Back to Journals » Clinical Ophthalmology » Volume 18
A Preservative-Free Approach – Effects on Dry Eye Signs and Symptoms After Cataract Surgery
Authors Jensen P , Nilsen C, Gundersen M , Gundersen KG , Potvin R , Gazerani P , Chen X , Utheim TP, Utheim ØA
Received 26 October 2023
Accepted for publication 15 February 2024
Published 26 February 2024 Volume 2024:18 Pages 591—604
DOI https://doi.org/10.2147/OPTH.S446804
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Per Jensen,1 Christian Nilsen,1 Morten Gundersen,1 Kjell Gunnar Gundersen,1 Rick Potvin,2 Parisa Gazerani,3 Xiangjun Chen,4– 6 Tor P Utheim,3– 8 Øygunn A Utheim4,7,8
1Ifocus Eye Clinic, Haugesund, Norway; 2Science in Vision, Frisco, TX, USA; 3Faculty of Health Sciences, Oslo Metropolitan University, Oslo, Norway; 4Department of Medical Biochemistry, Oslo University Hospital, Oslo, Norway; 5Department of Ophthalmology, Sørlandet Hospital Arendal, Arendal, Norway; 6Department of Ophthalmology, Vestre Viken Hospital Trust, Drammen, Norway; 7Department of Ophthalmology, Oslo University Hospital, Oslo, Norway; 8The Norwegian Dry Eye Clinic, Oslo, Norway
Correspondence: Per Jensen, Ifocus Eye Clinic, Sørhauggata 111, Haugesund, 5527, Norway, Tel +47 906 13 685, Email [email protected]
Purpose: To compare the effect of treatment with preservative-free dexamethasone, NSAIDs and trehalose/hyaluronic acid eye drops with the preservative benzalkonium chloride containing dexamethasone and NSAIDs after cataract surgery in dry versus non-dry eyes.
Patients and Methods: In this prospective randomized intervention study, dry eye tests were performed before and 6 weeks after cataract surgery. Patients were considered as having dry eye, SDE (sign of dry eye), if at least one of the following dry eye tests were abnormal; corneal fluorescein staining (CFS), non-invasive keratograph breakup time (NIKBUT) or tear osmolarity. Patients with SDE were randomly assigned to one of two groups. Group 1 patients were treated with dexamethasone and bromfenac eye drops with the preservative benzalkonium chloride (BAC). Group 2 patients were treated with preservative-free dexamethasone and preservative-free diclofenac, as well as a preservative-free lubricant with trehalose and hyaluronic acid both before and after surgery. Patients with normal tear film status acted as the control group (group 3) and received same treatment as group 1.
Results: A total of 215 patients were enrolled six weeks after surgery, the number of patients with SDE decreased significantly in groups 1 and 2 (p < 0.001). Subjective symptoms and objective measures including osmolarity, NIKBUT, CFS, and tear film thickness (TFT) improved after surgery, tear production remained unchanged, while corneal sensitivity and meibomian gland dysfunction (MGD) parameters worsened. In the control group with normal tear-film status, SDE increased significantly after the surgery (p < 0.001). There were no statistically significant differences in tear film parameters between the three groups after surgery.
Conclusion: After cataract surgery, patients with mild to moderate dry eyes may experience improved tear film status and reduced symptoms. However, we found no additional beneficial effect on dry eye parameters with treatment with preservative-free dexamethasone, NSAIDs, and lubricants compared to preservative-containing eye drops.
Keywords: sign of dry eye, cataract surgery, osmolarity, corneal fluorescein staining, non-invasive keratograph tear break-up time, ocular surface disease index, meibomian gland dysfunction
Introduction
Cataract surgery is a commonly performed procedure that can significantly improve the quality of life for most patients. One of the most common complications of cataract surgery is the development of dry eye disease (DED).1–3 DED is characterized by an imbalance in the tear film resulting in ocular discomfort, visual disturbances, and potential damage to the ocular surface.4 There are several hypotheses about the mechanisms underlying the development of DED after cataract surgery, for instance: the use of eye drops with harmful preservatives, weakened reflex stimuli for tear secretion because the surgical incisions affect nerve endings, damage to the corneal epithelium, the increase of inflammatory markers and loss of mucus-producing cells (goblet cells) caused by ocular damage.2,3,5 In addition, studies have reported that a common contributor to postoperative discomfort is pre-existing DED.6–8
The use of combined topical corticosteroids and nonsteroidal anti-inflammatory drugs (NSAIDs) is common after cataract surgery, for prophylactic management and to reduce the risk of inflammation and cystoid macular edema (CME).9,10 However, many of these medications contain preservatives, which in some patients have been shown to exacerbate dry eye symptoms and signs, such as increasing ocular surface staining and shortened tear break-up time.11,12 The most commonly used preservative in eye drops is benzalkonium chloride (BAC),13,14 which demonstrates antimicrobial efficacy against a wide variety of common pathogens. There is considerable evidence from its use in glaucoma medications of its deleterious effect on the ocular surface, especially when used over an extended period.11,15
In the short term, exposure to BAC can cause ocular surface irritation, redness, and consequently surface inflammation.11 This is because BAC has been shown to disrupt the lipid layer of the tear film, leading to increased evaporation and dryness of the eye.16,17 Additionally, BAC can damage the epithelial cells of the cornea, leading to further inflammation and tissue damage.13,18 The threshold concentration at which toxicity occurs has been estimated to be ~0.005%.11
In the longer term, chronic exposure to BAC has been linked to more serious ocular conditions, such as subepithelial inflammation and punctate keratopathy.11 This is because BAC can penetrate the deeper layers of the cornea, causing damage to corneal nerves and endothelial cells.19–21 The development of DED and the cytotoxic effect of BAC have been linked to the number of medications, the number of drops per day and the duration of therapy.11
Other options than mere preservative-free eyedrops would be the use of preservatives that were non-toxic to the ocular surface, eg, Polyquaternium and oxidizing preservatives like Sodium perborate and Stabilized Oxychloro Complexes. These preservatives are traditionally used in contact lens solutions, but are also successfully used in lubricants as reviewed by Walsh and Jones.15 However, to our knowledge, NSAIDs and topical steroids for the eye are not available in other forms than preservative-free versions and BAC-containing versions. In addition, for the most severe dry eyes, preservative-free lubricants are preferable to preservative-containing artificial tears.22 Therefore, we decided to use preservative-free single-dose containers with NSAIDs and dexamethasone and preservative-free lubricants with a disinfectant bottle system.
