Back to Journals » Clinical Ophthalmology » Volume 14
A Prospective, Post-Market, Multicenter Trial (CHEETAH) Suggested TearCare® System as a Safe and Effective Blink-Assisted Eyelid Device for the Treatment of Dry Eye Disease
Authors Karpecki P , Wirta D, Osmanovic S, Dhamdhere K
Received 6 November 2020
Accepted for publication 23 December 2020
Published 30 December 2020 Volume 2020:14 Pages 4551—4559
DOI https://doi.org/10.2147/OPTH.S285953
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
Paul Karpecki,1 David Wirta,2 Smajo Osmanovic,3 Kavita Dhamdhere4
1Kentucky Eye Institute, Lexington, KY, USA; 2Eye Research Foundation, Newport Beach, CA, USA; 3Arlington Eye Physicians Llc, Arlington Heights, IL, USA; 4Sight Sciences, Inc., Menlo Park, CA, USA
Correspondence: Kavita Dhamdhere 4040 Campbell Avenue, Suite 100, Menlo Park, CA 94025, USA
Tel +1 650-223-4062
Fax +1 877-266-1144
Email [email protected]
Purpose: This study evaluated the safety and effectiveness of TearCare® System to treat the signs and symptoms of dry eye disease (DED).
Methods: In this multicenter, prospective, post-market, exploratory, interventional trial, 58 eyes (29 subjects) received a single TearCare procedure and were assessed at baseline, post-procedure 1-week and 1-month. Effectiveness was assessed as mean change from baseline in tear break-up time (TBUT), Ocular Surface Disease Index (OSDI), total Meibomian Gland Secretion Score (MGSS), and corneal/conjunctival staining. Adverse events (AE) and changes in visual acuity were used to asses safety.
Results: The baseline TBUT of 3.7± 1.1 seconds was improved by 2.6± 1.6 (70%) seconds at 1-week and by 3.1± 2.2 (84%) seconds at 1-month (p < 0.0001). Mean baseline OSDI of 54.9± 20.2 improved by 17.9± 20.9 at 1-week and 25.8± 24.3 at 1-month (p < 0.001). A clinically meaningful improvement was seen in 83% of subjects as per the Miller-Plugfelder definition and 66% of subjects improved by at least 1 OSDI category. The baseline MGSS of 5.6± 4.0 improved by 9.3± 4.0 at 1-week and 8.8± 5.8 at 1-month (p < 0.0001). Corneal and conjunctival staining improved by 1.4± 2.8 and 1.2± 2.9 from a mean baseline of 4.8± 2.5 and 5.9± 3.2, respectively. Moreover, similar trajectories of improvement were observed for subgroups of subjects stratified by severity. Subjects with more severe gland obstruction at baseline had greater improvements in TBUT and staining compared to the less severe subgroup. No device-related adverse events or significant changes in visual acuity were observed.
Conclusion: These results suggest that single TearCare procedure is safe and effective in treating signs/symptoms of DED. Significant improvements were seen in all subjects (100%) in all signs and symptoms of DED within 1-week of treatment and 83% subjects experienced clinically meaningful symptom relief. Additionally, TearCare seems to be effective in treating DED associated with all severities of meibomian gland obstruction.
Keywords: meibomian gland dysfunction, MGD, thermal therapy, TearCare procedure, evaporative dry eye disease, DED
Introduction
DED is a chronic, multi-factorial disease causing many symptoms including corneal inflammation, scarring, and vision loss.1,2 It is a highly prevalent condition with approximately one-third of patients who visit their eye care provider are suffering from DED or DED-related symptoms. The prevalence of dry eye increases with age, specifically amongst post-menopausal women, and is continuing to become increasingly prevalent as the global population ages and experiences increased environmental pollutants and technological stressors like ever increasing screen usage. In tandem with high prevalence, DED bears a high individual management cost ($783/month average) and societal cost leading to an estimated $3.84 billion burden to the US healthcare system and $55.4 billion in lost productivity.3
DED is widely accepted to present in two forms, evaporative and aqueous tear deficient, which result in nearly identical symptoms but differ in the underlying etiology of the disease. The American Academy of Ophthalmology (AAO) guidelines for the treatment of Dry Eye underscores that both forms coexist in a majority of patients suffering from DED; however, evaporative DED is most common and reported in a majority of dry eye cases.4,5 Evaporative DED leads to a vicious cycle of reduced tear quality and increased inflammation. Normal tears form a protective barrier over the ocular surface which acts to lubricate, protect from infection, provide vital nutrients, and maintain an optically clear surface for proper light refraction which influences visual performance/acuity. Disruption of any of the three tear layers results in a disruption of these protective and optical properties in turn leading to an array of symptoms and induction of multi-factorial inflammatory processes leading to an exacerbation of DED.
