Back to Journals » International Journal of General Medicine » Volume 15
A Randomized Clinical Trial Comparing Different Concentrations of Chloroprocaine with Lidocaine for Activating Epidural Analgesia During Labor
Authors Zhu HJ , He Y , Wang SY , Han B , Zhang Y
Received 2 December 2021
Accepted for publication 21 January 2022
Published 9 February 2022 Volume 2022:15 Pages 1307—1317
DOI https://doi.org/10.2147/IJGM.S351030
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Hai-Juan Zhu,1,2,* Yan He,3,* Sheng-You Wang,2 Bo Han,2 Ye Zhang1
1Department of Anesthesiology and Perioperative Medicine, The Second Hospital of Anhui Medical University, Key Laboratory of Anesthesiology and Perioperative Medicine of Anhui Higher Education Institutes, Anhui Medical University, Hefei, 230601, People’s Republic of China; 2Department of Anesthesiology, Anhui Maternal and Child Health Care Hospital, Maternal and Child Health Care Hospital of Anhui Medical University, Hefei, 230001, People’s Republic of China; 3Department of Anesthesiology, Wannan Medical College, Wuhu, Anhui Province, 241002, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Ye Zhang
Department of Anesthesiology and Perioperative Medicine, The Second Hospital of Anhui Medical University, Key Laboratory of Anesthesiology and Perioperative Medicine of Anhui Higher Education Institutes, Anhui Medical University, Hefei, 230601, People’s Republic of China
, Tel +86-551-63869485
; +86-13966768081
, Fax +86 551-63869400
, Email [email protected]
Purpose: This study aimed to explore the efficacy and safety of chloroprocaine for activating labor analgesia and the optimal concentration compared to lidocaine.
Patients and Methods: Ninety-six nulliparous parturients were randomly assigned to three groups: LD group, patients received the conventional initial dose of 6 mL of 1% lidocaine; CP1.5 group, patients received 6 mL of 1.5% chloroprocaine as the initial dose; and CP1.2 group, patients received 7.5 mL of 1.2% chloroprocaine as initial dose. Labor analgesia was maintained in all patients via a programmed intermittent epidural bolus (PIEB). The primary outcome was the analgesia onset time. Secondary outcomes included the visual analog scale (VAS) scores, the interval and duration of uterine contractions during the first 12 contractions, failure to reach adequate analgesia, labor and neonatal outcomes, maternal satisfaction and adverse effects.
Results: Parturients in the CP1.5 and CP1.2 groups achieved a shorter onset time than those in the LD group (hazard ratio (HR) = 6.540; 95% confidence interval (CI), 3.503– 12.210; P < 0.001 and HR = 3.460; 95% CI, 1.905– 6.282; P < 0.001, respectively). The median time (95% CIs) to adequate analgesia was 12.0 (10.9– 13.1), 7.0 (6.2– 7.8) and 8.0 (7.5– 8.5) minutes in the LD, CP1.5 and CP1.2 groups, respectively. PIEB in the CP1.5 group was associated with lower VAS scores, patient-controlled epidural analgesia (PCEA) boluses, and analgesic consumption; a shorter time from epidural initiation to the first PCEA demand; and higher maternal satisfaction scores than the other two groups (P < 0.01). The interval and duration of uterine contractions, labor and newborn outcomes and adverse effects were comparable among the three groups.
Conclusion: Chloroprocaine provided a faster onset of labor analgesia than lidocaine. Thus, 6 mL of 1.5% chloroprocaine might be a superior volume and concentration regimen to 7.5 mL of 1.2% chloroprocaine for activating labor analgesia.
Clinical Trial Registration Statement: The study was registered prior to subject enrollment at www.chictr.org.cn (ChiCTR2100049113).
Keywords: epidural, labor analgesia, onset time, activation, chloroprocaine, lidocaine
Plain Language Summary
Epidural analgesia is the most effective and safe technique for alleviating labor pain, but it has the drawback of a slow onset time. Chloroprocaine is a fast-acting local anesthetic and is used safely for cesarean section and surgical anesthesia for infants. This study found that chloroprocaine achieved a faster onset of labor analgesia without increasing side effects, and 6 mL of 1.5% chloroprocaine might be the optimal volume and concentration for activating labor analgesia.