Preservative-free formulations of ocular medications are better tolerated and cause less discomfort but can be more expensive and difficult to handle.23,24 Several studies have focused on the benefits of using preservative-free eye drops after cataract surgery. In addition, treatment with lubricants has been described as effective in resolving postoperative signs and symptoms of DED.25–27 Jee et al hypothesized that the combination of preservative-free steroid and lubricant eye drops may relieve pre-existing DED by decreasing the oxidative and inflammatory damage to the ocular surface in patients after cataract surgery.28
Even though the potential toxicity of preservatives and the benefits of using preservative-free medications and lubricants for dry eye are known, most clinics are still using traditional treatment formulations with preservatives. The aim of the present study was to compare the effect on dry eye of treatment with dexamethasone, NSAIDs and trehalose/hyaluronic acid, all preservative-free, to the effect of dexamethasone and NSAIDs both with preservatives after cataract surgery. We hypothesized that the use of a preservative-free treatment regimen after cataract surgery would be more effective in preventing symptoms and signs of DED. To our knowledge, no previous studies have evaluated the effect of these treatment regimens on dry eye symptoms and signs following cataract surgery.
Materials and Methods
This prospective randomized interventional trial is part of a larger study, where a cohort of patients scheduled for cataract surgery were examined for DED at baseline before and after surgery. The patients were randomized to different treatment arms based on ocular surface signs of DED. The prevalence of DED, biometric precision, and variability related to DED, the significance of osmolarity as a diagnostic tool of DED, and dry eye examinations before and after surgery were discussed. In addition, tear film samples were collected for metabolomic and lipidomic analysis. Two articles from this project have already been published.29,30
In this manuscript, the changes in DED signs and symptoms after surgery are presented, the results from the different treatment arms are compared and the effect on DED of using a preservative-free treatment regimen is discussed. The study was conducted from August 2020 to January 2022 at one clinical site in Haugesund, Norway. The study followed the tenets of the Declaration of Helsinki and adhered to good clinical practice. It was approved by the Regional Committee for Medical and Health Research Ethics in Norway (Ref. 2020/64847). The original data and anonymous patient files are stored on a secured server (Tjenester for Sensitive Data, TSD) at the University of Oslo. The data are available on request. The study protocol (Metabolomic Profile in Dry Eye Syndrome Patients) was first submitted to Clinicaltrials.gov on 10.08.2020 and re-posted on 27.06.2022 (NCT 05433428). The reasons for the delay were extended data collection time, administrative issues with the submission, and the fact that the clinic had to downscale its operations due to the Covid-19 pandemic. Written informed consent was obtained from all participants at their first visit.
Inclusion criteria included consecutive patients who were referred for age-related cataract surgery, 18 years or older, and were willing and able to participate in the study. Exclusion criteria were current corneal disease, scarring or corneal ectasia, lid deformities or previously performed corneal refractive procedures. Patients were instructed not to wear contact lenses on the days of examination and not to use any eye drops at least two hours before the examination.
Test Protocol
A preoperative assessment with a focus on dry eyes was conducted as described in the following paragraph by two trained technicians. One eye was randomly selected as the study eye and dry eye examinations were performed only on the study eye, except for osmolarity testing that was performed in both eyes as required by the manufacturer’s instructions.31 These examinations were also performed at the 6-week follow-up visit. Slit-lamp examination, subjective refraction, and biometry were also performed as part of a standard preoperative cataract surgery evaluation.
Tear osmolarity from both eyes was obtained using the TearLab osmolarity system,32 and was performed as the first of the examinations to avoid influence from other measures. The microchip was placed in the lateral meniscus while the patient was instructed to gaze superonasally to avoid touching the conjunctiva.33 Symptom scoring was performed by asking the subjects to answer two questionnaires, the Ocular Surface Disease Index (OSDI), and the Standard Patient Evaluation of Eye Dryness (SPEED).34
The Non-Invasive Keratograph Break-Up Time (NIKBUT) was obtained using the Keratograph 5M (OCULUS, Wetzlar, Germany) device.35 The subject was instructed to blink twice and then keep their eyes open as long as possible during the automatic sequence with infrared illumination. Three consecutive NIKBUT measurements were performed, and the average was recorded. The Lipiview instrument (Lipiview II Interferometer, TearScience Inc., Morrisville, NC) was used to measure the tear film thickness (TFT).36,37 Assessment of corneal fluorescein staining (CFS) was performed using a slit lamp biomicroscope with cobalt blue light and a yellow barrier filter. Five µL of fluorescein sodium 2% (without anesthesia) was instilled into the inferior fornix. After ½ to 1 minute, the corneal staining was graded according to the Oxford grading scheme (0–5).38 To evaluate tear secretion, the Schirmer test was performed without topical anesthesia (TearFlo, MDT, Krakow, Poland). Results were evaluated after 5 minutes.31 Schirmer tests were performed approximately 5 minutes after 5 µL of fluorescein sodium was instilled in the conjunctival sac.
