Back to Journals » International Journal of General Medicine » Volume 16
A Preliminary Study on Sympathetic Skin Response in Acute Ischemic Cerebrovascular Disease
Authors Chen W, Chen Y, Ye W, Wang T
Received 28 February 2023
Accepted for publication 19 April 2023
Published 28 April 2023 Volume 2023:16 Pages 1581—1587
DOI https://doi.org/10.2147/IJGM.S405495
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Wenhong Chen,1 Yunping Chen,2 Wenjuan Ye,3 Ting Wang1
1Department of Pediatric Neurology, Maternal and Child Health Hospital of Hubei Province, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, People’s Republic of China; 2Department of Neurology, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, People’s Republic of China; 3Department of Pediatric, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, People’s Republic of China
Correspondence: Ting Wang, Department of Pediatric Neurology, Maternal and Child Health Hospital of Hubei Province, Tongji Medical College, Huazhong University of Science and Technology, 745 Wuluo Road, Hongshan District, Wuhan, Hubei, People’s Republic of China, Tel +86-27-87169308, Email [email protected]
Purpose: The study aims to identify the characteristics of SSR in patients with AICVD and their correlation with clinical presentations.
Methods: SSR of the upper limbs, the National Institute of Health stroke scale (NIHSS), the Barthel index (BI), the Essen stroke risk score (ESRS), and imaging examinations, was evaluated in 30 healthy subjects and 66 patients with AICVD. All results were recorded and analyzed via Statistical Package for the Social Sciences (SPSS 22.0) software. t-test and Spearman rank correlation were used.
Results: Compared to the control group, SSR of upper limbs in patients with AICVD showed prolonged latency, reduced amplitude, and disappeared waveform (p=0.000, p=0.015, p=0.004), No statistically significant difference was observed between the affected side and the healthy side (p=0.068, p=0.661). In the case group, the higher the abnormal rate of SSR, the more severe the neurological impairment (NIHSS and ADL scores) and the worse the long-term prognosis. Specific results are as follows: Firstly, the total abnormality rate of SSR, prolonged SSR latency were positively related to the NIHSS, also the ESRS (r=0.347, p=0.004; r=0.437, p< 0.001), (r=0.371, p=0.005; r=0.433, p=0.001), the reduced amplitude was positively related to the NIHSS (r=0.341, p=0.012) while the disappeared waveform was positively related to the ESRS (r=0.299, p=0.015); Secondly, the total abnormality rate of SSR, prolonged SSR latency and reduced amplitude were negatively related to the BI (r=− 0.346, p=0.004) (r=− 0.426, p=0.001) (r=− 0.316, p=0.020).
Conclusion: There may be inhibition of sympathetic reflex activity in patients with AICVD, SSR abnormality rate in patients with AICVD may be correlated with the degree of neurological impairment and long-term prognosis.
Keywords: acute ischemic cerebrovascular disease, AICVD, sympathetic skin response, SSR, autonomic nervous system, ANS
Acute ischemic cerebrovascular disease (AICVD) is one of the most common cerebrovascular diseases with relatively high morbidity and disability rates. Although the development of modern medical imaging has made a great breakthrough in the diagnosis and treatment of AICVD, it remains difficult to evaluate the cerebral function and prognosis of patients in a comprehensive and objective manner. Neuroelectrophysiological technologies, such as sympathetic skin Response (SSR), are expected to fill this gap.