Introduction
Labor pain is widely described to be the most severe and unbearable pain suffered by women. In clinical practice, each mother is eager to relieve pain immediately during labor. Exploring ideal local anesthetics for rapid-onset and highly efficient pain relief via epidural analgesia (EA) during labor with minimal adverse effects and without altering maternal or fetal outcomes is extremely important in the clinic.1 According to a recent study, a longer EA onset time significantly correlates with a higher pain score at 60 and 120 min and the administration of a greater number of patient-controlled EA (PCEA) boluses during the first 120 min.2 Hence, studies exploring appropriate methods to shorten the onset time as much as possible are needed.
The epidural technique is the most effective and practical technique to relieve labor pain with minimal side effects and is routinely used in most hospitals.3 However, it has a drawback of delayed analgesia onset. Various factors affect analgesia onset, such as the pharmacological and pharmacokinetic properties of local anesthetics, the infused concentration and volume, and the pH and temperature of the anesthetic solution.2 In our clinical practice, 6 mL of 1% lidocaine is conventionally used for initiation followed by 8 mL of 0.08–0.125% ropivacaine plus sufentanil for a total loading dose consisting of 14–16 mL of solution. The mean EA onset time (time from the initial bolus to VAS score≤3) is approximately 15 min, which is significantly longer than that obtained with the combined spinal-epidural (CSE) technique and dural puncture epidural (DPE) technique.4
Chloroprocaine is a local ester anesthetic that is approved by the US Food and Drug Administration (FDA) for neuraxial analgesia due to its rapid onset and short duration.5 In recent years, chloroprocaine has been reintroduced into clinical practice and is widely used in emergency lower abdominal surgery and ambulatory lower limb surgery via epidural or spinal access.6–8 Coppens et al reported that the onset of action was 2–3 min when the drug was administered epidurally for labor analgesia.8 It has also been used for intrapartum emergency cesarean delivery in patients after labor EA with an onset time of 3–5 minutes, while that of lidocaine is 10–12 minutes.9 Kamath et al reported that chloroprocaine was safely used for labor EA in several patients who were allergic to lidocaine.10 However, limited evidence is available for the usefulness of chloroprocaine in epidural labor activation.
Hence, we hypothesized that chloroprocaine provides faster and better analgesia without increasing the incidence of adverse effects on the mother or fetus. As a method to test this hypothesis, we compared 1% lidocaine, 1.5% chloroprocaine and 1.2% chloroprocaine as initial doses to activate epidural analgesia in a prospective, double-blind, randomized controlled trial. The primary outcome was the time to analgesia onset, and the secondary outcomes were the effects of local anesthetic loading on uterine contraction activity, labor and infant outcomes and the incidence of side effects. We present the following article in accordance with the CONSORT 2010 reporting checklist.
Materials and Methods
Ethics Statement
This study was approved by the Institutional Ethics Committee of the Anhui Women and Child Health Care Hospital (NO. YYLL-2019-03-01) and was performed in accordance with the Declaration of Helsinki (1964); written informed consent was obtained from all parturients participating in the trial. The study was registered prior to subject enrollment at http://www.chictr.org.cn/index.aspx (ChiCTR2100049113) with a minor revision of drug dosages and pain evaluation intervals. This manuscript adheres to the applicable CONSORT guidelines.
Study Population
All nulliparous patients who presented for vaginal delivery and requested neuraxial analgesia at our hospital from July to October 2021 were recruited for this trial. The inclusion criteria were as follows: American Society of Anesthesiologists (ASA) physical status I or II, aged 20 to 40 years, body mass index (BMI) 20–35 kg/cm2, term pregnancy of a singleton fetus with spontaneous labor, cervical dilatation 2–3 cm, and provided written informed consent. The exclusion criteria were as follows: ASA physical status ≥III, aged <20 or >40 years, pregnancy-related disease (gestational diabetes, hypertension or preeclampsia), chronic pain, drug abuse, contraindications to neuraxial block, known fetal abnormality, and refusal to participate in the trial. Participants were also excluded if the epidural catheter was dislodged or delivery occurred within 1 hour after epidural catheter placement.11
Randomization and Concealment of Group Allocation
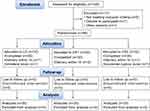
One hundred nine parturients were eligible; 13 parturients were excluded, and 96 parturients were randomly divided into three groups using a random number: 1) patients in the LD group received the conventional initial dose of 6 mL of 1% lidocaine; 2) patients in the CP1.5 group received 6 mL of 1.5% chloroprocaine (9 mg); and 3) patients in the CP1.2 group received 7.5 mL of 1.2% chloroprocaine (9 mg). The CP1.5 and CP1.2 groups were designed to receive the same total dosage and different concentrations of chloroprocaine.