In 86% of DED cases, meibomian gland dysfunction (MGD) plays a causative role in the disruption of the lipid layer of tears.6,7 Despite the high prevalence of evaporative DED and MGD, the International Dry Eye Workshop (DEWS) II has suggested that increasingly the severity of DED signs and symptoms forms the basis of treatment decisions made by clinicians rather than an aim to ameliorate the discrete deficiency, aqueous or evaporative, experienced by the patient.1,2 Namely, standard of care therapies for DED are used broadly and have largely focused on providing relief through instillation of artificial tears, ie, palliative treatment, and anti-inflammatories (Restasis® [cyclosporine ophthalmic emulsion], Xiidra® [lifitegast ophthalmic solution]), ie, symptomatic relief.8 Recently, restoring meibomian gland function and natural flow of meibum has come to the forefront as a therapeutic strategy among clinicians.9,10 Towards the treatment of MGD specific etiologies, patients are often treated with warm compresses coupled with lid massaging to improve lipid production and flow to the tear film; however, these standard of care treatments are limited, amongst the long list, by lack of sufficient therapeutic capability and efficacy in practice.11
The TearCare System (Sight Sciences, Inc, Menlo Park, CA, USA) represents an innovation that overcomes these limitations to enable long-lasting safe and effective treatment of both signs and symptoms of DED.12,13 The TearCare procedure couples a custom-designed medical device which delivers a safe, precisely controlled, and maximum therapeutic level of heat to the meibomian glands to thoroughly melt all hardened meibum obstructions and manual gland expression in a medical office to fully evacuate all meibomian glands and restore the natural flow of meibum, thus effectively interrupting the vicious cycle of evaporative DED. The objective of the study presented herein was to further evaluate the safety and effectiveness of a single TearCare procedure to treat adult patients with DED.
Materials and Methods
Study Design
This was a prospective, single-arm, post-market, exploratory interventional study (NCT03588624) conducted at three sites in the United States with enrollment between July 12, 2018 and October 11, 2018. All subjects eligible for enrollment received a single TearCare procedure comprised an in-office 15-minute meibum-melting session accompanied by natural blink mechanism followed by a manual, lid-by-lid meibum-evacuation session of the meibomian glands using the TearCare Clearance Assistant. Subjects were followed at study visits 1 week and 1-month post-procedure (Figure 1). At all visits, effectiveness was assessed using OSDI©, BSCVA (Snellen), slit-lamp biomicroscopy findings, TBUT, corneal staining, conjunctival staining, and Meibomian Gland Secretion Score (MGSS). Safety endpoints included the nature and incidence of adverse events identified by patient report, solicitation by study staff and by examination. The severity and device relatability of adverse events was assessed by the investigator. At the 1-month follow-up visit, a log for use of lubricant drops was collected for each subject. To avoid implicit biases, the efficacy endpoint assessment was masked by having an independent study staff that did not perform the TearCare procedure on the Study subjects perform the endpoint assessments.
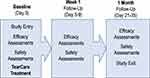 |
Figure 1 Schematic of the clinical study plan. |
This study was conducted under the approval of the Aspire Institutional Review Board as a post-market study and in compliance with The Declaration of Helsinki for the protection of human subjects in medical research. Informed consent was obtained from all eligible subjects prior to study commencement.
