Back to Journals » Clinical Ophthalmology » Volume 17
A Prospective Study of Cyclosporine A 0.1% Combined with Loteprednol 0.2% vs Cyclosporine A 0.05% Alone in the Treatment of Dry Eye
Authors Hovanesian J , Chester T , Sorenson RC
Received 17 May 2023
Accepted for publication 7 July 2023
Published 2 August 2023 Volume 2023:17 Pages 2181—2191
DOI https://doi.org/10.2147/OPTH.S419600
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
John Hovanesian,1 Thomas Chester,2 Robert C Sorenson3
1Harvard Eye Associates, Laguna Hills, CA, USA; 2Cleveland Eye Clinic, Cleveland, OH, USA; 3Inland Eye Specialists, Hemet, CA, USA
Correspondence: John Hovanesian, Harvard Eye Associates, 23961 Calle Magdalena, Suite 300, Laguna Hills, CA, 92653, USA, Tel +1 949 951 2020, Email [email protected]
Purpose: To examine the efficacy and tolerability of a combination of cyclosporine 0.1% and loteprednol 0.2% (CsA–LE; Klarity CL) in comparison to commercially available cyclosporine 0.05% (CsA; Restasis) in improving signs and symptoms of dry eye.
Methods: This multicenter, prospective, randomized, controlled, open-label study evaluated 60 patients randomized to a single treatment for 4 weeks and evaluated at day 0, day 14, and day 28. Comparison was made of corneal higher-order aberrations (HOAs), dry-eye symptoms (SPEED score), tear-breakup time (TBUT), corneal staining, and ocular hyperemia, as well as tolerability of each medication with the validated COMTOL instrument.
Results: A total of 56 patients completed enrollment. Corneal HOAs improved significantly with CsA–LE, but not CsA alone. Both groups showed significant improvement (with no significant differences between groups) in SPEED scores, corneal staining, TBUT, and conjunctival hyperemia. Tolerability was similar between the drugs, and no significant safety issues were identified.
Conclusion: The combination of CsA 0.1%–LE 0.2% provided significant improvement in corneal HOAs, while CsA 0.05% did not. For all other measures of ocular surface improvement, both medications showed similar benefits. Tolerability was comparable between the formulations. When rapid rehabilitation of the ocular surface is needed to reduce aberrations, CsA–LE is an appropriate choice.
Keywords: dry eye, higher-order aberrations, loteprednol, cyclosporine
Plain Language Summary
This study compared treatment of dry eyes with two regimens: one with cyclosporine 0.05% alone and one with a combination of cyclosporine 0.1% and loteprednol 0.2%. Both regimens improved ocular surface health, but the combination regimen also improved irregularities of the cornea more effectively than cyclosporine alone.
Introduction
Thirty million people in the US are thought to suffer from dry eye.1 Patients with this condition manifest with ocular surface disruption, such as corneal fluorescein staining and reduced tear-breakup time (TBUT).2 Successful treatment is a high priority for both patients and physicians, reducing symptoms and improving visual acuity and corneal higher-order aberrations (HOAs), which have been closely linked with visual benefits and treatment satisfaction when refractive or cataract surgery is performed.3,4 A number of studies have shown the benefit of both cyclosporine and steroids in managing dry eye.5 However, existing formulations of these products are challenged by limited tolerability, high out-of-pocket cost, or both. Combination therapy with these agents in an advanced formulation has the potential to improve both tolerability and cost. However, the available formulation of preservative-free Klarity CL (cyclosporine 0.1% and loteprednol 0.2% in a chondroitin sulfate vehicle) has not been studied rigorously. The purpose of this study was to examine the efficacy and tolerability of a combination of cyclosporine 0.1% and loteprednol 0.2% (Klarity CL) in comparison to commercially available cyclosporine 0.05% (Restasis) in improving signs and symptoms of dry eye.