 |
Table 1 Demographic and Baseline Data of Parturients |
Prior to the start of the study, 96 cards were created (32 cards per group) and placed in sealed envelopes to assure proper blinding. Each envelope was assigned a number from 1–96 via a computer-generated random sequence. The CONSORT trial flow diagram is shown in Figure 1. The anesthesiologist completed epidural puncture and connected the analgesic pump, and the anesthesia nurse was responsible for outcome assessment and recording. The parturients and the outcome assessors (anesthesia nurses) were blinded to the group allocation. All procedures were performed by a senior anesthesiologist-in-charge who had previously completed over 500 neuraxial procedures.
Procedure of Labor Analgesia
All participants received 500 mL of lactated Ringer’s solution as an intravenous fluid bolus. Blood pressure, heart rate, oxygen saturation (SpO2) determined using pulse oximetry, and fetal heart rate determined using echocardiography were monitored beginning immediately after entering the delivery room. When cervical dilatation reached 2–3 cm, epidural puncture was performed at the L2-3 interspace using an 18 G epidural needle, and a soft-tip catheter was inserted 4–4.5 cm into the epidural space. After aspiration of the catheter to exclude intravascular and intrathecal placement, the patient was administered an initial bolus (1st bolus) according to the grouping scheme described above via the syringe. If no abnormal response was reported (eg, dizziness, tinnitus, metallic taste, lower limb atony or unconsciousness) after 5 minutes, a second dose of 8 mL of 0.083% ropivacaine combined with 0.4 µg/mL sufentanil (2nd bolus) was injected via the PCEA pump in programmed intermittent epidural bolus (PIEB) mode. The same solution was used for the maintenance of labor analgesia. The parameters of the PIEB pump were set as follows: a bolus dose of 8 mL, a lockout time of 30 min and a PCEA bolus of 8 mL.
Data Collection
The demographic and baseline characteristics, including maternal age, height, weight, BMI, gestational weeks, and cervical dilation at the time of neuraxial placement, were monitored and recorded.
The primary outcome was the analgesia onset time, which was defined as the time from the end of the administration of the initial dose to adequate analgesia.4 Parturients were asked to indicate their level of pain based on a score ranging from 0 (no pain) to 10 (worst imaginable pain). After neuraxial analgesia had been completed, the visual analog scale (VAS) score was measured in a blinded manner during each active uterine contraction. Contractions were confirmed by tracing on a tocodynamometer, and the interval and duration of uterine contractions were noted. “Adequate analgesia” was defined as a VAS score ≤3 during each active uterine contraction.12
Secondary outcomes were as follows: the VAS score, the interval and duration of uterine contractions during the first 12 contractions (baseline, 2nd, 4th, 6th, 8th, 10th and 12th contractions) after the administration of the initial dose; the number of failures to reach adequate analgesia in the first 15 min; cold sensory blockade level; motor blockade score; total number of PCEA boluses administered during labor; the time from epidural initiating dose to first demand for PCEA; epidural drug consumption; delivery mode; durations of the first and second labor stages; Apgar score; umbilical vein pH; score of analgesia satisfaction; and adverse effects, including maternal hypotension, shivering, pruritus, nausea and vomiting, fetal bradycardia within 30 min after analgesia, and active phase stagnation.
The uterine contractions and fetal heart rate were monitored and recorded using an external tocodynamometry (TOCO) system (SRF618B5, Sunary Company, Guangdong, China).13 The degree of motor blockade was assessed using the modified Bromage score as follows: 0, no impairment; 1, unable to raise the extended leg but able to move the knee and foot; 2, unable to raise the extended leg or flex the knee but able to move the foot; and 3, unable to flex the ankle, foot or knee (complete blockade).14 Maternal satisfaction with the onset time and overall analgesia was measured on a Likert scale ranging from 0 to 10 points, with 0 and 10 representing the minimum and maximum satisfaction, respectively.15
Respiratory depression was defined as SpO2<90% when inhaling air. Maternal hypotension was defined as a systolic blood pressure (SBP) <90 mmHg or a decrease of >20% from baseline (before analgesia). Fetal bradycardia was defined as a fetal heart rate <110 bpm for more than 10 min.12
Statistical Analysis
The sample size was estimated using G*Power 3.1 software based on data from our preliminary study.16 In the preliminary study, the mean onset times in the LD, CP1.5 and CP1.2 groups were 11.3, 8.7 and 9.3 min, with standard deviations (SD) of 2.4, 2.2 and 1.8, respectively. For a power of 90% and two-sided statistical significance set to P<0.05, the minimum sample size was calculated as 63 patients. Given an expected patient dropout rate of 20%, the total sample size was increased to 90 participants (30 per group).