Study Population
English-speaking individuals at least 22 years of age willing and able to provide informed consent, comply with all study procedures, and follow-up visits who had reported dry eye symptoms within the past 3 months and had used artificial tears or lubricants regularly over 1 month were enrolled in the study. Subjects were required to have an OSDI score of ≥23 (ie, moderate to severe symptoms), TBUT of ≤7 seconds in both eyes, MGSS of ≤15 in each eye, at least 15 expressible glands in each lower eyelid, and BSCVA of at least 20/100 in both eyes.
Prior to the baseline visit, subjects were required to discontinue use of systemic antihistamines or Accutane for at least 1 month, cyclosporine ophthalmic solution or lifitegrast ophthalmic solution for at least 2 months, and other dry eye or MGD-related medication or treatments (eg, antibiotics, non-steroidal and anti-inflammatory drugs, corticosteroids and TrueTear) for at least 2 weeks. Ocular lubricants and nutritional supplements were not restricted.
Subjects were excluded if there was evidence of co-existing ocular conditions potentially posing an increased risk of procedure-related injury (eg, active ocular infection or inflammation in either eye); ocular surgery or trauma within 3 months of the baseline examination; ocular surface abnormality potentially compromising corneal integrity in either eye; eyelid abnormalities affecting lid function in either eye; systemic disease resulting in dry eye; and an unwillingness to abstain from systemic medications known to cause dryness for the study duration. Subjects were also excluded for co-existing conditions that could interfere with the assessment of safety and effectiveness of the treatment (eg, macular disease; women who were pregnant, nursing or not using adequate birth control measures; etc.). Subjects who had systemic diseases resulting in dry eye or allergies to silicone tissue adhesives were excluded.
TearCare Procedure
The TearCare System is comprised of a SmartHub controller, a charging nest, a charging adaptor, and the single use, therapeutic SmartLids. The SmartLids, which have an integral medical grade silicon-based adhesive, were affixed using a medical grade adhesive to the external surface of the upper and lower eyelids of both eyes along the lid margins of each enrolled subject. The custom, flexible, and wearable design of the SmartLids facilitates consistent conformance of the devices to the tarsal plate of each unique eyelid to enable precise targeted delivery of optimal thermal therapy to the meibomian glands while allowing the patients to maintain normal blinking. It is noteworthy that no eligible patients had to be excluded from the study due to inability of the SmartLids to appropriately fit on the eyelids because of condition, shape, size, contour of the eyelids or the size of the palpebral fissure.
The meibum-melting session was initiated by activation of the SmartHub controller; following which, the system gradually increased the temperature of SmartLids over 2‐3 minutes until the maximum, and therapeutically optimal temperature of 45°C, is reached. Maintaining a tarsal temperature of 45°C for several minutes is required to achieve the therapeutic melting temperature of 41°C within the meibomian glands at the inner eyelid. This requisite 45°C external eyelid and 41°C internal eyelid treatment temperature was maintained through constant communication (240× per second) between the SmartHub and the SmartLid sensors on an eyelid-by-eyelid basis to ensure that each eyelid and all meibomian glands were being maximally treated throughout the 15-minute meibum-melting session. Adhesion to the 4 separate eyelid-worn devices to the external surface of each eyelid allowed for normal blinking during thermal therapy to not only facilitate natural expression of thermally liquefied meibum but also spare the cornea of any undesirable or unsafe temperature rise possible with closed-eye heating solutions.
Immediately following the meibum-melting session, the investigator individually and comprehensively evacuated each meibomian gland in all four eyelids using the TearCare Clearance Assistance under direct visualization. The investigators ensured that the complete evacuation of liquefied meibum had occurred by performing at least two passes; thus, completing the meibum-evacuation session for all 4 eyelids during the standard office visit.