Methods
This was a multicenter, prospective, randomized, controlled, open-label study of two commercially available products used to treat dry eye: cyclosporine A 0.1% combined with loteprednol etabonate 0.2% in a chondroitin sulfate emulsion containing dextran, glycerol, and hydroxypropylmethylcellulose (Klarity CL, ImprimisRx, Carlsbad, California), and cyclosporine A 0.05% in a castor oil emulsion (Restasis, AbbVie, Irvine, California).
The study was registered on ClinicalTrials.gov as NCT05322148 and conducted under US FDA IND number 161424. The study was conducted under the approval of WCG IRB as protocol 2010 CLEAN Klarity CL Dry Eye Success. None of the authors has any affiliation with WCG IRB. The study adhered to principles of both the Declaration of Helsinki and good clinical practices as defined by the US Food and Drug Administration. All patients provided informed consent prior to enrollment using an informed consent document that was approved by the IRB.
To calculate sample size, we relied on data from an earlier study we published on refractive accuracy after treating dry eye with lifitegrast.2 In that study, we measured the change in HOAs after 1 month of lifitegrast treatment and found a mean difference from pre- to posttreatment measurements of 0.11±0.26 µm at 1 month. If we assume the same mean ± standard deviation for 1-month results with this study and a rate of type 1 error of 0.05 and type 2 error rate of 0.2, we would require a sample size of 56 patients (using a paired t-test calculator at sample-size.net).
Sixty sequential patients who presented to the clinic, met the inclusion/exclusion criteria, and gave informed consent were to be enrolled. Inclusion criteria included age >18 years and the presence at baseline of central or inferior corneal fluorescein staining defined by the Oxford scale,6 reduced TBUT ≤10 seconds, the ability to comprehend and sign a statement of informed consent, willingness to take an electronic survey about their tolerability of either study medication, and willingness to complete all required study visits.
Exclusion criteria are listed in Box 1 and were designed to exclude eyes with conditions that would confound the study’s outcome measures, such as recent (within the last 3 months) surgery, trauma, infection, inflammation, or other abnormality, or systemic treatment that might alter the course of dry-eye management.
 |
Box 1 Exclusion criteria |
Enrolled patients were randomized in a 2:1 proportion, with 40 in the cyclosporine A–loteprednol etabonate (CsA–LE) group and 20 in the cyclosporine A 0.05% (CsA) group. Randomization was accomplished by assigning each patient a number sequentially according to the order of enrollment in the study. A randomization key document assigned each patient number to a study drug. All patients in both groups were dosed with medication BID of one drop per dose, and all patients were treated in both eyes. At each assessment visit, patients were queried about dryness symptoms according to the Standardized Patient Evaluation of Eye Dryness (SPEED) questionnaire.7 Following this assessment, conjunctival redness was evaluated with the Schulze scale.8 Corneal staining was next assessed using the Oxford scale6 (0–5). Finally, TBUT was assessed as described by Lee and Kee.9
If both eyes met the study inclusion and not exclusion criteria, the eye with the more severe corneal staining at the baseline assessment was chosen as the study eye. If both eyes met the enrollment criteria and had the same severity of staining, the right eye was chosen as the study eye. Three sites enrolled patients, and the duration of treatment was 4 weeks. Subjects were examined and evaluated at baseline (day 0), 2 weeks (day 10–18), and 4 weeks (day 21–35). Patients who were already using anti-inflammatory medications for dry eye, such as cyclosporine, steroids, or nonsteroidals, were not enrolled in the study. Patients taking lubricant drops or using other treatment measures for dry eye, such as eyelid hygiene, were permitted to continue these regimens with no changes so as not to confound the study outcome measures.