Kaplan–Meier survival curves were compared among groups using a Log rank test, and a univariate Cox regression model was used to analyze the hazard ratios of the primary outcome. A 95% confidence interval (CI) for the median to adequate analgesia was also reported.17
The one-sample Kolmogorov–Smirnov test was used to assess the normality of quantitative data. Quantitative variables with a normal distribution were presented as the means (SD) and were analyzed using one-way ANOVA followed by the Bonferroni post hoc test. Quantitative variables with an abnormal distribution were presented as medians (interquartile ranges; IQRs) and were analyzed using the nonparametric Kruskal–Wallis test. Categorical variables were presented as numbers (n, %) and were analyzed using Pearson’s chi-squared or Fisher’s exact test. VAS scores and the interval and duration of active uterine contractions were assessed longitudinally among groups with a linear mixed model using the restricted maximum likelihood method and accounting for patient-level clustering (random intercept) under an unstructured model.18 The baseline variables were treated as a covariate to ensure statistical balance and reduce error variance. The models consisted of main effects of the treatment group and contractions. The group by contraction interaction term was tested first. An adjusted Bonferroni correction was used for multiple comparisons between-group differences at each time point. P < 0.05 indicated that the difference was statistically significant. Statistical analyses were performed using SPSS software (version 23.0, IBM Corp, USA). All analyses followed the intention-to-treat principle.
Results
From July to September 2021, 109 women were screened, and 96 subjects were recruited and randomized into the three groups. Six participants were excluded due to delivery within one hour, unilateral block or accidental rupture of the dura; thus, data were collected from 90 subjects (Figure 1). No significant differences in demographic and baseline characteristics were observed among the three groups (P>0.05, Table 1).
Primary Outcome
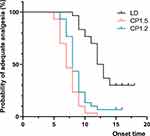
Kaplan–Meier curves for the onset time of adequate analgesia as the primary outcome are shown in Figure 2. According to the univariate Cox regression analysis, parturients in the CP1.5 and CP1.2 groups achieved a VAS pain score≤3 significantly faster than those in the LD group (hazard ratio (HR)=6.540; 95% CI, 3.503–12.210; P<0.001 and HR=3.460; 95% CI, 1.905–6.282; P<0.001, respectively). The median time (95% CI) to adequate analgesia was 12.0 (10.9–13.1) min in the LD group, 7.0 (6.2–7.8) min in the CP1.5 group and 8.0 (7.5–8.5) min in the CP1.2 group (Table 2).
 |
Table 2 Analgesic Characteristics and Adverse Effects |
Secondary Outcomes
Figure 3A shows the observed mean VAS scores at each data collection point when patients were experiencing an active contraction. According to the linear mixed-effect model analysis, the VAS scores over different beats of active contraction were significantly different (F=246.113, P<0.001), and the main effects on differences among the groups were significant (F=42.804, P<0.001). When examining the interaction of groups with contractions, the difference was also statistically significant (F=4.029, P<0.001). The VAS scores before EA were not significantly different among the three groups (P=0.953). The lowest VAS scores (median (IQR)) achieved during the first 12 contractions were significantly higher in the LD group than in the CP1.5 and CP1.2 groups (2.0 (2.0–3.0) vs 1.0 (1.0–2.0), P<0.001) and (2.0 (2.0–3.0) vs 2.0 (1.0–2.0), P=0.005), but the difference between the CP1.5 and CP1.2 groups was not significant (P=0.056), as shown in Table 2.
The interval and duration of uterine contractions are shown in Figure 3B and 3C. The interval between two consecutive uterine contractions and the duration of uterine contractions were significantly different over every beat of active contractions (F=7.526, P<0.001; F=8.658, P<0.001). No significant differences were observed among the groups (F=1.621, P=0.204; F=2.438, P=0.093). The effect of the group by contractions interaction was statistically significant (F=2.165, P=0.027; F=4.194, P<0.001).
