Back to Journals » International Journal of General Medicine » Volume 16
A Prospective Observational Study of Children with FS-Associated Hospitalization: The Implication and Outcomes of Pathogen Detection in Cerebrospinal Fluid
Authors Chen F, Feng F, You D, Guo Y, Yang S, Zhao T, Sun S, Wang L
Received 27 February 2023
Accepted for publication 11 May 2023
Published 18 May 2023 Volume 2023:16 Pages 1891—1898
DOI https://doi.org/10.2147/IJGM.S410337
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
Fang Chen,* Fan Feng,* Dianping You, Yinghui Guo, Shuo Yang, Tong Zhao, Suzhen Sun,* Le Wang*
Institute of Pediatric Research, Children’s Hospital of Hebei Province, Shijiazhuang, 050031, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Le Wang, Institute of Pediatric Research, Children’s Hospital of Hebei Province, 133 Jianhua South Street, Shijiazhuang, Hebei Province, 050031, People’s Republic of China, Tel +86-0311-85911389, Email [email protected]
Background: Febrile seizures (FS) are a common cause of paediatric emergencies, but research on their aetiology and epidemiology are limited. The aim of this study was to investigate the prevalence of central nervous system (CNS) pathogenic infections in patients with FS-associated hospitalization.
Methods: A prospective observational study was conducted in children under 16 years of age with FS-associated hospitalization. Demographic, clinical and laboratory data were recorded. Multiplex-PCR was performed on cerebrospinal fluid (CSF) samples for nine viruses, nine bacteria and one fungus.
Results: A total of 119 children were enrolled between June 2021 and June 2022. Of these, 83.2% had a final diagnosis of FS (69.7%) or FS plus (13.4%). In addition, epilepsy and encephalitis/meningitis were also found in 16.8% (20/119). Seven pathogens were identified from 9 CSF samples (7.6%), including viruses (EV, EBV, HHV-6) and bacteria (H. influenzae, S. pneumoniae, M. tuberculosis, S. putrefaciens). There were no significant clinical or laboratory differences between children who tested positive or negative for pathogens in the CSF, except for the presentation of herpes pharyngitis. Children with encephalitis/meningitis had longer hospital stays compared with those diagnosed with FS at discharge; abnormal EEG findings were significantly more common in patients with epilepsy.
Conclusion: FS-associated hospitalized children may have viral or bacterial intracranial infections. Pathogen testing of CSF is an important basis for timely antibiotic or antiviral therapy when clinical and laboratory findings make FS indistinguishable from other CNS disorders.
Keywords: febrile seizure, children, CSF, infection
Introduction
Febrile seizures (FS) are one of the major neurological emergencies that require prompt diagnosis and treatment.1 Most cases are benign but may be associated with multiple clinical sequelae, including a higher risk of epilepsy and FS recurrence.2 If this acute condition is not adequately evaluated and treated at the time of first presentation, the opportunities to alter the poor prognosis may be missed.3 Identifying the cause of fever is the first task in the assessment of such children.4 FS caused by infections of respiratory or intestinal systems have been well recognized.5–10 However, evidence on central nervous system (CNS) pathogens in children with primary symptom of seizure accompanied with fever is limited, which creates a risk of confusion between FS and other CNS disorders.6 Over the past decade, nucleic acid amplification tests have been used to clinically diagnose various common causes of meningitis.11 If cultures of suspected encephalitis/meningitis remain negative, highly sensitive PCR or multiplex-PCR can be used to amplify conserved regions of pathogenic DNA/RNA to identify the causative organism.12–14 The aim of this study was to investigate the role of common CNS pathogens in CSF specimens from FS-associated hospitalized children and to identify susceptibility factors and specific characteristics of children who tested positive for CSF.
Materials and Methods
Ethics
Ethics approval (protocol 2019202) was granted by the Ethics Committee of Children’ s Hospital of Hebei in compliance with the principles of the Declaration of Helsinki and the Code of Ethics of the World Medical Association. The child’s legal guardian(s) or parent(s) provided written informed consent for sample collection and clinical record review. Each enrolled child is coded with a unique number that does not reflect their personal information.