The corneal sensitivity (CS) was assessed using the Cochet-Bonnet esthesiometer (Luneau SAS France).39
Meibomian gland dropout was evaluated from infrared images of the lower eyelid (Meiboscore, OCULUS Keratograph 5M). Finally, a slit lamp was used to assess the meibum quality and expressibility. A gentle application of a cotton swab was employed towards the edge of the lower eyelid, and an evaluation was conducted based on the visual characteristics of the meibum secretion. Expressibility was assessed on a scale from 0 to 3, with a grade of 0 indicating that all glands were easily expressible, a grade of 1 signifying that 3–4 glands could be expressed, a grade of 2 denoting that 1–2 glands were expressible, and a grade of 3 representing a condition where none of the glands could be expressed. Additionally, the quality of the meibum was rated on a scale from 0 to 3: grade 0 represented a clear meibum fluid, grade 1 indicated a cloudy appearance, grade 2 described a granular texture, and grade 3 characterized a thick, toothpaste-like consistency of the meibum.40 A summary of the order of testing with normal and abnormal values for each test is presented in Table 1.
 |
Table 1 Summary of Testing Order with Normal and Abnormal Values for Each Test |
Treatment Protocol
Since in the diagnostic process, we only considered a selection of objective tests for dry eyes and not subjective symptoms, we chose to use the term “sign of dry eye” (SDE) for those patients with abnormal values, identified as having dry eyes, furthermore, patients who presented with normal values, did not have dry eyes (normal).
Patients were considered as having dry eyes (SDE) if at least one of the following dry eye tests were abnormal: CFS, NIKBUT average or Osmolarity. Patients with SDE were randomly assigned to one of two groups. Group 1 patients were treated with preserved dexamethasone 0.1% (Spersadex, Blumont Ofta Trading Ltd, Gzira, Malta) and preserved bromfenac 0.09% (Yellox, Bausch & Lomb, Dublin, Ireland). Both eye drops contained benzalkonium chloride (BAC), with a concentration of 0.01% in Spersadex and 0.005% in Yellox. Group 2 patients were treated with preservative-free dexamethasone 0.1% (Monopex, Laboratories Thèa, Clermont-Ferrand, France) and preservative-free diclofenac 0.1% (Voltaren Ophtha, Laboratories Thèa, Clermont-Ferrand, France). In addition, group 2 patients were instructed to use the preservative-free lubricant (3% trehalose, 0.15% hyaluronic acid) Thealoz Duo (Laboratories Thèa, Clermont-Ferrand, France) both before and after surgery. Patients with a normal preoperative tear film acted as a control group (Group 3) and received the same treatment as group 1. An overview of the detailed treatment plan is presented in Table 2.
 |
Table 2 Overview of the Medications Used in the Study |
All patients underwent bilateral phacoemulsification and intraocular lens implantation in the capsular bag performed by the same surgeon. Preoperative disinfection with Betadine 5% (Alcon, Fort Worth, Texas, USA) and anesthesia with Tetracaine 1% (Bausch & Lomb, Dublin, Ireland) were applied. A 2.2 mm clear corneal incision and two side ports of 1 mm each were used. Prophylactic intracameral cefuroxime solution (Aprokam®) was administered. The operating microscope used was the NGENUITY 3D Visualization System (Alcon, Fort Worth, TX, USA).
Statistical Analysis
Statistical analysis was performed using the Statistical Package for the Social Sciences (IBM, SPSS statistics, version 14.0). Testing for normal distributions was performed by using the Shapiro–Wilk test. Non-normally distributed data were described as median with interquartile range (IQR), and categorical data were reported as numbers and percentages. The Kruskal–Wallis test was used to compare inter-group differences for continuous values. Pearson’s chi-square test was used to compare categorical values between groups. Statistical analysis of changes from baseline to 6 weeks by groups, was made using the non-parametric related samples McNemar change test. P-values for the comparison of dry eye tests before and after surgery and categorical values between group 1 and 2 were adjusted with the Bonferroni method for multiple comparisons. A p-value less than 0.05 was considered statistically significant.
Results
There were 224 patients enrolled in the study, but 5 patients were excluded because they were unable to complete the examinations. Four additional patients were excluded due to intra or postoperative complications. A total of 215 patients were included in the post hoc analyses, 76 patients in group 1, 95 in group 2, and 44 in group 3. The median age was 75 years in group 1 and 2, and 76 years in group 3. Forty-two patients (55.2%) were women in group 1, 59 (62.1%) in group 2, and 20 (45.4%) in group 3. There were no statistically significant differences between the 3 groups with regard to age and sex.
Dry Eye Diagnosis Before and After Surgery
Table 3 displays the number of patients who were diagnosed with dry eyes according to specifications defined for this study as diagnostic criteria (SDE and normal) after cataract surgery for groups 1, 2, and 3. Six weeks after surgery the number of patients with dry eyes decreased significantly (p < 0.001) in group 1 and 2, with no statistically significant difference between the two groups (76.3 and 75.7%, p =0.936). Again, based on the diagnostic criteria for the study, no subjects in group 3 had dry eyes preoperatively, however, fifty percent of patients in this group were diagnosed as having dry eyes after surgery, and this increase was statistically significant (p < 0.001). With no difference between groups 1 and 2, their data could be pooled and compared against group 3 postoperatively. With that, we found a statistically significant difference (p = 0.001), showing patients diagnosed with dry eyes preoperatively, regardless of treatment, improved after surgery compared to the non-dry eye patients.
