Accessed February 2, 2017. | ||
Allegra C, Antignani PL, Will K, Allaert F. Acceptance, compliance and effects of compression stockings on venous functional symptoms and quality of life of Italian pregnant women. Int Angiol. 2014;33(4):357–364. | ||
Magee LA, Chandra K, Mazzotta P, Stewart D, Koren G, Guyatt GH. Development of a health-related quality of life instrument for nausea and vomiting of pregnancy. Am J Obstet Gynecol. 2002;186 (Suppl 5):S232–S238. | ||
Lacasse A, Berard A. Validation of the nausea and vomiting of pregnancy specific health related quality of life questionnaire. Health Qual Life Outcomes. 2008;6:32. | ||
Launois R, Reboul-Marty J, Henry B. Construction and validation of a quality of life questionnaire in chronic lower limb venous insufficiency (CIVIQ). Qual Life Res. 1996;5(6):539–554. | ||
Lacasse A, Rey E, Ferreira E, Morin C, Berard A. Validity of a modified Pregnancy-Unique Quantification of Emesis and Nausea (PUQE) scoring index to assess severity of nausea and vomiting of pregnancy. Am J Obstet Gynecol. 2008;198(1):71. | ||
Koren G, Clark S, Hankins GD, et al. Effectiveness of delayed-release doxylamine and pyridoxine for nausea and vomiting of pregnancy: a randomized placebo controlled trial. Am J Obstet Gynecol. 2010;203(6):571. | ||
Lacroix R, Eason E, Melzack R. Nausea and vomiting during pregnancy: a prospective study of its frequency, intensity, and patterns of change. Am J Obstet Gynecol. 2000;182(4):931–937. | ||
Verberg MF, Gillott DJ, Al-Fardan N, Grudzinskas JG. Hyperemesis gravidarum, a literature review. Hum Reprod Update. 2005;11(5):527–539. | ||
Austrell C, Nilsson L, Norgren L. Maternal and fetal haemodynamics during late pregnancy: effect of compression hosiery treatment. Phlebology. 1993;8(4):155–157. | ||
Mendoza E, Proebstle TM, Mumme A, Breu FX, Morrison N, Lattimer CR. Duplex Ultrasound of Superficial Leg Veins. Berlin: Springer; 2014. | ||
Blazek C, Amsler F, Blaettler W, Keo HH, Baumgartner I, Willenberg T. Compression hosiery for occupational leg symptoms and leg volume: a randomized crossover trial in a cohort of hairdressers. Phlebology. 2013;28(5):239–247. |
Supplementary materials
  | Table S1 Validated NVPQOL questionnaire |
  | Table S2 Validated and modified PUQE questionnaire |
  | Table S3 Questions to be answered at study end |
  | Figure S2 PUQE scores over time. |
References
Magee LA, Chandra K, Mazzotta P, Stewart D, Koren G, Guyatt GH. Development of a health-related quality of life instrument for nausea and vomiting of pregnancy. Am J Obstet Gynecol. 2002;186 (Suppl 5):S232–S238. | ||
Koren G, Boskovic R, Hard M, Maltepe C, Navioz Y, Einarson A. Motherisk-PUQE (pregnancy-unique quantification of emesis and nausea) scoring system for nausea and vomiting of pregnancy. Am J Obstet Gynecol. 2002;186 (Suppl 5):S228–S231. | ||
Lacasse A, Berard A. Validation of the nausea and vomiting of pregnancy specific health related quality of life questionnaire. Health Qual Life Outcomes. 2008;6:32. |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.