Nine patients in the LD group (30.0%), 0 in the CP1.5 group (0. 0%) and 2 in the CP1.2 group (6.7%) did not achieve a consistent VAS score ≤ 3 in the 15-minute study period (LD vs CP1.5, P=0.004; LD vs CP1.2, P=0.020; CP1.5 vs CP1.2, P=0.472). The number of total PCEA doses administered during the course of labor, the time from epidural initiation to the first PCEA demand, and the consumption of analgesic solution in the CP1.5 groups were significantly lower than those in the LD group (P<0.01). Maternal satisfaction with the onset of analgesia was significantly higher in the CP1.5 and CP1.2 groups than that in the LD group (P<0.01). A significant difference was not observed between the CP1.5 and CP1.2 groups (P>0.05) (Table 2). The degree of sensory and motor blockade, maternal satisfaction with overall analgesia and incidence of adverse effects did not differ among the three groups (P>0.05), as shown in Table 2.
There were no significant differences in the first or second labor stage duration, delivery mode, Apgar score at 1 or 5 min after birth, or umbilical artery pH or lactate level among the three groups (P>0.05) (Table 3).
 |
Table 3 Outcomes of Labor, Parturients and Fetuses |
Discussion
The main finding of this study was that activating analgesia with 6 mL of 1.5% chloroprocaine followed by 8 mL of 0.083% ropivacaine combined with 0.4 µg/mL sufentanil as the loading dose provided a more rapid onset and resulted in greater maternal satisfaction than 6 mL of 1% lidocaine and 7.5 mL of 1.2% chloroprocaine.
Powell et al suggested that an ideal local anesthetic for the initial bolus was needed for “activation” to acquire rapid-onset EA.19 In the present study, we compared the “activation” efficacy of lidocaine and chloroprocaine. We chose an epidural loading dose of approximately 15 mL in all patients according to previous reports of EA, consisting of 6–7.5 mL of lidocaine or chloroprocaine followed by 8 mL of PIEB solution.2,4 A lidocaine concentration of 1% was chosen according to a report by Shahram Nafisi.20 Based on preliminary findings and reports in the literature, 6 mL of 1.5% and 7.5 mL of 1.2% chloroprocaine were the volumes and concentrations selected to explore an optimal volume and concentration with an equal total dosage (9 mg).21,22
Lidocaine and chloroprocaine are both fast-acting local anesthetics that have been applied for EA in many outpatient procedures and intrapartum emergency cesarean delivery with labor EA.9,23 A recent Bayesian network meta-analysis including 24 randomized clinical trials reported the rank order of EA onset for cesarean section with 2% lidocaine plus bicarbonate as the fastest, followed by 3% 2-chloroprocaine and 2% lidocaine.24 Ituk et al found that the onset times of 2% lidocaine with epinephrine and 3% chloroprocaine were 10–12 min and 3–5 min, respectively, when extending labor analgesia to anesthesia.9 Few direct comparisons of the onset time of lidocaine and chloroprocaine in labor EA are available. The data obtained in this study showed that the median onset times of 6 mL of 1.5% chloroprocaine, 7.5 mL of 1.2% chloroprocaine, and 1% lidocaine were 7.0 min, 8.0 min, and 12.0 min, respectively, which are approximately consistent with reports in the literature.9,22
The dissociation constant (pKa) is associated with the action onset time of amide-type local anesthetics.25 Lidocaine, with a lower pKa, has a greater portion of molecules in the free (active) form, leading to a more rapid onset than ropivacaine, which has a higher pKa.25 These weakly basic drugs principally bind to α-1-acid glycoprotein.21 Additionally, a high affinity for plasma proteins (ropivacaine, 94%; lidocaine, 55%) is associated with a prolonged duration of action and reduced amount of free local anesthetic diffusing across the placenta. The high protein binding of ropivacaine results in its poor diffusion across the placenta, whereas the greater diffusion of lidocaine results in higher fetal blood levels. Therefore, lidocaine is a rapid-onset agent that exhibits high placental transfer and fetal blood levels.