Symptoms
Table 4 displays the number and percentage of patients with subjective symptoms of dry eyes measured with the OSDI and SPEED questionnaire before and after surgery for group 1, 2 and 3 respectively. There were no reported differences in the number of patients experiencing symptoms of dry eyes between group 1 and group 2 before surgery for both OSDI and SPEED (p-values of 0.677 and 0.909, respectively). The incidence of patients with symptoms of dry eyes in group 3 was high for both OSDI and SPEED (68.1% and 59%, respectively) before surgery. Postoperatively, the number of patients having subjective symptoms of dry eyes with the OSDI questionnaire decreased significantly in all groups. However, a comparison of pooled results from group 1 and 2 to results from group 3 showed no statistically significant difference (p=0.677).
With SPEED, the analyses showed a statistically significant decrease in symptoms in Group 1 (p = 0.001), but in group 2 the decrease was not statistically significant (p = 0.067). The symptoms of DED increased in group 3 after surgery, but the increase was not statistically significant (p = 0.549). Overall, there were no statistically significant differences between groups 1 and 2 after surgery.
Looking at the severity of subjective symptoms with OSDI (Figure 1), the percentage of patients with severe subjective symptoms of dry eyes (OSDI scores ≥ 33) was 38% in group 1, 40% in group 2, and 22% in group 3 at baseline. The postoperative incidence was 12.8%, 12.6% and 4% respectively. The reduction of severe symptoms from baseline to 6 weeks after surgery was statistically significant in all 3 groups (p<0.001), however, there was no statistically significant difference between groups 1 and 2.
Dry Eye Tests
Tables 5 and 6 shows the number and percentage of patients with normal and abnormal dry eye tests according to the dry eye tests specific diagnostic criteria (Table 1) before and after surgery for group 1, 2 and 3. No statistical significance differences were observed between groups 1 and 2 in patients having abnormal osmolarity at baseline and 6 weeks after surgery. The number of patients with abnormal osmolarity at 6 weeks decreased in group 1 and 2. The decrease was not statistically significant in group 1 (from 78.9% to 65.7%, p=0.064), however, it was statistically significant in group 2 (from 86.3% to 62.1%, p <0.001). In contrast, in group 3, the number of patients with abnormal osmolarity increased significantly (36.3%, p<0.001) postoperatively.
 |
Table 5 The Number and Percentage of Patients with Normal and Abnormal Signs of Dry Eye (SDE) at Baseline |
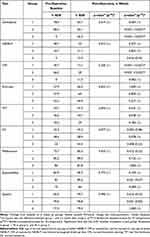 |
Table 6 The Percentages of Patients with Abnormal Dry Eye Sign (SDE) Before and 6 Weeks After Surgery |
No statistical significance difference was observed between group 1 and 2, in patients having abnormal NIKBUT at baseline or 6 weeks after surgery. After surgery, there was a significant increase in the number of patients with an unstable tear film (NIKBUT) in group 3.
Pre-operatively, the number of patients having abnormal CFS was statistically significantly lower in group 1 compared to group 2 (p=0.001). Postoperatively, the number of patients in group 1 and 2 having abnormal CFS decreased statistically significantly compared to baseline (p<0.001) in both groups, however, no statistically significant difference was observed between the two groups (p=0.236). The proportion of patients who had abnormal CFS after surgery in group 3 increased, though the increase was not statistically significant (p=0.63).
Analysis of the Schirmer test results showed no statistically significant difference between groups 1 and 2 at baseline or after surgery (p=1.00 and 0.652 respectively). Similarly, the proportion of patients with abnormal Schirmer values after surgery compared to baseline was the same for group 1 (p=1.00) and group 2 (p=0.850). In group 3 fewer patients had abnormal tear production 6 weeks after surgery compared to baseline, but the decrease was not statistically significant (p=0.227). However, when comparing groups 1 and 2 (pooled together) with group 3, there was a statistically significant difference (p=0.037).
The number of patients having an abnormal tear film thickness decreased in all groups after surgery, the decrease was statistically significant in group 2 (p=0.038). However, no significant inter-group difference between groups 1 and 2 was observed 6 weeks after surgery (p=0.094).
Pre-operatively, no differences were observed between group 1 and 2 in the number of patients having abnormal CS. An increase in the number of patients with reduced CS was observed at 6 weeks compared to baseline in groups 1, 2 and 3, but the increase was not statistically significant.
Looking at the meibomian gland dysfunction (MGD) parameters, the difference in the number of patients having an abnormal meiboscore between groups 1 and 2 at baseline was not statistically significant (padj=0.531). The number of patients with an abnormal meiboscore after surgery increased in both groups 1 and 2, but the change was not statistically significant. Preoperative and postoperative meibomian expressibility was unchanged in groups 1, 2 and 3. For all MGD parameters, there were no statistically significant differences between groups 1 and 2 postoperatively.
Discussion
Overall findings of this study showed that patients who initially had clinical signs of dry eyes improved after cataract surgery when treated with topical steroids and NSAIDs. The use of preservative-free drops and artificial tears did not appear to provide any additional benefit. Conversely, patients who initially had a normal tear film worsened after cataract surgery when treated with preservative-containing topical steroids and NSAIDs.
We found a lower percentage of postoperative patients with severe OSDI scores compared to preoperative, including those with a normal tear film. These results suggest that patients with severe symptoms of dry eyes may improve after cataract surgery, regardless of whether their drops contained preservatives or not. This may be a function of treatment with steroids and NSAIDs. The combination of topical NSAIDs and topical steroids with preservatives is used routinely after cataract surgery to prevent postoperative inflammation and CME,10 even though there is not an established consensus regarding post-operative treatment.41 The use of NSAIDs in treating dry eyes is controversial.42–44 However, low-dose steroid treatment is often recommended for those with severe dry eye, to manage inflammation.45,46 Avunduk et al concluded that topical corticosteroids, but not topical NSAIDs, had a beneficial effect on both symptoms and signs of moderate to severe dry eye.47
When subjective symptoms were measured with the SPEED questionnaire, dry eye patients who received preservative-free drops and artificial tears improved after surgery compared to those who received preservative-containing drops. The non-dry group at baseline had an unchanged symptom score after surgery when SPEED was used. This suggests that there could be a benefit to dry eye symptoms with preservative-free drops and lubricants. OSDI includes questions regarding visual function that may be affected by the presence of a cataract, while SPEED does not address any vision complaints. This may explain the differences between SPEED and OSDI results in our study and may indicate that the SPEED questionnaire may be a more accurate measure of DED symptoms in patients scheduled for cataract surgery.