Study Population
Children aged under 16 years of age attending our hospital (the only tertiary care children’s hospital in Hebei, northern China) were prospectively enrolled in this study between June 2021 and June 2022. Children were recruited based on a symptom of seizure accompanied by fever (axillary temperature ≥38°C) within the preceding 96 hours. An FS is diagnosed as a seizure accompanied by fever without CNS infection, that occurs in infants and children 6 through 60 months of age.15 A FS-plus is diagnosed when the child with seizure accompanied by fever is older than 5 years old.16 Multiple episodes of FS during a single febrile illness were defined as recurrent FS.16 Complex FS are diagnosed if one of the following conditions is met: 1) seizures with focal semiology; 2) duration longer than 15 minutes; 3) recurrent FS.16 The following data were collected: age, sex, biochemical and microbiological culture results of CSF specimens, antimicrobial therapy, duration of illness prior to admission and length of hospital stay. Children have previous diagnosis of epilepsy, suspected of having CNS infection, encephalopathy, metabolic, toxic or neurodegenerative diseases on admission will be excluded. The final diagnosis at discharge is determined by an expert group based on the patient’s clinical manifestation, laboratory tests and response to treatment.
Pathogens Detection
Obtained by lumbar puncture, CSF samples were used for routine biochemical testing and culture, while the remaining samples were stored at −80°C for subsequent molecular analysis. Nucleic acids were extracted and purified on an automated extraction workstation Smart LabAssist-16/32 (TANBead, Taiwan, China) using an extraction kit (HGT, Ningbo, China) for a total of 300µL CSF samples. Extracts were immediately used as template for PCR amplification or stored below −20°C. Pathogen detection was performed as previous reported.17 Briefly, multiplex RT-PCR was performed using an ABI Veriti 96 Thermal Cycler. The PCR products were added into the platform for capillary electrophoresis and fragment analysis. This multiple detection kit can detect 18 microorganisms, including 9 bacteria as Neisseria meningitidis, Mycobacterium tuberculosis, Listeria monocytogenes, Streptococcus pneumoniae, Mycoplasma pneumoniae, Streptococcus agalactiae, Acinetobacter baumannii, Haemophilus influenzae, Escherichia coli K1, 8 viruses: Enterovirus (EV), herpes simplex virus type 1 (HSV-1), herpes simplex virus type 2 (HSV-2), varicella zoster virus (VZV), Epstein–Barr virus (EBV), mumps virus (MuV), cytomegalovirus (CMV), human herpesvirus type 6 (HHV-6) and Cryptococcus neoformans.
Statistical Analysis
In this study, categorical variables were tested using Chi-square or Fisher’s exact test. The Kolmogorov–Smirnov test was used to check whether continuous variables conformed to a normal distribution, and the Mann–Whitney U-test was used to determine statistical significance. We perform multi-comparisons within the FS (including FS plus), epilepsy and encephalitis/meningitis groups by Bonferroni method. Adjusted p values are indicated in the table Statistical analysis was performed by SPSS 25 (SPSS Inc., Chicago, USA). Differences were considered statistically significant if p<0.05.
Results
Study Population
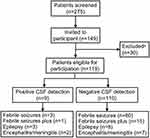
During the study period, 275 patients presenting to the emergency department (ED) were documented as having seizures accompanied by fever in the last 96 hours. Children could be hospitalized if they met one of the following points: i) complex febrile seizures; ii) poor mental status after seizure remission.18,19 Finally, 126 of 275 children were assessed by physicians as not requiring hospitalization, and the remaining 149 children were hospitalized and enrolled in our study. Of the 149 children, i) 13 were found ineligible because they had a history of afebrile seizures, an underlying neurological disease or a suspected acute infection involving the CNS; ii) 5 were eligible but did not have adequate CSF volume, and iii) 12 declined the consents during the research. In total, CSF samples from 119 cases were successfully tested by multiplex-PCR (Figure 1).