The data collected at each visit included manifest refraction and best-corrected visual acuity (BCVA), corneal topography with the Zeiss Atlas topographer used to calculate total HOAs in the central 6 mm of the cornea, slit-lamp examination for corneal staining (Oxford scale), intraocular pressure (IOP), TBUT, and conjunctival hyperemia using the Schulze scale.10 Patients also completed the validated SPEED questionnaire7 and at week 2 and 4 visits a modified version of the validated Comparison of Ophthalmic Medications for Tolerability (COMTOL) to assess tolerability symptoms at 4 weeks.
The primary outcome measures of the study were the change in corneal HOAs from baseline to 2 and 4 weeks of treatment. Secondary outcome measures included the change in SPEED score at baseline vs 2 and 4 weeks as well as the change in TBUT, corneal staining, and ocular hyperemia. An exploratory outcome measure was the tolerability scores for individual symptoms on the validated COMTOL scale at 4 weeks. Side effects queried included burning, redness, blurred vision, bitter taste, unusual taste, itchy eyes, discharge, swelling of eyelids, browache, dimming of vision, difficulty focusing, and dry eyes.
Results
Sixty eyes of sixty patients were enrolled in the study. Of these, 56 (93%) completed all visits. These were evenly distributed between the three study sites, and each site had a 2:1 distribution of patients in the CsA–LE and CsA groups, respectively. The distribution of age and sex favored women over men at all sites and in both treatment cohorts (Table 1 and Table 2). Of the four patients who were not included in the analysis because they did not complete all study visits, two (3.3%) withdrew after the enrollment visit without reportedly taking any drops, one (1.6%) reported medication intolerance with the combination drop (stinging), and one (1.6%) had incomplete data that could not be recovered.
 |
Table 1 Enrollment by site |
 |
Table 2 Enrollment by Treatment Group |
Primary Outcome Measure — Higher-Order Aberrations
Statistically significant improvement was seen in total corneal HOAs in the central 6 mm of the cornea between baseline and each of week 2 and week 4 for the CsA–LE group (P<0.014 and P<0.012 vs baseline, respectively, paired t-test). No significant improvement was seen among patients treated with CsA alone (Table 3, P<0.18 and P<0.48 vs baseline, respectively, paired t-test). From baseline to week 2, HOAs moved in a positive direction in 23 (62%) of eyes taking CsA–LE vs 8 (42%) with CsA. HOAs remained unchanged in six (16%) vs five (26%), and were worse in eight (22%) vs six (32%) in each group, respectively. From baseline to week 4, HOA improvement was seen in 20 (54%) with CsA–LE vs seven (37%) with CsA, the same in five (14%) vs 5 (26%), and worse in 12 (32%) vs seven (37%), respectively. These differences were not statistically significant. Figure 1 shows the change in HOAs among patients in each cohort at the three study visits.
 |
Figure 1 Significant improvements in HOAs were seen from baseline to week 2 and week 4 in eyes treated with the combination drop, whereas no significant change in HOAs was seen with cyclosporine. |
Among the special population of cataract patients with dry eye who are being considered for multifocal IOL candidacy, an HOA threshold of 0.5 µm is considered a soft criterion to safely allow multifocal use. The proportion of eyes in this study that met this criterion is shown in Figure 2 for each cohort and each study visit. Although this study was not powered to demonstrate statistical significance for this outcome measure, a clear trend favoring CsA–LE over CsA alone is shown in Figure 2, with an increase in “candidacy” from 35% to 60% for CsA–LE vs 47% to 43% at baseline vs 4 weeks (Figure 2).