Endpoint Assessments
Following the TearCare procedure, subjects were assessed for the primary and secondary effectiveness endpoints by an independent assessor. To reduce potential bias in subjective endpoint assessments, the investigator performing the TearCare procedure did not perform the endpoint assessments. The discrepancies amongst the endpoint assessors were minimized by rigorous training on assessment methods. All investigators acting as endpoint assessors were required to perform at least 10 procedures and 10 assessment sessions prior to examining the first study subject. Additionally, during training, all investigators were subjected to a quiz developed by ophthalmologists at Sight Sciences, Inc. that was used to standardize parameters used for TBUT and staining scores. Assessors at each site were specifically and extensively trained and practiced in the performance of less frequently used tests such as the MGSS evaluation.
Subjects were first assessed for the primary endpoint measure, TBUT, using a standardized amount measured by a micropipettor (5 μL) of 0.5% fluorescein dye, cobalt blue illumination, and a stopwatch for keeping time. TBUT was assessed as the number of seconds between a blink and the appearance of the first dry spot or negative staining in the tear film. A total of three measurements were recorded and subsequently averaged. Evaluation of secondary endpoints began immediately following TBUT assessment, within 1–4 minutes of fluorescein dye instillation. Within this time range, corneal staining was assessed for each of the 5 corneal areas (central, temporal, nasal, inferior, and superior) using blue light and a Wratten filter according to the NEI/Industry grading system,14 where, grade 0 = no dots, 1 = 1–15 dots, 2 = 16–40 dots, and 3 ≥ 41 dots. Following fluorescein corneal staining assessments, a standard amount (10 μL) of lissamine green diagnostic dye was instilled and used to assess conjunctival staining within 1–4 minutes of stain instillation using increasing illumination and red barrier filters to highlight the staining patterns. Staining was scored according to the NEI/Industry grading system as previously described. The staining procedures instituted in this study minimized false-positive staining resulting from contact of the dye strips with the conjunctiva and cornea as well as by standardizing the amounts of dye instilled to perform staining, as it is well established that the dye amount and saturation can affect the results.15
Following staining, MGSS were collected using a Meibomian Gland Evaluator placed immediately inferior to the eyelashes of the lower eyelid. A total of 15 glands (5 nasal, 5 central, and 5 temporal) were scored in the lower eyelid of each eye according to the grading scale where 0 = no secretion, 1 = toothpaste-like secretion, 2 = cloudy secretion, and 3 = clear; a higher number indicates more normal meibomian gland function.16 OSDI questionnaire was self-administered by subjects to assess self-reported dry eye symptoms and subjects’ quality of life. OSDI is a validated tool that consists of 12 questions assessing ocular symptoms, their impact on patient’s vision-related functioning, and environmental factors triggering the symptoms on a scale from 0 = no experience of symptoms to 4 = experience of symptoms all of the time.17
Statistical Analyses
Given that this study was designed as an exploratory study, no sample size or power was calculated. A sample size of approximately 30 subjects (60 eyes) was chosen as a reasonable number of subjects to evaluate the safety and effectiveness of the TearCare System for the treatment of patients with DED. The per-protocol analysis population included all subjects (eyes) that completed at least 1 follow-up visit and had no major protocol deviations. All primary and secondary effectiveness endpoint analyses were performed using the per-protocol population. Each continuous and ordinal primary and secondary variable was summarized descriptively with mean, standard deviation, minimum and maximum calculated for each eye. No imputation of missing data was required in this study. Safety measurements including evaluation of device-related adverse events and changes from baseline Snellen BCVA were considered evaluable for the safety analysis on all subjects enrolled in the study.
For the primary and secondary endpoints, changes in signs and symptoms of DED were calculated as mean differences from baseline such that Treatment Effectn = Follow-upn- baseline, where n = 1 week or 1 month. Paired T-tests were performed to test for statistically significant (p < 0.05) differences between baseline and follow-up timepoints. Corrections for multiplicity were performed using a post hoc Tukey’s test.