Patients with a normal tear film did not receive treatment with tear film substitutes in our study. A statistically significant increase in the number of patients diagnosed with SDE after surgery was found in the group with a normal tear film at baseline. In a study involving patients undergoing cataract surgery, Cagini et al enrolled individuals with a healthy ocular surface. They discovered that a tear film substitute containing trehalose 3% and hyaluronic acid 15% effectively reduced inflammation and alleviated dry eye symptoms.25 Several studies support the importance of using tear film substitutes in the treatment regimen before and after cataract surgery, and suggest that preservative-free formulations are superior to those with preservatives.48–50
Before cataract surgery, non-dry eye patients might not have exhibited significant dry eye symptoms or signs because their tear film and ocular surface were relatively healthy. However, after cataract surgery, various factors associated with the surgical procedure could have triggered or exacerbated dry eye symptoms and signs in these patients, such as pre and perioperative anesthetics, antibiotics and disinfection medications, corneal incisions, light exposure, and postoperative medications.51–53 This could result in symptoms and signs that are more severe compared to patients who already had some degree of dry eye before the surgery.
With the dry eye tests performed in this study, we found no statistically significant differences between patients treated with preservative-free eyedrops versus preserved. However, our results show that patients diagnosed with preoperative SDE improved in both symptoms and objective signs, regardless of treatment, and that patients with preoperative non-dry eye worsened. For patients with pre-existing dry eyes, treatment with corticosteroids can provide rapid and effective relief of both signs and symptoms.54 Along with the fact that dry eyes after cataract surgery can be a transient condition with gradual improvement 4–6 weeks after surgery, can explain our findings.55,56 Patients who did not have SDE preoperatively (group 3), had higher osmolarity and an aggravation of CFS after surgery. This is in line with other studies that concluded that cataract surgery causes the onset or worsening of dry eyes for patients without preexisting dry eyes.50,57,58
Interestingly, all meibomian gland measurements remained unchanged after surgery regardless of preoperative dry eye status and use of preservatives. A recent study conducted by Malmin et al finds that repeated intravitreal injections (IVs) with anti-vascular endothelial growth factor (anti-VEGF) with preoperative povidone-iodine (PVP-I) application was associated with reduced meibomian gland (MG) loss, increased tear volume and reduced signs of inflammation.59 A suggested cause was the antibacterial properties of PVP-I, which is also used before cataract surgery. This is an area for potential future research.
Previous studies of signs and symptoms of dry eye patients after cataract surgery suggest a short-time aggravation of dry eyes, with a peak around 1 week, and a gradual improvement, returning to preoperative values 1–3 months after cataract surgery.57,60,61 On the other hand, some authors propose that preservative toxicity depends on daily dosing, the duration of the treatment and the concentration of preservatives in the administered solution. This is documented in numerous studies where a majority are glaucoma patients, who have long and chronic exposure to BAC.20,51,62,63 We did use eye-drop formulations with BAC concentrations of 0.01 and 0.005%, with a total exposure time of 3 weeks after surgery for patients in group 1 and 3. In addition, the postoperative examinations were performed 3 weeks after discontinuation of eye drop treatment, which might suggest that the tear function returned to preoperative levels. This may explain our findings of little difference between group 1 and 2. Several studies suggest a correlation between preservatives and dry eyes after cataract surgery. Jun et al showed that preservative-free 3% diquafosol, had better efficacy in treating dry eyes than a similar solution with preservatives, with a total exposure time of 12 weeks after cataract surgery.64 In addition Jee et al compared preservative-free sodium hyaluronate 0.1% eyedrops and preservative-free fluorometholone 0.1% eye drops to eye drops containing preservatives with a total exposure time of 8 weeks after surgery, showing that patients receiving preservative-free eyedrops improved in symptoms and signs of DED.28 Both studies had longer exposure time to preservatives and longer observation time than our study, potentially explaining the different results.
There are limitations to this study. First, we had only one postoperative study visit, at 6 weeks. Observation at several additional time periods might have been helpful. Second, the introduction of an eye drop containing hyaluronic acid and trehalose in the preservative-free group, might have masked the effect of using preservative-free corticosteroids and NSAIDs. Third, we did not note the total duration of surgery and the cumulative dissipated energy (CDE) for each procedure. Increased duration of surgery, prolonged microscopic light exposure and CDE energy used are some of the risk factors for developing postoperative dry eyes.57
Conclusion
After cataract surgery, patients with mild to moderate dry eyes may experience improved tear film status and reduced symptoms. However, we found no additional beneficial effect on dry eye parameters with treatment using preservative-free dexamethasone, NSAIDs, and trehalose/hyaluronic acid compared to dexamethasone and NSAID with preservatives.
Data Sharing Statement
The original data and anonymous patient files are stored on a secured server (Tjenester for Sensitive Data, TSD) at the University of Oslo for 10 years. Supplemental information can be available from the Corresponding Author upon reasonable request.
Research Ethics and Consent
The study followed the tenets of the Declaration of Helsinki, adhered to good clinical practice, and was approved by the Regional Committee for Medical and Health Research Ethics in Norway (Ref. 2020/64847). The project protocol was registered in Clinicaltrials.gov: NCT 05433428 (first submitted 10.08.2023, first posted 27.06.2022). Written informed consent was obtained from all participants at their first visit.