Chloroprocaine is an amino-ester local anesthetic that is rapidly metabolized by plasma cholinesterases, and hence is known for a fast onset time and short duration of action with high efficacy.9,11 As it is eliminated rapidly by plasma cholinesterase, chloroprocaine has several advantages over local amide anesthetics as follows: (1) it has the least placental transfer because it is eliminated rapidly by plasma cholinesterase; (2) it has been suggested to be safe for fetuses and infants because it does not rely on hepatic microsomal enzymes, which do not reach full maturity until one year after birth; and (3) it has a high safety profile, with rare local anesthetic systemic toxic effects.26
In this study, we used chloroprocaine as the initial bolus to “activate” EA for the first time and found that 6 mL of 1.5% chloroprocaine was superior to the other two treatments. Although a low concentration and high volume have been recommended for labor EA, we found that a relatively high concentration was needed to “activate” EA, followed by a lower concentration for maintenance.22
Unlike bupivacaine and ropivacaine, which exert separate effects on sensory and motor function, lidocaine and chloroprocaine result in some degree of motor blockade and muscle relaxation. Chloroprocaine (1.5%) at 7 and 8 mL was also used in the preliminary experiment and was abandoned due to the high incidence of lower limb atony and the effect of maternal mobility. We also observed alterations in uterine contraction activity induced by lidocaine and chloroprocaine. The interval between two consecutive uterine contractions was longer and the duration of uterine contractions was transiently shorter than at baseline during the first six uterine contractions (approximately the first 20 min) after the administration of the initial bolus.
According to the literature, the continuous epidural infusion of chloroprocaine decreases the efficacy of epidural opioids due to the antagonistic pharmacological properties of opioids for EA.27 The fast offset of chloroprocaine anesthesia and the slow onset of opioid EA result in an analgesic window. An analgesic bridging dose of a low-concentration, long-acting local anesthetic (ropivacaine or bupivacaine) can be used to fill the analgesic window. In this study, the analgesic window was not observed, potentially due to the bridging effect of ropivacaine, and opioids were used as adjuvants at a very small dosage.9
A recent review suggested that a “standard” test dose (3 mL of 1.5% lidocaine plus 1:200,000 epinephrine) in labor analgesia is associated with serious adverse events, such as extensive sensory and motor block with the need for airway management or urgent cesarean section, as well as the fluctuation of the maternal blood pressure and reduction of the fetal heart rate and fetal umbilical arterial blood pH induced by epinephrine, due to physiological alterations during pregnancy and labor as a result of increased sensitivity in parturients.28,29 In contemporary practice, mobile EA relies on the use of low-dose, low-concentration local anesthetics with opioid adjuncts.30 At such a low dosage, the analgesic bolus itself might be considered a test dose.29 In our clinical practice, we use soft-tip catheters to reduce the incidence of accidental intravascular insertion and aspiration tests and fractionated administration of the initial analgesic dose to avoid undetected intravascular or intrathecal catheterization.29,31
Three patients in the CP1.5 group and four in the CP1.2 group exhibited mild shivering, while fewer patients exhibited shivering in the LD group, and no significant difference was observed. The lack of a significant difference may be related to the insufficient sample size, as we calculated the sample size based on the primary outcome of the onset time, which might be insufficient for the analysis of variables with a relatively low incidence, such as shivering. Therefore, although no statistically significant differences in the incidence of shivering were observed among the three groups a relevant difference could not be excluded.
This study had some limitations. First, the onset of adequate labor analgesia remains difficult to define precisely. Pain scores are only able to be assessed during uterine contractions, which differs by parturient given the uncertain cyclical nature of labor and the progress of childbirth. Second, we did not record the time of PCEA administration via pump or physician-administered rescue boluses. Some deviation in the analgesic efficacy was observed between administration by PCEA and by the anesthesiologist via the injection syringe. Third, the dosage and concentration of chloroprocaine were determined based on preliminary experiments rather than a sequential method, which might affect the accuracy and precision of the data. Further studies are warranted to clarify the efficacy and safety of chloroprocaine for labor analgesia, such as its effect on breakthrough pain and maintenance of labor analgesia.
Conclusions
In this study, we found for the first time that initiation with chloroprocaine followed by PIEB achieved a shorter analgesia onset, greater analgesic-sparing effect, and higher maternal satisfaction without affecting the outcomes of labor than lidocaine. Six milliliters of 1.5% chloroprocaine might be a volume and concentration regimen superior to 7.5 mL of 1.2% chloroprocaine for activating labor analgesia.
Abbreviations
FDA, Food and Drug Administration; EA, epidural analgesia; PCEA, patient-controlled epidural analgesia; PIEB, programmed intermittent epidural bolus; CP, chloroprocaine; LD, lidocaine; IQR, interquartile range; CI, confidence interval; HR, hazard ratio; ASA, American Society of Anesthesiology; VAS, visual analog scale; SBP, systolic blood pressure.
Data Sharing Statement
The data that support the findings of this study are available from the corresponding author upon request.