 |
Figure 2 Multifocal candidacy almost doubled among eyes receiving CsA–LE vs those taking CsA alone. |
Other Symptoms and Signs of Dry Eye
SPEED scores (Figure 3) significantly improved after treatment with both CsA–LE and CsA, with mean SPEED scores of 11.8±5.7 and 13.1±6.1 at baseline, 7.6±6.1 and 8.6±5.8 at 2 weeks, and 6.4±5.9 and 6.8±5.8 and at 4 weeks, respectively (P<0.0001 and P<0.0005 for baseline vs 2 weeks and P<0.0001 for both drugs for baseline vs 4 weeks, paired t-test). Among the patients treated with CsA–LE and CsA, SPEED scores of 5 or less were observed significantly more commonly at 2 and 4 weeks than at baseline. These scores occurred in five (14%) and one (5%) patients before treatment, 15 (41%) and eight (42%) after 2 weeks, and 17 (46%) and nine (47%) after 4 weeks, respectively (P<0.01 and P<0.008 for baseline vs 2 weeks and P<0.003 and P<0.0004 for baseline vs 4 weeks, respectively, McNemar’s 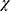 2 test). No significant difference in SPEED score improvement was noted comparing the two drugs.
2 test). No significant difference in SPEED score improvement was noted comparing the two drugs.
 |
Figure 3 Both cyclosporine/–loteprednol and cyclosporine alone significantly reduced corneal staining (P<0.0001 for both drops for baseline vs 2 and 4 weeks, paired t-test). |
Corneal staining (Figure 4), measured by the Oxford scale, significantly improved among patients treated with CsA–LE from a mean grade of 1.86±0.59 to 0.73±0.84 and 0.59±0.76 at baseline, week 2 and week 4, respectively (P<0.0001 for baseline vs both 2 and 4 weeks, paired t-test). Among patients treated with CsA alone, similar improvement was seen from 1.73±0.45 at baseline to 0.78±0.63 after 2 weeks and 0.63±0.96 after 4 weeks (P<0.0001 for baseline vs both 2 and 4 weeks, paired t-test).
 |
Figure 4 Corneal staining improved significantly in both groups from baseline to both week 2 and 4 (P<0.00001, paired t-test for all comparisons). |
All eyes had at least grade 1 staining before treatment, which was an inclusion criterion for the study. Of these, improvement to grade 0 was seen in 18 (49%) with CsA–LE and six (32%) with CsA alone at 2 weeks, and 20 (54%) with CsA–LE and 11 (58%) with CsA at 4 weeks. This improvement was statistically significant for each drug (P<0.0001, McNemar’s test for baseline vs both 2 and 4 weeks for both drugs), and the difference in improvement between CsA–LE and CsA was not statistically significant.
Mean TBUT improved significantly for patients treated with both drug regimens. These changes were statistically significant compared to baseline at each follow-up visit (McNemar’s 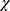 2 test, Table 4). The proportion of patients with an abnormally low TBUT (<5 seconds) decreased in all groups. This change was statistically significant for CsA–LE at both 2 and 4 weeks (P<0.003 and P<0.0001, respectively, McNemar’s
2 test, Table 4). The proportion of patients with an abnormally low TBUT (<5 seconds) decreased in all groups. This change was statistically significant for CsA–LE at both 2 and 4 weeks (P<0.003 and P<0.0001, respectively, McNemar’s 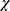 2 test) and for CsA alone at 4 weeks (P<0.01, Figure 5).
2 test) and for CsA alone at 4 weeks (P<0.01, Figure 5).
 |
Table 4 Mean tear-breakup time (TBUT) compared to baseline had improved significantly at each follow-up visit |
Both cohorts had significantly reduced conjunctival redness (Figure 6), with significant improvement in CsA–LE and CsA groups from baseline to week 2 (P<0.0002 and P<0.01, respectively, McNemar’s  2 test) and from baseline to week 4 (P<0.0005 and P<0.02). BCVA is reported in Table 5. There were no statistically significant differences in logMAR BCVA between treatment groups at any visit. Mean BCVA did improve significantly in the CsA–LE group between baseline and week 2, but the improvement from baseline to week 4 was not statistically significant.