Severity of dry eye symptoms was graded by OSDI score such that scores ≤12 were classified as “normal”, scores ≤ 22 were classified as “mild”, scores ≤ 32 were classified as “moderate” and score >32 were classified as “severe”. Clinically meaningful improvements in OSDI were classified as improvements of 7.3 units for moderate symptoms, and 10.4 units for severe symptoms.18
Post-hoc analyses to further investigate the effectiveness of the TearCare procedure were conducted on the per-protocol population stratified by median baseline MGSS into two subgroups reflecting the severity of meibum and meibomian gland dysfunction at baseline: one group with MGSS above the median representing less severe dysfunction and one with MGSS below the median indicating more severe dysfunction. The primary effectiveness endpoint, TBUT, and secondary endpoints, corneal staining and MGSS were analyzed with these subgroups.
Results
Subject Demographics and Disposition
A total of 32 subjects were screened and 29 eligible subjects (58 eyes) with an average age of 60.6 ± 12.4 years were enrolled in the study. Two sites enrolled 10 subjects per site, and one site enrolled 9 subjects. Three subjects did not meet the eligibility criteria and were exited from the study. All enrolled subjects completed the study through the 1-month follow-up visit. Twenty-two female (76% of study population) and seven male (24% of study population) subjects were enrolled and all subjects were white, non-Hispanic/Latino. All subjects received a single TearCare procedure. No subjects withdrew or were discontinued from the study for any reason. During the course of the study, 3 non-significant protocol deviations occurred; visits out of window were reported for 2 subjects. One subject met the exclusion criteria due to reported use of anti-glaucoma medications at baseline (Visit 1) and was exited prior to receiving the TearCare procedure.
The ocular and medical history of subjects enrolled was similar across the study population. Use of contact lenses across the analysis population demonstrated 14 subjects (48%) were former contact lens users, of these 6 subjects (21%) had attempted use of contact lenses prior to the study, 1 subject (3%) was a current user of contact lenses prior to study enrollment, and 7 subjects (24%) had a past history of use of contact lenses. Fifteen subjects had a history of use of prescription DED therapeutics including debridement (2 subjects, 7%), cyclosporine ophthalmic solution (4 subjects, 14%), lifitegrast ophthalmic solution (2 subjects, 7%), and punctual plugs (7 subjects, 24%). On average, subjects reported 4.3 ± 3.0 hours of screen usage (Table 1).
 |
Table 1 Baseline Disease Characteristics |
Baseline Disease Characteristics
As pre-requisites for enrollment in the study, all subjects reported dry eye symptoms within 3 months of the baseline visit, a TBUT ≤ 7 seconds, OSDI score ≥ 23 and a meibomian gland secretion score ≤ 15 in both eyes. At baseline the subjects had the following baseline disease characteristics: an average TBUT of 3.7 ± 1.1 seconds, meibomian gland score was 5.6 ± 4.0, corneal staining score was 4.8 ± 2.5, conjunctival staining score was 5.9 ± 3.2, and an OSDI score of 54.9 ± 20.2 (Table 1). At baseline the subject population had DED with the following signs and symptoms: TBUT (seconds) = 3.7 ± 1.1; Meibomian Gland Score (cumulative for 15 lower eyelid meibomian glands) = 5.6 ± 4.0; Corneal Staining Score = 4.8 ± 2.5; Conjunctival Staining Score = 5.9 ± 3.2 and symptom (OSDI = 54.9 ± 20.2).
Effectiveness Analysis
Following administration of a single TearCare procedure, subjects demonstrated a statistically significant improvement in TBUT of 2.6 seconds (ranging from 2.2 to 6.4 seconds) longer than baseline at 1 week that continued to improve to an average of 3.1 seconds (ranging from 2.6 to 8.3 seconds) longer than baseline at 1 month following the procedure; both p < 0.001 (Figure 2A). Similarly, the consistency of meibomian gland secretions was significantly improved from baseline following treatment with the TearCare procedure at all time points; a cumulative score for secretions from 15 lower lid meibomian glands of 14.9 ± 7.0 was observed at 1 week and remained stable at 1 month (cumulative score = 14.4 ± 7.3) (Figure 2B). Subjects also saw improvement in mean corneal and conjunctival staining following treatment (Figure 2C). Mean corneal staining was reduced from 4.8 ± 2.5 at baseline to 3.5 ± 2.2 at 1 week and 4.1 ± 2.8 at 1-month post-treatment. Similarly, mean conjunctival staining was reduced from 5.9 ± 3.2 at baseline to 3.4 ± 3.1 and 4.7 ± 3.0 at 1 week and 1-month post-treatment, respectively.