Ethical Approval and Consent to Participate
This study was approved by the Institutional Ethics Committee of the Anhui Women and Child Health Care Hospital (YYLL-2019-03-01). All study participants, or their legal guardians, provided written informed consent prior to study enrollment.
Acknowledgments
We would like to thank Qi Xue for her assistance with the statistical analysis and Chunxia Huang for her invaluable assistance with the review of the manuscript. The authors thank American Journal Experts (http://www.journalexperts.com) for providing editing services for this manuscript.
Funding
This study was funded by the Applied Medical Research Project of Hefei Health and Family Planning Commission (Hwk2021yb017).
Disclosure
The authors report no conflicts of interest related to this work.
References
1. Wu JP, Tang YZ, He LL, Zhao WX, An JX, Ni JX. Preprocedure ultrasound imaging combined with palpation technique in epidural labor analgesia. World J Clin Cases. 2021;9(21):5900–5908. doi:10.12998/wjcc.v9.i21.5900
2. Nevo A, Aptekman B, Goren O, Matot I, Weiniger CF. Labor epidural analgesia onset time and subsequent analgesic requirements: a prospective observational single-center cohort study. Int J Obstet Anesth. 2019;40:39–44. doi:10.1016/j.ijoa.2019.05.008
3. Wang TT, Sun S, Huang SQ. Effects of epidural labor analgesia with low concentrations of local anesthetics on obstetric outcomes: a systematic review and meta-analysis of randomized controlled trials. Anesth Analg. 2017;124(5):1571–1580. doi:10.1213/ANE.0000000000001709
4. Wang T, Lu Y, Zhou P, Huang S, Yu X. A randomized controlled comparison of epidural analgesia onset time and adverse reactions during labor with different dose combinations of bupivacaine and sufentanil. Clin J Pain. 2020;36(8):612–617. doi:10.1097/AJP.0000000000000837
5. Wang LL, Li X, Tian HT, Sun Y, Sun C. Comparison of effects of chloroprocaine and lidocaine epidural block on emergency response of gynecologic patients after pelvic surgery. J Biol Regul Homeost Agents. 2020;34(6):2097–2102. doi:10.23812/20-299-L
6. Wesselink E, Hurk GJD, Vegt RV, et al. Chloroprocaine versus prilocaine for spinal anesthesia in ambulatory knee arthroscopy: a double-blind randomized trial. Reg Anesth Pain Med. 2019:
7. Xu H, Li H, Zuo Y, et al. A multicenter study of the analgesic effects of epidural chloroprocaine after lower limb orthopedic surgery. J Clin Anesth. 2016;35:313–320. doi:10.1016/j.jclinane.2016.08.009
8. Coppens M, Anssens S, Parashchanka A, et al. Determination of the median effective dose (ED50) of spinal chloroprocaine in labor analgesia. Anaesthesia. 2017;72(5):598–602. doi:10.1111/anae.13808
9. Ituk U, Wong CA. Anesthetic choices for intrapartum cesarean delivery in patients with epidural labor analgesia. Adv Anesth. 2020;38:23–40. doi:10.1016/j.aan.2020.07.002
10. Kamath A, Raghove V, Kalstein A, Yarmush J. Labor epidural in a patient who is allergic to lidocaine: a case series. Local Reg Anesth. 2021;14:21–23. doi:10.2147/LRA.S253087
11. Zhang T, Yu Y, Zhang W, Zhu J. Comparison of dexmedetomidine and sufentanil as adjuvants to local anesthetic for epidural labor analgesia: a randomized controlled trial. Drug Des Devel Ther. 2019;13:1171–1175. doi:10.2147/DDDT.S197431
12. Song Y, Du W, Zhou S, et al. Effect of dural puncture epidural technique combined with programmed intermittent epidural bolus on labor analgesia onset and maintenance: a randomized controlled trial. Anesth Analg. 2021;132(4):971–978. doi:10.1213/ANE.0000000000004768
13. Hao D, An Y, Qiao X, Qiu Q, Zhou X, Peng J. Development of electrohysterogram recording system for monitoring uterine contraction. J Healthc Eng. 2019;2019:4230157. doi:10.1155/2019/4230157
14. Roofthooft E, Barbé A, Schildermans J, et al. Programmed intermittent epidural bolus vs. patient-controlled epidural analgesia for maintenance of labour analgesia: a two-centre, double-blind, randomised study. Anaesthesia. 2020;75(12):1635–1642. doi:10.1111/anae.15149