2 test) and from baseline to week 4 (P<0.0005 and P<0.02). BCVA is reported in Table 5. There were no statistically significant differences in logMAR BCVA between treatment groups at any visit. Mean BCVA did improve significantly in the CsA–LE group between baseline and week 2, but the improvement from baseline to week 4 was not statistically significant.
 |
Table 5 Best-corrected visual acuity improved significantly between baseline and week 2 in patients taking CsA–LE, but not CsA alone, while no other differences were statistically significant |
 |
Figure 6 Conjunctival redness (Schulze scale) improved significantly in both groups. |
Tolerability
The validated COMTOL questionnaire includes questions about burning/stinging, redness, blurred vision, bitter taste, unusual taste, itchy eyes, discharge, swelling of eyelids, brow ache, dimming of vision, difficulty in focusing from near to far, dry eyes, trouble reading, trouble seeing at night, and tearing. Of these symptoms, only burning/stinging was reported at any level in a number of patients to allow a meaningful comparison between the CsA–LE and CsA groups, which each included 37 and 19 patients, respectively. Burning/stinging was reported by 26 (70%) of patients with CsA–LE and nine (47%) patients with CsA alone. The distribution of frequency of burning/stinging symptoms is shown in Figure 7. The overall frequency of side effects is shown in Figure 8.
 |
Figure 7 Frequency distribution of side effects of each medication. |
 |
Figure 8 Frequency of all side effects queried in the validated COMTOL questionnaire. |
Safety
IOP did not rise significantly in either group. Abnormal pressure readings (>21 mmHg) were limited to a single patient (3%) in the CsA–LE group with an IOP of 30 mmHg at visit 3 (Table 6).
 |
Table 6 IOP did not rise significantly in either group: abnormal pressure readings (>21 mmHg) were limited to a single patient (3%) in the combination drop group with an IOP of 30 MmHg at visit 3 |
Discussion
To our knowledge, this randomized, controlled, multicenter study is the first comparison of a commercially available novel formulation of cyclosporine 0.1%–loteprednol 0.2% (Klarity CL) to cyclosporine 0.05% (Restasis) for treatment of dry eye. This study showed that the CsA–LE combination drop significantly reduced HOAs at both 2 and 4 weeks in a dry-eye population. Improvement in HOAs in patients taking the comparator drug, CsA alone, did not show significant improvement in HOAs. Both drugs led to significant improvements in SPEED scores, corneal staining (Oxford scale), TBUT, and conjunctival redness (Schulze scale). Both drugs were well tolerated with no significant differences between them in their tolerability profile (validatedCOMTOL instrument). No significant safety concerns were raised by the study in either drug group.
Improvement in HOAs positively influences visual quality in all patients, but has particular relevance for patients preparing for cataract surgery because it can lead to a more accurate prediction of what lens-implant power will achieve the desired refractive result.2 Patients with lower degrees of HOAs are also more likely to have a satisfactory result with today’s popular multifocal implants.3 A treatment like CsA–LE being shown to rapidly and significantly reduce HOAs, therefore, is particularly valuable in preparing patients for surgery, just as it is important for all patients with dry eye.
Cyclosporine is a well-proven treatment for dry eye, but its therapeutic benefit is thought to begin only after 4 weeks of treatment.11 We have unpublished data from other work suggesting this therapeutic effect may occur more quickly, and in this study, outcome measures such as corneal staining, TBUT, SPEED scores, and conjunctival redness all improved significantly as early as 14 days. Cyclosporine’s vehicle might have contributed to this treatment success, and this study’s design did not examine vehicles alone. However, our previous experience suggests that the cyclosporine component of Restasis was at least somewhat to credit for ocular surface improvement. Further study on the speed of onset of cyclosporine is merited.
The differences between groups are most likely attributable to the addition of loteprednol in Klarity CL, but there are other differences between these drug regimens: Klarity CL’s cyclosporine at 0.1% is double the concentration of that in Restasis (0.05%). For cyclosporine, a drug with historically poor tolerability, a significantly higher concentration would be expected to significantly decrease its tolerability compared to Restasis.12 The observed lack of significant difference may be attributable to the presence of loteprednol 0.2%, the difference in vehicle, or both. Klarity has a vehicle of chondroitin sulfate emulsion containing dextran, glycerol, and hydroxypropylmethylcellulose, while Restasis uses a caster-oil emulsion as vehicle. With this study design, which did not include separate vehicle-only groups, it is impossible to distinguish the effect of each of these differences. Nevertheless, this was designed to be a “real world” study comparing the commercially available formulation of each drug, rather than just the drug components themselves. Whatever the difference in outcomes is attributable to is of more academic than practical interest.