Funding
The study was supported by The Research Council of Norway (project nr. 306649).
Disclosure
Richard (Rick) Potvin reports consulting fees from Alcon and Hoya. Dr Tor Utheim reports being one of around 20 owners of the Norwegian Dry Eye Clinic, during the conduct of the study. The authors report no other conflicts of interest in this work.
References
1. Rossi T, Romano MR, Iannetta D, et al. Cataract surgery practice patterns worldwide: a survey. BMJ Open Ophthalmol. 2021;6(1):e000464. doi:10.1136/bmjophth-2020-000464
2. Li XM, Hu L, Hu J, Wang W. Investigation of dry eye disease and analysis of the pathogenic factors in patients after cataract surgery. Cornea. 2007;26(9 Suppl 1):S16–S20. doi:10.1097/ICO.0b013e31812f67ca
3. Naderi K, Gormley J, O’Brart D. Cataract surgery and dry eye disease: a review. Eur J Ophthalmol. 2020;30(5):840–855. doi:10.1177/1120672120929958
4. Craig JP, Nichols KK, Akpek EK, et al. TFOS DEWS II definition and classification report. Ocul Surf. 2017;15(3):276–283. doi:10.1016/j.jtos.2017.05.008
5. Khanal S, Tomlinson A, Esakowitz L, et al. Changes in corneal sensitivity and tear physiology after phacoemulsification. Ophthalmic Physiol Opt. 2008;28(2):127–134. doi:10.1111/j.1475-1313.2008.00539.x
6. Zhao X, Xia S, Chen Y. Comparison of the efficacy between topical diquafosol and artificial tears in the treatment of dry eye following cataract surgery: a meta-analysis of randomized controlled trials. Medicine. 2017;96(39):e8174. doi:10.1097/md.0000000000008174
7. Kasetsuwan N, Satitpitakul V, Changul T, Jariyakosol S, Wedrich A. Incidence and pattern of dry eye after cataract surgery. PLoS One. 2013;8(11):e78657. doi:10.1371/journal.pone.0078657
8. Lu Q, Lu Y, Zhu X. Dry eye and phacoemulsification cataract surgery: a systematic review and meta-analysis. Front Med. 2021;8:649030. doi:10.3389/fmed.2021.649030
9. Wingert AM, Liu SH, Lin JC, Sridhar J. Non-steroidal anti-inflammatory agents for treating cystoid macular edema following cataract surgery. Cochrane Database Syst Rev. 2022;12(12):Cd004239. doi:10.1002/14651858.CD004239.pub4
10. Kato K, Miyake K, Hirano K, Kondo M. Management of postoperative inflammation and dry eye after cataract surgery. Cornea. 2019;38(Suppl 1):S25–S33. doi:10.1097/ico.0000000000002125
11. Goldstein MH, Silva FQ, Blender N, Tran T, Vantipalli S. Ocular benzalkonium chloride exposure: problems and solutions. Eye. 2022;36(2):361–368. doi:10.1038/s41433-021-01668-x
12. Zaleska-żmijewska A, Strzemecka E, Wawrzyniak ZM, Szaflik JP. Extracellular MMP-9-based assessment of ocular surface inflammation in patients with primary open-angle glaucoma. J Ophthalmol. 2019;2019:1240537. doi:10.1155/2019/1240537
13. Baudouin C, Labbé A, Liang H, Pauly A, Brignole-Baudouin F. Preservatives in eyedrops: the good, the bad and the ugly. Prog Retin Eye Res. 2010;29(4):312–334. doi:10.1016/j.preteyeres.2010.03.001
14. Actis AG, Rolle T. Ocular surface alterations and topical antiglaucomatous therapy: a review. Open Ophthalmol J. 2014;8(1):67–72. doi:10.2174/1874364101408010067
15. Walsh K, Jones L. The use of preservatives in dry eye drops. Clin Ophthalmol. 2019;13:1409–1425. doi:10.2147/opth.S211611
16. Maca SM, Amon M, Findl O, Kahraman G, Barisani-Asenbauer T. Efficacy and tolerability of preservative-free and preserved diclofenac and preserved ketorolac eyedrops after cataract surgery. Am J Ophthalmol. 2010;149(5):777–784. doi:10.1016/j.ajo.2009.12.010
17. Ishibashi T, Yokoi N, Kinoshita S. Comparison of the short-term effects on the human corneal surface of topical timolol maleate with and without benzalkonium chloride. J Glaucoma. 2003;12(6):486–490. doi:10.1097/00061198-200312000-00008
18. Porela-Tiihonen S, Kokki H, Kaarniranta K, Kokki M. Recovery after cataract surgery. Acta Ophthalmol. 2016;94(Suppl 2):1–34. doi:10.1111/aos.13055
19. Baudouin C, Hamard P, Liang H, Creuzot-Garcher C, Bensoussan L, Brignole F. Conjunctival epithelial cell expression of interleukins and inflammatory markers in glaucoma patients treated over the long term. Ophthalmology. 2004;111(12):2186–2192. doi:10.1016/j.ophtha.2004.06.023
20. Rossi GC, Pasinetti GM, Scudeller L, Raimondi M, Lanteri S, Bianchi PE. Risk factors to develop ocular surface disease in treated glaucoma or ocular hypertension patients. Eur J Ophthalmol. 2013;23(3):296–302. doi:10.5301/ejo.5000220
21. Ha JY, Sung MS, Park SW. Effects of preservative on the meibomian gland in glaucoma patients treated with prostaglandin analogues. Chonnam Med J. 2019;55(3):156–162. doi:10.4068/cmj.2019.55.3.156
22. Jones L, Downie LE, Korb D, et al. TFOS DEWS II management and therapy report. Ocul Surf. 2017;15(3):575–628. doi:10.1016/j.jtos.2017.05.006