Improvements in DED symptoms were evaluated with the OSDI questionnaire to assess ocular symptoms, their impact on patient vision-related functioning, and environmental factors triggering DED symptoms.17 Following the TearCare procedure, subjects saw clinically and statistically significant improvements in DED severity as scored by OSDI at both 1 week and 1 month following (Figure 2D). Importantly, per the Miller-Plugfelder definition, 83% of subjects showed clinically meaningful improvements with OSDI score improvements ≥ 13.4 and 66% of subjects saw an improvement in severity from severe DED to moderate DED.
To further understand the treatment effect of a single TearCare procedure to provide immediate intervention of MGD-related DED signs and symptoms, post hoc subgroup analyses were conducted on the subject population stratified by MGD severity using median baseline MGSS. Based on the median baseline MGSS of 2, less severe group included subjects with baseline MGSS ≤ 2 and more severe group included subjects with baseline MGSS >2. Both groups demonstrated a similar trajectory of improvement in the quality of meibum as measured by the average MGSS at 1 week and 1 month after receiving the TearCare procedure as such (Figure 3A). This suggests TearCare has a broad acting treatment effect that provides relief of gland dysfunction regardless of severity of gland obstruction.
Analysis of the more and less severe MGD subgroups at the primary effectiveness endpoint demonstrated that subjects in both subgroups experienced improvement from baseline TBUT at 1 week and 1-month post-procedure; however, subjects in the more severe gland-dysfunction subgroup trended towards experiencing greater improvements in comparison to the less severe group with 1 month average ± standard error of mean (SEM) TBUT of 7.93 ± 0.86 seconds and 6.09 ± 0.53 seconds, respectively (Figure 3B). Similarly, corneal staining analyses of the subgroups demonstrated that subjects in both groups experienced improvements from baseline corneal staining at 1 week and 1-month post-procedure while those subjects with more severe MGD at baseline also experienced a similar trend towards greater improvements in corneal staining 1-month post-procedure compared to subjects with less severe dysfunction with average ± SEM corneal staining scores of 3.06 ± 0.90 and 4.36 ± 0.60, respectively (Figure 3C).
Safety Analysis
The safety of the TearCare procedure was assessed by evaluating the following measures over time: device-related adverse events and Snellen BSCVA. Worsening of two or more lines from baseline on the Snellen BSCVA scale was observed in 4 eyes of 2 subjects. For 1 subject the observed worsening of BSCVA was recorded at 1 week following the TearCare procedure and continued to worsen through the 1-month follow-up visit. For the other subject worsening of BSCVA was observed at 1 month following the TearCare procedure. No observations of worsening BSCVA were considered by investigators to be serious or device-related. No ocular or device-related AEs were reported in the study.
Discussion
From the results of this study, it is evident that the TearCare system, leveraging maximally controlled and optimized therapeutic levels of penetrating heat at the site of disease, unencumbered blinking for natural gland priming, and manual gland evacuation, is safe, well tolerated, and significantly effective, both statistically and clinically, in the management of the signs and symptoms of DED in adult patients. The results indicate that a single TearCare treatment safely and consistently delivers improvements in both the signs (TBUT, MGSS, corneal and conjunctival staining) and symptoms (OSDI) of DED.
It is notable that the subjects enrolled in this study were at a moderate to severe stage of DED based on TBUT, MGSS and OSDI measures. Interestingly, more than half the (n = 15; 52%) subjects had history of use of DED therapeutics; prescription drops being most common. At the time of enrollment, these subjects were at a moderate to severe stage of DED, suggesting failure to achieve relief. Subgroup analysis of these subjects stratified by MGD severity showed the same level of improvement in signs and symptoms of DED pointing to the potential of TearCare to successfully treat cases where alternative DED treatments, such as prescription therapeutics, failed.