The Diagnose at Discharge
Ninety-nine (83.2%) of the children admitted were diagnosed with FS (83, 69.7%) or FS plus (16, 13.4%) at discharge. Besides that, patients were diagnosed with epilepsy (9.2%) and encephalitis/meningitis (7.6%, Table 1). Of the children with FS, 71 had seizures within 24 hours of fever, and 12 had seizures 3 days after fever. Twenty-three patients had simple FS and 60 had complex FS. The median age of patients with FS was 2 years (IQR 1–3.3 years), and 57% were male. Thirty-seven percent had a previous history of FS and 34% had a family history of febrile and/or afebrile seizures. Almost every patient with FS or FS plus had other sources of infection outside the CNS, including respiratory infections, gastrointestinal infections, septic tonsillitis and sinusitis. The total number of children with a first seizure less than 1 year of age accounted for 53.7% (64/119). Seven cases had underlying medical conditions, including congenital heart disease, anaemia, pervasive dysplasia and microcephaly.
 |
Table 1 Demographics of Pediatric Patients with FS-Associated Hospitalization |
Clinical Features Between FS and Other CNS Disorders
There were no significant differences between the three groups in terms of age, gender or extra-CNS manifestations on admission (Table 1). Patients with encephalitis/meningitis had longer hospital stays compared with those discharged with a diagnosis of FS or FS plus (12 vs 6 days, p<0.001). EEG abnormalities were significantly more common in patients with epilepsy (89% vs 22%, p<0.001). Most children with FS or FS plus received supportive treatment, whereas those with intracranial infections received antiviral therapy (81% vs 9%, p<0.001). Children with epilepsy were significantly more liable to receive anticonvulsant medication to terminate seizures than those with FS or FS plus (55% vs 9%, p<0.001). No patients were admitted to the intensive care unit and all patients made a full neurological recovery.
Pathogen Detection in CSF
Seven pathogens were identified in nine CSF samples (Table 2, 7.6%), including viruses (EV, EBV and HHV-6) and bacteria (H. influenzae, S. pneumoniae, M. tuberculosis and S. putrefaciens). At discharge, four patients were diagnosed as FS or FS plus, while the other five children were diagnosed with encephalitis/meningitis or epilepsy. There were no significant differences between children with and without detectable pathogens in CSF in terms of age, sex, laboratory or clinical presentations. The only exception was the manifestation of herpetic pharyngitis, which was observed more frequently in patients who tested positive for CSF (56% vs 15%, p=0.002).
 |
Table 2 Patients with Positive Detection in the CSF |
Among the nine children who tested positive in CSF, S. putrefaciens was found in case No. 60, who had suffered a traumatic brain injury 3 days earlier (Table 2). He was the only of these children to have an abnormal CSF biochemical result. Enteroviruses were found in CSF of cases No. 17 and No. 48. Although these was a lack of evidence of typical inflammatory changes in CSF, both patients were diagnosed with encephalitis based on their clinical manifestation and response to treatment. M. tuberculosis was found in CSF of case No. 86, and enterococcus avium was also detected in urine cultures of this child. The CD4+ lymphocytes in the blood were counted as 86/uL, which was below the reference interval, indicating a lower immune function.
Discussion
Febrile seizures (FS) are an ongoing challenge for parents and clinicians. For parents, FS can be a traumatic and frightening experience, which may explain why most of the children in our study were taken to our emergency department within 24 hours after seizure. For clinicians, the primary consideration is the need to exclude CNS infection as the cause of fever.20 Although the need for lumbar puncture in children with FS has been debated for many years, clinical features such as fever and new onset seizures need to be discriminated from encephalitis/meningitis.21–23 Recent studies have highlighted the importance of etiology in children with FS, as 16% of patients with meningitis have seizures and a third of them have no meningeal signs or symptoms.4,24 Previous studies have assessed extracerebral causes of fever by using respiratory and faecal specimens to detect common respiratory or enteric pathogens, respectively.25 However, intracranial bacterial or viral infections as a cause of fever remain largely unknown.9 A major goal of this work was to explore the pathogens in CSF specimens from children with FS-associated hospitalization.