SSR is a multisynaptic sudomotor reaction involving the participation of brain and spinal cord. It is a transient potential response recorded on the skin surface by exciting the sympathetic nerves to change the permeability of sweat gland cell membrane to the K+ in the body. SSR is an electrophysiological method to objectively measure sympathetic sudomotor pathway reflex activities, and is currently considered as a method that can comprehensively reflect the functional status of the entire sympathetic sudomotor pathways (both peripheral and central parts).1–3 The clinical parameters of SSR test are mainly latency, amplitude, and waveform. An SSR waveform usually starts with a positive phase component, followed by a negative phase or a negative-positive phase.4 The first waveform is usually taken as the observation index. The amplitude is mostly considered to represent the number of neurons activated by the stimulation or the density of sweat glands with local secretory activities.5 It is highly variable and adaptive, so the change in amplitude alone has little significance. Latency refers to the time course of the whole conductive pathways from stimulation to sweating of sweat glands, including afferent, central, and efferent parts. Latency is mainly correlated to the form of stimulation5,6 and is relatively stable under the same stimulation, mainly reflecting the abnormalities in efferent and central parts.7 We all know that the autonomic nervous system (ANS), mainly the sympathetic and parasympathetic nervous systems, regulates the functions of smooth muscles, myocardia, and glands under the action of central nervous system, and widely participates in the physiological activities of multiple tissues and organs such as circulatory, respiratory, urinary, and reproductive activities, as well as in the systemic metabolism. Numerous studies over the past several years have shown that many diseases are accompanied by ANS dysfunction. Among them, the sympathetic nervous system (SNS) dysfunction is involved in a series of pathophysiological processes such as vascular remodeling, endothelial dysfunction, arteriosclerosis, and insulin resistance, and is one of the factors for the onset and progression of acute cardio-cerebrovascular events,8 which is the largest contributor to premature death in the world.9 ANS function tests include invasive (direct) examination and non-invasive (indirect) examination.10,11 The invasive examination was mainly based on radiotracer techniques or microelectrode recording of superficial nerves.11 Because of its invasiveness and limited clinical application, it was gradually replaced by non-invasive examination. By contrast, there are many non-invasive examination methods, and one of the commonly used methods is to test the sympathetic nerve potential, such as potential test of muscle sympathetic nerves,12 and potential test of kidney sympathetic nerves,13 and SSR. Under normal physiological conditions, the sympathetic and parasympathetic nervous systems play their roles in stabilizing the milieu interne under the regulation of the cortex, thalamus, and brain stem14 pathways. Under stress conditions, the regulation of the ANS is imbalanced,15 resulting in multiple organ dysfunction syndrome and even sudden death.
We measured SSR in patients with AICVD in this study, to identify the characteristics of SSR in patients with AICVD and its correlation with clinical presentation of patients, as well as to assess the value of SSR in evaluating the condition and long-term prognosis in patients with AICVD.
Materials and Methods
Clinical Data
We retrospectively analyzed the patients admitted to the Department of Neurology of Wuhan Union Hospital and Maternal and Child Health Hospital of Hubei Province from March 2015 to December 2015, among which 66 patients with AICVD and 30 patients without AICVD were selected.
AICVD group: 66 patients with AICVD, including 46 cases of acute ischemic stroke (AIS) with a male-to-female ratio of approximately 2.00 and an average age of 60.70 years old, and 20 cases of transient ischemic attack (TIA) with a male-to-female ratio of approximately 1.80 and an average age of 58.65 years old. All diagnoses were confirmed by Magnetic Resonance Imaging (MRI) and Computed Tomography (CT) of the brain, which met the diagnostic criteria for various cerebrovascular diseases first established in 1996. Patients excluded from this study included those who had diseases (thyroid dysfunction, Parkinson’s disease, diabetes and peripheral neuropathy, etc.) that clearly affected the function of autonomic nervous system (ANS), those who took drugs that affect ANS activities, such as epinephrine, before the test, and those who did not cooperate with the tests.
Control group: 30 non-AICVD patients visited in the same time period with a male-to-female ratio of 2.20 and an average age of 58.23 years old. All patients voluntarily participated in the study and had no specific history of AICVD, no specific symptoms of autonomic nervous dysfunction, and no other serious diseases or stress events. All other exclusion criteria were the same as those for the case group.
SSR Test and Scale Evaluation
The Keypoint 4 EMG/EP apparatus manufactured by Medtronic, Inc. was used. Electrical stimulation was applied and both hands were recorded. The experiments were conducted in a quiet and bright environment: the room temperature was maintained at 20 to 25°C, and the skin temperature was maintained at ≥32°C. The subjects were sufficiently relaxed and were instructed to rest comfortably in a supine or sitting position. The intensity of current stimulation was controlled within the range from 10 mA to 30 mA, and the duration of each stimulus was 0.1 ms to 0.2 ms. To avoid habituation, stimuli were administered at irregular intervals of more than 60 seconds. The duration of the whole experiment was approximately 15 min. All subjects selected have their SSR test performed by the designated neuroelectrophysiological technicians within 72 h of admission; the National Institute of Health stroke scale (NIHSS), the Barthel index (BI), and the Essen stroke risk score (ESRS) were recorded and analyzed by the designated neurologists within 24 h of admission.
Evaluation Criteria and Statistical Processing
The statistical analysis of this study was performed using SPSS 22.0, with the level of significance set at 0.05. The analysis of variance was used for inter-group comparisons of quantitative data, and the Chi-Square (Χ2) test was used for inter-group comparisons of qualitative data. The t-test was used for comparisons of two groups of independent sample data. The Spearman’s rank correlation was used to analyze the relationship between indexes.
The statistical results of the control group were taken as the reference standard. Criteria for SSR abnormality: (1) the onset latency was 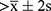 ; (2) the amplitude was below the lowest value of the control group; (3) no complete SSR waveform was obtained.