23. Assil KK, Greenwood MD, Gibson A, Vantipalli S, Metzinger JL, Goldstein MH. Dropless cataract surgery: modernizing perioperative medical therapy to improve outcomes and patient satisfaction. Curr Opinion Ophthalmol. 2021;32(Suppl 1):S1–S12. doi:10.1097/icu.0000000000000708
24. Rosin LM, Bell NP. Preservative toxicity in glaucoma medication: clinical evaluation of benzalkonium chloride-free 0.5% timolol eye drops. Clin Ophthalmol. 2013;7:2131–2135. doi:10.2147/opth.S41358
25. Cagini C, Di Lascio G, Torroni G, et al. Dry eye and inflammation of the ocular surface after cataract surgery: effectiveness of a tear film substitute based on trehalose/hyaluronic acid vs hyaluronic acid to resolve signs and symptoms. J Cataract Refract Surg. 2021;47(11):1430–1435. doi:10.1097/j.jcrs.0000000000000652
26. Labiris G, Ntonti P, Sideroudi H, Kozobolis V. Impact of polyethylene glycol 400/propylene glycol/hydroxypropyl-guar and 0.1% sodium hyaluronate on postoperative discomfort following cataract extraction surgery: a comparative study. Eye Vis. 2017;4(1):13. doi:10.1186/s40662-017-0079-5
27. Mencucci R, Boccalini C, Caputo R, Favuzza E. Effect of a hyaluronic acid and carboxymethylcellulose ophthalmic solution on ocular comfort and tear-film instability after cataract surgery. J Cataract Refract Surg. 2015;41(8):1699–1704. doi:10.1016/j.jcrs.2014.12.056
28. Jee D, Park M, Lee HJ, Kim MS, Kim EC. Comparison of treatment with preservative-free versus preserved sodium hyaluronate 0.1% and fluorometholone 0.1% eyedrops after cataract surgery in patients with preexisting dry-eye syndrome. J Cataract Refract Surg. 2015;41(4):756–763. doi:10.1016/j.jcrs.2014.11.034
29. Graae Jensen P, Gundersen M, Nilsen C, et al. Prevalence of dry eye disease among individuals scheduled for cataract surgery in a Norwegian Cataract Clinic. Clin Ophthalmol. 2023;17:1233–1243. doi:10.2147/opth.S407805
30. Nilsen C, Graae Jensen P, Gundersen M, et al. The significance of inter-eye osmolarity difference in dry eye diagnostics. Clin Ophthalmol. 2023;17:829–835. doi:10.2147/opth.S402556
31. de Monchy I, Gendron G, Miceli C, Pogorzalek N, Mariette X, Labetoulle M. Combination of the Schirmer I and phenol red thread tests as a rescue strategy for diagnosis of ocular dryness associated with Sjögren’s syndrome. Invest Ophthalmol Vis Sci. 2011;52(8):5167–5173. doi:10.1167/iovs.10-6671
32. Osmolarity system - user manual. San Diego, CA, USA: TearLab Corporation; 2017.
33. Craig JP, Nelson JD, Azar DT, et al. TFOS DEWS II Report Executive Summary. Ocul Surf. 2017;15(4):802–812. doi:10.1016/j.jtos.2017.08.003
34. Asiedu K, Kyei S, Mensah SN, Ocansey S, Abu LS, Kyere EA. Ocular Surface Disease Index (OSDI) versus the Standard Patient Evaluation of Eye Dryness (SPEED): a study of a nonclinical sample. Cornea. 2016;35(2):175–180. doi:10.1097/ico.0000000000000712
35. Tian L, Qu J, Zhang X and Sun X. Repeatability and reproducibility of noninvasive keratograph 5M measurements in patients with dry eye disease. J Ophthalmol. 2016;2016:6. doi:10.1155/2016/8013621
36. Herbaut A, Liang H, Denoyer A, Baudouin C, Labbé A. Tear film analysis and evaluation of optical quality: a review of the literature. J Fr Ophtalmol. 2019;42(2):e21–e35. doi:10.1016/j.jfo.2018.12.001
37. Finis D, Pischel N, Schrader S, Geerling G. Evaluation of lipid layer thickness measurement of the tear film as a diagnostic tool for Meibomian gland dysfunction. Cornea. 2013;32(12):1549–1553. doi:10.1097/ICO.0b013e3182a7f3e1
38. Bron AJ, Evans VE, Smith JA. Grading of corneal and conjunctival staining in the context of other dry eye tests. Cornea. 2003;22(7):640–650. doi:10.1097/00003226-200310000-00008
39. Chao C, Stapleton F, Badarudin E, Golebiowski B. Ocular surface sensitivity repeatability with Cochet-Bonnet esthesiometer. Optom Vis Sci. 2015;92(2):183–189. doi:10.1097/opx.0000000000000472
40. Stonecipher K, Abell TG, Chotiner B, Chotiner E, Potvin R. Combined low level light therapy and intense pulsed light therapy for the treatment of meibomian gland dysfunction. Clin Ophthalmol. 2019;13:993–999. doi:10.2147/opth.S213664
41. Juthani VV, Clearfield E, Chuck RS. Non-steroidal anti-inflammatory drugs versus corticosteroids for controlling inflammation after uncomplicated cataract surgery. Cochrane Database Syst Rev. 2017;7(7):Cd010516. doi:10.1002/14651858.CD010516.pub2
42. Singer DD, Kennedy J, Wittpenn JR. Topical NSAIDs effect on corneal sensitivity. Cornea. 2015;34(5):541–543. doi:10.1097/ico.0000000000000309
43. Isawi H, Dhaliwal DK. Corneal melting and perforation in Stevens Johnson syndrome following topical bromfenac use. J Cataract Refract Surg. 2007;33(9):1644–1646. doi:10.1016/j.jcrs.2007.04.041