As we know, 20–40% of the patients develop seizures early or during bacterial or viral encephalitis/meningitis.22,26–28 In the present study, approximately one in ten children was eventually diagnosed with encephalitis/meningitis even when CNS infection was ruled out based on laboratory and clinical findings on admission. Of these, no typical inflammatory changes were seen on CSF biochemical examination in children with positive CSF detection, except for one child who had S. putrefaciens infection. Classically, in encephalitis or meningitis, the glucose concentrations and white cell counts (WCC) in CSF will increase. However, these parameters have been shown not to be reliable predictors in recent years.29–33 In our study, eight of nine children with viral or bacterial infection in CSF did not show typical inflammatory changes. Mohammad et al investigated HHV-6 infection in hospitalized patients with FS and observed no differences in age, gender, and clinical presentation between HHV-infected and non-infected children.30 Kondo et a suggested that HHV-6 invades the brain during the acute phase of infection and the recurrence of FS may be associated with reactivation of HHV-6.34 Rantala et al performed PCR in 144 children with febrile convulsions and isolated 9 viruses from their CSF, including adenovirus, parainfluenza virus, respiratory syncytial virus, echovirus, herpes simplex virus, and influenza B viruses.35 Doja et al reviewed 21 children diagnosed with infectious mononucleosis, 5 of whom had fever and convulsions, and detected EBV in the CSF of 3 of them and Mycoplasma pneumoniae from 1 of them.36 Among 340 children with complex FS, Kimia et al found two cases of S. pneumoniae without typical laboratory manifestations.31 One possible reason for this phenomenon is that the virus or bacteria may invade the nervous system directly without causing an inflammatory response. Therefore, in the absence of typical clinical signs or CSF pathogenesis, encephalitis/meningitis can be excluded due to normal CSF biochemical findings, which emphasizes the importance of differential diagnosis between this group of patients from viral encephalitis in the clinical setting.
The only discrepancy between patients with and without detectable pathogens is the manifestation of herpes cheilitis, as we found that children who present with herpangina had a higher chance of positive pathogen detection in CSF. As we know, herpes cheilitis can occur with enteroviral ectopia and a number of neurological disorders, including aseptic meningitis and encephalitis. Such manifestations are known to be caused by enterovirus, EBV and HHV-6.37–39 Taken together, these findings lead us to speculate the role of previously unknown pathogenic infections in CSF specimens from children with FS. The observed clinical manifestations help to distinguish encephalitis from FS.
Recent studies have highlighted that, fever following respiratory or intestinal infections in children are the main cause of convulsions.7,24,40 Francis et al used PCR tests and showed that approximately 70% of the children with FS were positive for respiratory virus, including rhinovirus, adenovirus, and influenza virus.5 Similarly, our study observed that nearly 80% of children with FS had respiratory infections. In our study, pathogens such as H. influenzae and S. pneumoniae were detected within the CSF, which can also cause symptoms of respiratory infections, suggesting that all these pathogens, once in the lungs, can spread throughout the body and eventually reach the CNS.6,41 Miyazaki et al showed that respiratory infections caused by H. influenzae produced TNF-α, which promote this pathogen to across the BBB into CSF.41 These findings therefore alert paediatricians to the importance of preventing hyperthermia following respiratory or intestinal infections, especially in children with a previous history or a family history of FS. To initiate appropriate antimicrobial therapy and to reduce the likelihood of neurological sequelae, it is recommended that children with FS be tested for pathogens that may infect multiple systems.