The equivalent treatment-related improvement in MGSS experienced by subjects with more severe and less severe meibomian gland dysfunction underscores the global, broad-acting effectiveness of the TearCare procedure to ameliorate the dysfunction of meibomian glands by evacuating hardened meibum. As such, TearCare treatments can be helpful for all patients regardless of MGD severity. Importantly, subjects with more severe gland dysfunction trended towards experiencing greater therapeutic benefit via improved TBUT and decreased corneal staining at 1-month post-procedure compared to the less severe group. In general, more severe gland dysfunction experienced by a subject at baseline trended towards correlation with greater sustained improvement and therapeutic benefit gained from a single TearCare procedure; thus, enabling clinically meaningful improvements in meibomian gland health and disease modification in these subjects needing immediate intervention. This trend, resulting from the clearance of gland obstructions and restoration of functional meibum, emphasizes the critical role of meibum and functional meibomian glands in the maintenance of the tear-film and corresponding corneal surface integrity.
A common limitation of many clinical studies of DED are the endpoints requiring subjective grading by study investigators. To overcome this, grading by investigators was controlled through masking of the endpoint assessor to the performance of the TearCare procedure. Additionally, investigators and endpoint assessors were required to undergo rigorous training and adhere to standardization procedures such as instillation of standard amounts of vital stains. This research was only an exploratory study to investigate the safety and effectiveness of TearCare; thus, the sample size was not powered for hypothesis testing and statistical analysis. However, results from this study are informative, suggesting the favorable safety profile and effectiveness of TearCare that will serve as essential preliminary data and foundation for future robust randomized controlled trials.
Results of this exploratory study show the TearCare system achieved optimized trans-tarsal heat transfer directly into the meibomian glands at the inner eyelid, leveraged the patient’s natural blink mechanism for natural gland priming and meibum flow, and facilitated the effective, lid-by-lid, manual gland evacuation of more easily expressed melted meibum. All subjects experienced immediate intervention and relief from MGD-related DED signs and symptoms with even greater therapeutic benefits being experienced by patients with severe MGD. This study demonstrates that TearCare is safe, well tolerated, and globally effective in the management of MGD and evaporative DED.
Conclusion
The TearCare procedure was evaluated in subjects with MGD-related DED at three clinical sites in the US. This study provided strong indications of safety and efficacy and demonstrated clinically significant improvements in all signs and symptoms of DED with no device-related adverse events or significant changes in visual acuity. Additionally, it is evident that TearCare has the potential to successfully treat patients who did not respond favorably to alternative treatments such as prescription dry eye medications. The results of this study will be leveraged for the generation of hypotheses to be further investigated in a larger randomized controlled trial.
Abbreviations
AAO, American Academy of Ophthalmology; BSCVA, ; DED, dry eye disease; DEWS, dry eye work shop; MGD, meibomian gland dysfunction; MGSS, Meibomian Gland Secretion Score; OSDI, Ocular Surface Disease Index; SEM, standard error of the mean; TBUT, tear break-up time.
Data Sharing Statement
The authors do not intend to share participant-level data.
Acknowledgments
The authors would like to acknowledge Anne-Marie Ripley for designing the protocol and Ora, Inc. for medical writing support.
Disclosure
Paul Karpecki reports personal fees from Sight Sciences, outside the submitted work and is a paid consultant and speaker for the Sight Sciences, Inc. David Wirta reports grants from TearCare, during the conduct of the study; and grants from Allergan, Novaliq, Novartis, Silk Tech, and Oyster Point, outside the submitted work. Kavita Dhamdhere is an employee of Sight Sciences, Inc.
The authors report no other potential conflicts of interest for this work.