15. Galati R, Simone M, Barile G, De Luca R, Cartanese C, Grassi G. Experimental setup employed in the operating room based on virtual and mixed reality: analysis of pros and cons in open abdomen surgery. J Healthc Eng. 2020;2020:8851964. doi:10.1155/2020/8851964
16. Faul F, Erdfelder E, Lang AG, Buchner A. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. 2007;39(2):175–191. doi:10.3758/bf03193146
17. Sharawi N, Bansal P, Williams M, Spencer H, Mhyre JM. Comparison of chloroprocaine versus lidocaine with epinephrine, sodium bicarbonate, and fentanyl for epidural extension anesthesia in elective cesarean delivery: a randomized, triple-blind, noninferiority study. Anesth Analg. 2021;132(3):666–675. doi:10.1213/ANE.0000000000005141
18. Kraus WE, Bhapkar M, Huffman KM, et al. 2 years of calorie restriction and cardiometabolic risk (CALERIE): exploratory outcomes of a multicentre, Phase 2, randomised controlled trial. Lancet Diabetes Endocrinol. 2019;7(9):673–683. doi:10.1016/S2213-8587(19)30151-2
19. Powell MF, Jarzombek KW, Venhuizen KJ, Tubinis MD, Morgan CJ, Frölich MA. Comparing bupivacaine, lidocaine, and a combination of bupivacaine and lidocaine for labor epidural activation: a prospective, randomized, double-blind study. Asian J Anesthesiol. 2019;57(2):55–60. doi:10.6859/aja.201906_57(2).0004
20. Nafisi S. Effects of epidural lidocaine analgesia on labor and delivery: a randomized, prospective, controlled trial. BMC Anesthesiol. 2006;6:15. doi:10.1186/1471-2253-6-15
21. Lee SC, Moll V. Continuous epidural analgesia using an ester-linked local anesthetic agent, 2-chloroprocaine, during labor: a case report. A a Case Rep. 2017;8(11):297–299. doi:10.1213/XAA.0000000000000494
22. Coffman JC, Brower KI, Small RH. Is low concentration 2-chloroprocaine for epidural labor analgesia a better option? A a Pract. 2018;10(4):95. doi:10.1213/XAA.0000000000000634
23. Yang Z, Li D, Zhang K, Yang F, Li M, Wang L. Comparison of epidural anesthesia with chloroprocaine and lidocaine for outpatient knee arthroscopy. J Orthop Surg. 2019;27(3):2309499019865534. doi:10.1177/2309499019865534
24. Reschke MM, Monks DT, Varaday SS, Ginosar Y, Palanisamy A, Singh PM. Choice of local anaesthetic for epidural caesarean section: a Bayesian network meta-analysis. Anaesthesia. 2020;75(5):674–682. doi:10.1111/anae.14966
25. Moore PA, Hersh EV. Local anesthetics: pharmacology and toxicity. Dent Clin North Am. 2010;54(4):587–599. doi:10.1016/j.cden.2010.06.015
26. Relland LM, Beltran R, Kim SS, et al. Continuous epidural chloroprocaine after abdominal surgery is associated with lower postoperative opioid exposure in NICU infants. J Pediatr Surg. 2021:
27. Karambelkar DJ, Ramanathan S. 2-Chloroprocaine antagonism of epidural morphine analgesia. Acta Anaesthesiol Scand. 1997;41(6):774–778. doi:10.1111/j.1399-6576.1997.tb04782.x
28. Okutomi T, Amano K, Morishima HO. Effect of standard diluted epinephrine infusion on epidural anesthesia in labor. Reg Anesth Pain Med. 2000;25(5):529–534. doi:10.1053/rapm.2000.7600
29. Massoth C, Wenk M. Epidural test dose in obstetric patients: should we still use it? Curr Opin Anaesthesiol. 2019;32(3):263–267. doi:10.1097/ACO.0000000000000721
30. Lv BS, Wang W, Wang ZQ, et al. Efficacy and safety of local anesthetics bupivacaine, ropivacaine and levobupivacaine in combination with sufentanil in epidural anesthesia for labor and delivery: a meta-analysis. Curr Med Res Opin. 2014;30(11):2279–2289. doi:10.1185/03007995.2014.946127
31. Shih CK, Wang FY, Shieh CF, et al. Soft catheters reduce the risk of intravascular cannulation during epidural block–a retrospective analysis of 1117 cases in a medical center. Kaohsiung J Med Sci. 2012;28(7):373–376. doi:10.1016/j.kjms.2012.02.004
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.