According to Chinese guideline,16 routine test for these children is biochemical examination on the CSF. The treatment of children in the acute phase of FS includes maintaining the airway open, monitoring vital signs, opening intravenous access if necessary, and taking measures to lower body temperature, initiate appropriate anti-infection and anti-shock therapy. The ancillary tests include peripheral blood electrolytes, blood glucose, blood gas analysis, white blood cell count and urine routine test as well as biochemical routine and white blood cell count in CSF. If seizures persist, resuscitation or hospitalization is recommended. During the interictal phase of seizures, the state of consciousness should be assessed first. If unconscious, the airway should be kept open, vital signs monitored, intravenous access opened. If consciousness is clear without positive features after the interictal phase, the child can leave the hospital. Furthermore, our study observed that more than 60% of the children with their first episode of FS were under 1 year of age. According to the guideline,16 children with FS younger than 1 year of age have a 50% chance of recurrence, so this group of children should be followed up. In addition, the majority of CSF-positive children had a history of FS in our study. Are these children more likely to be at risk of developing intracranial infections? Further studies are needed to understand the detailed mechanisms behind this phenomenon.
Limitations
We acknowledge several limitations of our study. 1) Not all patients with FS-associated hospitalization underwent lumbar puncture. 2) Although no patients returned to hospital with a diagnosis of encephalitis/meningitis, these patients may have gone to other institutions for treatment. 3) The number of children positive for CSF pathogens was small, so the significance of some parameters may have been underestimated.
Conclusions
In conclusion, our findings not only shed light on the discrimination between febrile seizures and other CNS disorders, but also remind us that in the absence of contraindications, lumbar puncture including cerebrospinal fluid PCR to identify the causative organisms is an important basis for timely antibiotic or antiviral therapy.
Abbreviations
FS, febrile seizures; CNS, central nervous system; CSF, cerebrospinal fluid; CHH, Children’s Hospital of Hebei; S. pneumoniae, Streptococcus pneumoniae; H. influenzae, Haemophilus influenza; M. tuberculosis, Mycobacterium Tuberculosis; S. putrefaciens, Staphylococcus putrefaciens; EV, enterovirus; EBV, Epstein–Barr virus; HHV, human herpesvirus type 6.
Data Sharing Statement
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
Ethics Approval and Consent to Participate
This study was approved by the Ethics Committee of Children’ s Hospital of Hebei (number 2019202), in compliance with the principles of the Declaration of Helsinki, the Code of Ethics of the World Medical Association. The legal guardian(s) or parent(s) of the children provided written informed consent for sample collection and clinical record review.
Acknowledgments
The study would not have been possible without the excellent support from clinical staff from the Neurology Department at Children’s Hospital of Hebei, and the laboratory staff from the Institute of Pediatric Research of Hebei.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This study was supported by the medical science research key project of Hebei province (20211225).
Disclosure
The authors declare that they have no competing interests.
References
1. Mukherjee A, Mukherjee A. Febrile convulsion--an overview. J Indian Med Assoc. 2002;100(5):317–9, 326.
2. Berg AT, Shinnar S. Unprovoked seizures in children with febrile seizures: short-term outcome. Neurology. 1996;47(2):562–568. doi:10.1212/WNL.47.2.562
3. Smith DK, Sadler KP, Benedum M. Febrile seizures: risks, evaluation, and prognosis. Am Fam Physician. 2019;99(7):445–450.