Four patients (6.7%) withdrew from the study. Of these, one (1.7%) withdrew because the combination drops were not tolerable, with reported stinging. The remaining three dropped out because of reasons not related to the medication. This low rate of medication intolerance is consistent with previous studies of CsA 0.05%.13–15
This study is not without its limitations. First, the sample size of 56 patients randomized to two treatment groups limits detection of more subtle trends that might appear in the data. One example relates to HOAs, which showed significant improvement from before to after treatment in the CsA–LE group, but not the CsA group. On a cursory view, this might be attributed to the smaller group of patients taking CsA alone, especially since the mean HOA measures were similar in both drug groups at baseline and week 2 (Table 3). However, further analysis showed that if the CsA group had been similar in size to the CsA–LE group, the results still would not be statistically significant at the 95% confidence interval.
Though the mean HOA scores were similar between groups, the percentage of patients who improved vs stayed the same or worsened were different between the groups. Since the significance of “improvement” depends on the aggregate change in each patient, rather than mean change in the group as a whole, the analysis showed CsA–LE to be significantly more effective in improving HOAs.
LogMAR BCVA improved in the CsA–LE group from baseline to week 2, but not vs week 4, and no other significant differences were noted in BCVA. This contrasts with the significant improvements in other measures of dry eye, but is not surprising. Though Snellen acuity has been a time-honored metric of visual performance, it is not highly sensitive to the small but visually important changes that occur in dry eye. Snellen acuity was included in this study because of its historical significance and its potential role in evaluating safety concerns, but it was not chosen as the primary outcome measure for this reason.
Another potential criticism of this study is its open-label design. While observer bias is a possibility in any study, this study’s primary outcome measure was HOAs, an objective measure that is derived from corneal topography. Also, to fully mask this study would have required dispensing each product in a similar bottle. This would not be possible with commercially available Restasis (the CsA group), which is provided in single-use tear-off dispensers, whereas Klarity CL (the CsA–LE group) is provided in a multidose bottle. Since the purpose of this study was to compare these two products as sold, some degree of masking was unavoidable by virtue of the design of the dispensers.
This study has shown that the combination of CsA–LE in a chondroitin sulfate vehicle is more effective in improving HOAs than the most widely used commercially available formulation of CsA alone. As expected, it also demonstrated consistent efficacy in improving corneal staining, TBUT, conjunctival redness, and patient symptoms (SPEED score). Perhaps more importantly, it achieved these goals in a time frame that suggests its use might be appropriate for patients with DED preparing to undergo ocular surgery.
Data Sharing
Reasonable requests for deidentified patient data relating to the study findings, including any outcome measures, will be available through the corresponding author for 5 years following the publication date.
Acknowledgments
Leslie Lemieux assisted in the administration of this study. Partial results of this study were presented at the annual meeting of the American Society of Cataract and Refractive Surgery in San Diego, California in May 2023.
Funding
This study was funded by Research InSight, LLC under an independent research grant from Imprimis Rx, a Harrow Company, which did not give direction in the design or conduct of this study. Dr. Hovanesian is a consultant to ImprimisRx.
Disclosure
Dr John Hovanesian reports grants and personal fees from ImprimisRx and personal fees from Allergan during the conduct of the study. Dr Thomas Chester reports personal fees from Allergan, personal fees from Dompe, personal fees from Glaukos, personal fees from Novartis, personal fees from Oyster Point, grants and personal fees from Sight Sciences, personal fees from Sun Ophthalmics, personal fees from Tarsus, and personal fees from Versea outside the submitted work. The authors report no other conflicts of interest in this work.