References
1. Craig JP, Nichols KK, Akpek EK, et al. TFOS DEWS II definition and classification report. Ocul Surf. 2017;15(3):276–283. doi:10.1016/j.jtos.2017.05.008
2. Lemp MA, Crews LA, Bron AJ, Foulks GN, Sullivan BD. Distribution of aqueous-deficient and evaporative dry eye in a clinic-based patient cohort: a retrospective study. Cornea. 2012;31(5):472–478. doi:10.1097/ICO.0b013e318225415a
3. McDonald M, Patel DA, Keith MS, Snedecor SJ. Economic and humanistic burden of dry eye disease in Europe, North America, and Asia: a systematic literature review. Ocul Surf. 2016;14(2):144–167. doi:10.1016/j.jtos.2015.11.002
4. Knop E, Knop N, Millar T, Obata H, Sullivan DA. The international workshop on meibomian gland dysfunction: report of the subcommittee on anatomy, physiology, and pathophysiology of the meibomian gland. Invest Ophthalmol Vis Sci. 2011;52(4):1938–1978. doi:10.1167/iovs.10-6997c
5. Nelson JD, Shimazaki J, Benitez-del-Castillo JM, et al. The international workshop on meibomian gland dysfunction: report of the definition and classification subcommittee. Invest Ophthalmol Vis Sci. 2011;52(4):1930–1937. doi:10.1167/iovs.10-6997b
6. Geerling G, Tauber J, Baudouin C, et al. The international workshop on meibomian gland dysfunction: report of the subcommittee on management and treatment of meibomian gland dysfunction. Invest Ophthalmol Vis Sci. 2011;52(4):2050–2064. doi:10.1167/iovs.10-6997g
7. Bron AJ, Tiffany JM. The contribution of meibomian disease to dry eye. Ocul Surf. 2004;2(2):149–165. doi:10.1016/S1542-0124(12)70150-7
8. Marshall LL, Roach JM. Treatment of dry eye disease. Consult Pharm. 2016;31(2):96–106. doi:10.4140/TCP.n.2016.96
9. Qiao J, Yan X. Emerging treatment options for meibomian gland dysfunction. Clin Ophthalmol. 2013;7:1797–1803. doi:10.2147/OPTH.S33182
10. Borchman D, Foulks GN, Yappert MC, et al. Human meibum lipid conformation and thermodynamic changes with meibomian-gland dysfunction. Invest Ophthalmol Vis Sci. 2011;52(6):3805–3817. doi:10.1167/iovs.10-6514
11. Arita R, Morishige N, Shirakawa R, Sato Y, Amano S. Effects of eyelid warming devices on tear film parameters in normal subjects and patients with meibomian gland dysfunction. Ocul Surf. 2015;13(4):321–330. doi:10.1016/j.jtos.2015.04.005
12. Badawi D. TearCare((R)) system extension study: evaluation of the safety, effectiveness, and durability through 12 months of a second TearCare((R)) treatment on subjects with dry eye disease. Clin Ophthalmol. 2019;13:189–198. doi:10.2147/OPTH.S191588
13. Badawi D. A novel system, TearCare((R)), for the treatment of the signs and symptoms of dry eye disease. Clin Ophthalmol. 2018;12:683–694. doi:10.2147/OPTH.S160403
14. Lemp MA. Report of the National Eye Institute/Industry workshop on clinical trials in dry eyes. CLAO J. 1995;21(4):221–232.
15. Begley C, Caffery B, Chalmers R, Situ P, Simpson T, Nelson JD. Review and analysis of grading scales for ocular surface staining. Ocul Surf. 2019;17(2):208–220. doi:10.1016/j.jtos.2019.01.004
16. Arita R, Minoura I, Morishige N, et al. Development of definitive and reliable grading scales for meibomian gland dysfunction. Am J Ophthalmol. 2016;169:125–137. doi:10.1016/j.ajo.2016.06.025
17. Schiffman RM, Christianson MD, Jacobsen G, Hirsch JD, Reis BL. Reliability and validity of the ocular surface disease index. Arch Ophthalmol. 2000;118(5):615–621. doi:10.1001/archopht.118.5.615
18. Miller KL, Walt JG, Mink DR, et al. Minimal clinically important difference for the ocular surface disease index. Arch Ophthalmol. 2010;128(1):94–101. doi:10.1001/archophthalmol.2009.356
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