44. Rolando M, Barabino S, Alongi S, Calabria G. Topical non-preserved diclofenac therapy for keratoconjunctivitis sicca. Adv Exp Med Biol. 2002;506(Pt B):1237–1240. doi:10.1007/978-1-4615-0717-8_177
45. Pinto-Fraga J, López-Miguel A, González-García MJ, et al. Topical fluorometholone protects the ocular surface of dry eye patients from desiccating stress: a randomized controlled clinical trial. Ophthalmology. 2016;123(1):141–153. doi:10.1016/j.ophtha.2015.09.029
46. Lin T, Gong L. Topical fluorometholone treatment for ocular dryness in patients with Sjögren syndrome: a randomized clinical trial in China. Medicine. 2015;94(7):e551. doi:10.1097/md.0000000000000551
47. Avunduk AM, Avunduk MC, Varnell ED, Kaufman HE. The comparison of efficacies of topical corticosteroids and nonsteroidal anti-inflammatory drops on dry eye patients: a clinical and immunocytochemical study. Am J Ophthalmol. 2003;136(4):593–602. doi:10.1016/s0002-9394(03)00326-x
48. Donthineni PR, Shanbhag SS, Basu S. An evidence-based strategic approach to prevention and treatment of dry eye disease, a modern global epidemic. Healthcare. 2021;9(1):89. doi:10.3390/healthcare9010089
49. Sánchez MA, Arriola-Villalobos P, Torralbo-Jiménez P, et al. The effect of preservative-free HP-Guar on dry eye after phacoemulsification: a flow cytometric study. Eye. 2010;24(8):1331–1337. doi:10.1038/eye.2010.24
50. Rossi GCM, Tinelli C, Milano G, et al. Randomised, single blind, controlled, three-month clinical trial on the evaluation and treatment of the ocular surface damage following phacoemulsification. Vision. 2022;6(3):42. doi:10.3390/vision6030042
51. Fraunfelder FT, Sciubba JJ, Mathers WD. The role of medications in causing dry eye. J Ophthalmol. 2012;2012:285851. doi:10.1155/2012/285851
52. Sarkar J, Chaudhary S, Namavari A, et al. Corneal neurotoxicity due to topical benzalkonium chloride. Invest Ophthalmol Vis Sci. 2012;53(4):1792–1802. doi:10.1167/iovs.11-8775
53. Hwang HB, Kim HS. Phototoxic effects of an operating microscope on the ocular surface and tear film. Cornea. 2014;33(1):82–90. doi:10.1097/ico.0000000000000001
54. Yang CQ, Sun W, Gu YS. A clinical study of the efficacy of topical corticosteroids on dry eye. J Zhejiang Univ Sci B. 2006;7(8):675–678. doi:10.1631/jzus.2006.B0675
55. Garg P, Gupta A, Tandon N, Raj P. Dry eye disease after cataract surgery: study of its determinants and risk factors. Turk J Ophthalmol. 2020;50(3):133–142. doi:10.4274/tjo.galenos.2019.45538
56. Prinz J, Maffulli N, Fuest M, Walter P, Bell A, Migliorini F. Efficacy of topical administration of corticosteroids for the management of dry eye disease: systematic review and meta-analysis. Life. 2022;12(11):1932. doi:10.3390/life12111932
57. Kohli P, Arya SK, Raj A, Handa U. Changes in ocular surface status after phacoemulsification in patients with senile cataract. Int Ophthalmol. 2019;39(6):1345–1353. doi:10.1007/s10792-018-0953-8
58. Sahu PK, Das GK, Malik A, Biakthangi L. Dry eye following phacoemulsification surgery and its relation to associated intraoperative risk factors. Middle East Afr J Ophthalmol. 2015;22(4):472–477. doi:10.4103/0974-9233.151871
59. Malmin A, Thomseth VM, Førland PT, et al. Associations between serial intravitreal injections and dry eye. Ophthalmology. 2023;130(5):509–515. doi:10.1016/j.ophtha.2023.01.009
60. Cetinkaya S, Mestan E, Acir NO, Cetinkaya YF, Dadaci Z, Yener HI. The course of dry eye after phacoemulsification surgery. BMC Ophthalmol. 2015;15(1):68. doi:10.1186/s12886-015-0058-3
61. Cung LX, Nga NTT, Nga DM, Hiep NX, Pham DT. Kataraktchirurgie destabilisiert vorübergehend die Benetzung der Augenoberflä che [Cataract surgery destabilises temporary the tear film of the ocular surface]. Klin Monbl Augenheilkd. 2021;238(3):282–287. German. doi:10.1055/a-1179-0373.
62. Kim JH, Kim EJ, Kim YH, et al. In vivo effects of preservative-free and preserved prostaglandin analogs: mouse ocular surface study. Korean j Ophthalmol. 2015;29(4):270–279. doi:10.3341/kjo.2015.29.4.270
63. Ghosh S, O’Hare F, Lamoureux E, Vajpayee RB, Crowston JG. Prevalence of signs and symptoms of ocular surface disease in individuals treated and not treated with glaucoma medication. Clin Exp Ophthalmol. 2012;40(7):675–681. doi:10.1111/j.1442-9071.2012.02781.x
64. Jun I, Choi S, Lee GY, et al. Effects of preservative-free 3% diquafosol in patients with pre-existing dry eye disease after cataract surgery: a randomized clinical trial. Sci Rep. 2019;9(1):12659. doi:10.1038/s41598-019-49159-0
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.