4. Rosman NP. Evaluation of the child who convulses with fever. Paediatr Drugs. 2003;5(7):457–461. doi:10.2165/00128072-200305070-00003
5. Francis JR, Richmond P, Robins C, et al. An observational study of febrile seizures: the importance of viral infection and immunization. BMC Pediatr. 2016;16(1):202. doi:10.1186/s12887-016-0740-5
6. Chung B, Wong V. Relationship between five common viruses and febrile seizure in children. Arch Dis Child. 2007;92(7):589–593. doi:10.1136/adc.2006.110221
7. Hautala M, Arvila J, Pokka T, et al. Respiratory viruses and febrile response in children with febrile seizures: a cohort study and embedded case-control study. Seizure. 2021;84:69–77. doi:10.1016/j.seizure.2020.11.007
8. Sawires R, Kuldorff M, Fahey M, et al. Snotwatch: an ecological analysis of the relationship between febrile seizures and respiratory virus activity. BMC Pediatr. 2022;22(1):359. doi:10.1186/s12887-022-03222-4
9. Millichap JG, Millichap JJ. Role of viral infections in the etiology of febrile seizures. Pediatr Neurol. 2006;35(3):165–172. doi:10.1016/j.pediatrneurol.2006.06.004
10. Manfredini R, Vergine G, Boari B, et al. Circadian and seasonal variation of first febrile seizures. J Pediatr. 2004;145(6):838–839. doi:10.1016/j.jpeds.2004.06.079
11. van de Beek D, Brouwer MC, Koedel U, et al. Community-acquired bacterial meningitis. Lancet. 2021;398(10306):1171–1183. doi:10.1016/S0140-6736(21)00883-7
12. Tansarli GS, Chapin KC. Diagnostic test accuracy of the BioFire(R) FilmArray(R) meningitis/encephalitis panel: a systematic review and meta-analysis. Clin Microbiol Infect. 2020;26(3):281–290. doi:10.1016/j.cmi.2019.11.016
13. Wang L, Chen F, You D, et al. Development and validation of a multiplex-PCR based assay for the detection of 18 pathogens in the cerebrospinal fluid of hospitalized children with viral encephalitis. J Virol Methods. 2020;277:113804. doi:10.1016/j.jviromet.2019.113804
14. Albuquerque RC, Moreno ACR, Dos Santos SR, et al. Multiplex-PCR for diagnosis of bacterial meningitis. Braz J Microbiol. 2019;50(2):435–443. doi:10.1007/s42770-019-00055-9
15. Subcommittee on Febrile, S. and P. American Academy of. Neurodiagnostic evaluation of the child with a simple febrile seizure. Pediatrics. 2011;127(2):389–394. doi:10.1542/peds.2010-3318
16. Subspecialty Group of Neurology, t.S.o.P.C.M.A. 热性惊厥诊断治疗与管理专家共识(2016) [Expert consensus on the diagnosis and management of febrile seizures(2016)]. Zhonghua Er Ke Za Zhi. 2016;54(10):723–727. Chinese. doi:10.3760/cma.j.issn.0578-1310.2016.10.002
17. You D, Chen F, Li J, et al. Prospective case-control study of enterovirus detection differences in children’s cerebrospinal fluid between multiplex PCR and real-time RT-PCR assay. J Clin Lab Anal. 2021;35(2):e23606. doi:10.1002/jcla.23606
18. Laino D, Mencaroni E, Esposito S. Management of pediatric febrile seizures. Int J Environ Res Public Health. 2018;15(10):2232. doi:10.3390/ijerph15102232
19. Capovilla G, Mastrangelo M, Romeo A, et al. Recommendations for the management of “febrile seizures”: ad Hoc Task Force of LICE guidelines commission. Epilepsia. 2009;50(Suppl 1):2–6. doi:10.1111/j.1528-1167.2008.01963.x
20. Auvin S, Antonios M, Benoist G, et al. Évaluation d’un enfant après une crise fébrile : focus sur trois problèmes de pratique clinique [Evaluating a child after a febrile seizure: insights on three important issues]. Arch Pediatr. 2017;24(11):1137–1146. French. doi:10.1016/j.arcped.2017.08.018
21. Son YY, Kim G-H, Byeon JH, et al. Need for lumbar puncture in children younger than 12 months presenting with simple febrile seizure. Pediatr Emerg Care. 2018;34(3):212–215. doi:10.1097/PEC.0000000000000779
22. Trainor JL, Hampers LC, Krug SE, et al. Children with first-time simple febrile seizures are at low risk of serious bacterial illness. Acad Emerg Med. 2001;8(8):781–787. doi:10.1111/j.1553-2712.2001.tb00207.x
23. Steering Committee on Quality, I. and S.o.F.S.A.A.o.P. Management. Febrile seizures: clinical practice guideline for the long-term management of the child with simple febrile seizures. Pediatrics. 2008;121(6):1281–1286. doi:10.1542/peds.2008-0939
24. American Academy of Pediatrics. Provisional committee on quality improvement, subcommittee on febrile seizures. Practice parameter: the neurodiagnostic evaluation of the child with a first simple febrile seizure. Pediatrics. 1996;97(5):769–72; discussion 773–5.