References
1. Farrand F, Fridman M, Stillman IÖ, Schaumberg DA. Prevalence of diagnosed dry eye disease in the United States among adults aged 18 years and older. Am J Ophthalmol. 2017;182:90–98. doi:10.1016/j.ajo.2017.06.033
2. Hovanesian JA, Epitropoulos A, Donnenfeld ED, Holladay JT. The effect of lifitegrast on refractive accuracy and symptoms in dry eye patients undergoing cataract surgery. Clin Ophthalmol. 2020;14:2709–2716. doi:10.2147/OPTH.S264520
3. McCormick GJ, Porter J, Cox IG, MacRae S. Higher-order aberrations in eyes with irregular corneas after laser refractive surgery. Ophthalmology. 2005;112:1699–1709. doi:10.1016/j.ophtha.2005.04.022
4. Williams D. Visual benefit of correcting higher order aberrations of the eye. J Refr Surg. 2013;16(5):S554–S559.
5. Matossian C, Trattler W, Loh J. Matossian, dry eye treatment with topical cyclosporine 0.1% in chondroitin sulfate ophthalmic emulsion. Clin Ophthalmol. 2021;15:1979–1984. doi:10.2147/OPTH.S308088
6. Bron A, Evans VE, Smith JA. Grading of corneal and conjunctival staining in the context of other dry eye tests. Cornea. 2003;22(7):640–650. doi:10.1097/00003226-200310000-00008
7. Ngo W, Situ P, Keir N, Korb D, Blackie C, Simpson T. Psychometric properties and validation of the standard patient evaluation of eye dryness questionnaire. Cornea. 2013;32(9):1204–1210. doi:10.1097/ICO.0b013e318294b0c0
8. Schulze M, Jones DA, Simpson TL. The development of validated bulbar redness grading scales. Optomet Vis Sci. 2007;84(10):976–983. doi:10.1097/OPX.0b013e318157ac9e
9. Lee JH, Kee CW. The significance of tear film break-up time in the diagnosis of dry eye syndrome. Korean J Ophthalmol. 1988;2:69–71. doi:10.3341/kjo.1988.2.2.69
10. Stelmack JA, Massof RW. Using the VA LV VFQ-48 and LV VFQ-20 in low vision rehabilitation. Optomet Vis Sci. 2007;84(8):705–709. doi:10.1097/OPX.0b013e3181339f1a
11. Wilson S, Perry HD. Long-term resolution of chronic dry eye symptoms and signs after topical cyclosporine treatment. Ophthalmology. 2007;114(1):76–79. doi:10.1016/j.ophtha.2006.05.077
12. Sheppard J, Scoper SV, Samudre S. Topical loteprednol pretreatment reduces cyclosporine stinging in chronic dry eye disease. J Ocul Pharmacol Therap. 2011;27(1):23–27. doi:10.1089/jop.2010.0085
13. Mah F, Donnenfeld E, Conway T, et al. PERSIST: physician’s evaluation of restasis® satisfaction in second trial of topical cyclosporine ophthalmic emulsion 0.05% for dry eye: a retrospective review. Clin Ophthalmol;2012. 1971–1976. doi:10.2147/OPTH.S30261
14. Perry Henry D, Solomon R, Donnenfeld ED, et al. Evaluation of topical cyclosporine for the treatment of dry eye disease. Arch Ophthalmol. 2008;126(8):1046–1050. doi:10.1001/archopht.126.8.1046
15. Sall K, Stevenson OD, Mundorf TK, et al. Two multicenter, randomized studies of the efficacy and safety of cyclosporine ophthalmic emulsion in moderate to severe dry eye disease. Ophthalmology. 2000;107(4):631–639. doi:10.1016/S0161-6420(99)00176-1
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.