25. Ueda H, Tajiri H, Kimura S, et al. Clinical characteristics of seizures associated with viral gastroenteritis in children. Epilepsy Res. 2015;109:146–154. doi:10.1016/j.eplepsyres.2014.10.021
26. Shah SS, Alpern ER, Zwerling L, et al. Low risk of bacteremia in children with febrile seizures. Arch Pediatr Adolesc Med. 2002;156(5):469–472. doi:10.1001/archpedi.156.5.469
27. McMinn P, Stratov I, Nagarajan L, et al. Neurological manifestations of enterovirus 71 infection in children during an outbreak of hand, foot. and Mouth Disease in Western Australia Clin Infect Dis. 2001;32(2):236–242.
28. Rotbart HA. Enteroviral infections of the central nervous system. Clin Infect Dis. 1995;20(4):971–981. doi:10.1093/clinids/20.4.971
29. Bijlsma MW, Brouwer MC, Kasanmoentalib ES, et al. Community-acquired bacterial meningitis in adults in the Netherlands, 2006–14: a prospective cohort study. Lancet Infect Dis. 2016;16(3):339–347. doi:10.1016/S1473-3099(15)00430-2
30. Houshmandi MM, Moayedi A, Rahmati MB, et al. Human herpes virus type 6 and febrile convulsion. Iran J Child Neurol. 2015;9(4):10–14.
31. Kimia A, Ben-Joseph EP, Rudloe T, et al. Yield of lumbar puncture among children who present with their first complex febrile seizure. Pediatrics. 2010;126(1):62–69. doi:10.1542/peds.2009-2741
32. Dubos F, Lamotte B, Bibi-Triki F, et al. Clinical decision rules to distinguish between bacterial and aseptic meningitis. Arch Dis Child. 2006;91(8):647–650. doi:10.1136/adc.2005.085704
33. Ray P, Badarou-Acossi G, Viallon A, et al. Accuracy of the cerebrospinal fluid results to differentiate bacterial from non bacterial meningitis, in case of negative gram-stained smear. Am J Emerg Med. 2007;25(2):179–184. doi:10.1016/j.ajem.2006.07.012
34. Kondo K, Nagafuji H, Hata A, et al. Association of human herpesvirus 6 infection of the central nervous system with recurrence of febrile convulsions. J Infect Dis. 1993;167(5):1197–1200.
35. Rantala H, Uhari M, Tuokko H. Viral infections and recurrences of febrile convulsions. J Pediatr. 1990;116(2):195–199. doi:10.1016/S0022-3476(05)82874-4
36. Doja A, Bitnun A, Ford Jones EL, et al. Pediatric Epstein-Barr virus-associated encephalitis: 10-year review. J Child Neurol. 2006;21(5):384–391. doi:10.1177/08830738060210051101
37. Domachowske JB, Cunningham CK, Cummings DL, et al. Acute manifestations and neurologic sequelae of Epstein-Barr virus encephalitis in children. Pediatr Infect Dis J. 1996;15(10):871–875. doi:10.1097/00006454-199610000-00008
38. Akkoc G, Kadayifci EK, Karaaslan A, et al. Epstein-Barr virus encephalitis in an immunocompetent child: a case report and management of Epstein-Barr virus encephalitis. Case Rep Infect Dis. 2016;2016:7549252. doi:10.1155/2016/7549252
39. Corsino CB, Ali R, Linklater DR. Herpangina. In: StatPearls. Treasure Island (FL): Statpearls Publishing; 2022.
40. Bohmwald K, Gálvez NMS, Ríos M, et al. Neurologic alterations due to respiratory virus infections. Front Cell Neurosci. 2018;12:386. doi:10.3389/fncel.2018.00386
41. Miyazaki Y, Yusa T, Matsuo S, et al. Zyxin modulates the transmigration of Haemophilus influenzae to the central nervous system. Virulence. 2014;5(6):665–672. doi:10.4161/viru.29786
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.