; (2) the amplitude was below the lowest value of the control group; (3) no complete SSR waveform was obtained.
Results
SSR Test Results of Control Group
No statistically significant difference was observed in the SSR latency and amplitude of the upper limbs of the 30 patients in the control group, and the latency was found to be more stable than the amplitude (see Table 1). For upper limbs, the latency was 1.37±0.07 s and the minimum amplitude was 0.25mV.
 |
Table 1 SSR Test Results of Upper Limbs in Control Group |
SSR Test Results of AICVD Group and Comparison with the Control Group
There were 20 cases (11 males and 9 females) in the TIA group with an average age of 58.65 years. Among them, 14 cases (70%) were found with SSR abnormalities: 9 cases (45%) with prolonged latency, and 5 cases (25%) with reduced amplitude; and 3 cases (15%) were found without complete waveform (or with disappeared waveform). No statistically significant difference was observed in the latency and amplitude of TIA patients’ upper limbs. No significant difference was found in regard to the gender and age compositions between the TIA group and the control group. The latency of both the affected and the healthy upper limbs were longer than those of the control group, and the amplitude of both the affected and the healthy upper limbs were lower than those of the control group (see Table 2).
 |
Table 2 Comparison of SSR Test Results of Upper Limbs in AICVD Group |
There were 46 cases (32 males and 14 females) in the AIS group with an average age of 60.70 years old. Among them, 32 patients (69.57%) were found with SSR abnormalities: 24 patients (52.17%) with prolonged latency, and 16 cases (34.78%) with reduced amplitude; and 11 cases (23.91%, including 2 cases with bilateral lesions) were found with waveform disappeared. No statistically significant difference was observed between the affected and the healthy limbs in the latency and amplitude. No significant difference was found in regard to the gender and age compositions between the AIS group and the control group. The latency of both the affected and the healthy limbs were longer than those of the control group, and the amplitude of both the affected and the healthy limbs were lower than those of the control group. No statistically significant difference was observed in gender and age compositions, or in bilateral SSR latency and amplitude between the AIS group and the TIA group (See Table 2).
SSR Test Results and Clinical Correlation Analysis
SSR Test Results and Scale Correlation Analysis
A correlation analysis was performed on the NIHSS, BI, and ESRS of patients in the groups with normal and abnormal results of SSR test (see Table 3). The analysis results showed that among patients with AICVD, the higher the abnormality rate of SSR was, the higher the NIHSS and ESRS scores were, and the lower the BI score was. Specifically, the abnormality rate of SSR was positively correlated with the degree of neurologic impairment and the risk of stroke recurrence (r=0.347, p=0.004; r=0.437, p=0.000), and was negatively correlated with the activities of daily living (ADL) (r=−0.346, p=0.004).
 |
Table 3 Analysis of Correlation Between SSR Results and NIHSS, BI and ESRS Scores |
SSR Abnormal Parameters and Clinical Correlation Analysis
The results of correlation analysis between SSR abnormal parameters and NIHSS, BI, or ESRS scores (see Table 4) showed that: Firstly, Compared with the normal group, patients in the group with prolonged SSR latency had higher NIHSS and ESRS scores and a lower BI score. The comparison between the two groups was statistically significant (p<0.05). The correlation parameters were r=0.371, p=0.005; r=−0.426, p=0.001; r=0.433, p=0.001, respectively; Secondly, Compared with the normal group, patients in the group with reduced SSR amplitude had a higher NIHSS score and a lower BI score, and there was a positive correlation (r=0.341, p=0.012) and a negative correlation (r=−0.316, p=0.020) between the two groups, respectively; Lastly, Compared with the group with waveform, patients in the group with disappeared waveform had a higher ESRS score, and there was a positive correlation between the two groups (r=0.299, p=0.015).
 |
Table 4 Analysis of Correlation Between SSR Abnormal Parameters and NIHSS, BI and ESRS Scores |
Discussion
Cerebrovascular diseases are common in neurology and are global public health issues.16 According to the latest statistics, cerebrovascular diseases have ranked first in the death cause spectrum of urban and rural residents in China17 and second around the world.18 In recent years, the widely used neuroelectrophysiological techniques, such as SSR, Somatosensory evoked potential (SEP), and Motor evoked potential (MEP), can sensitively display brain dysfunctions as well as small or subclinical lesions that cannot be found by imaging examinations. They have a great significance in the comprehensive evaluation of the condition and prognosis of patients with cerebrovascular diseases. In our study, SSR test and analysis were performed on the upper limbs of 66 patients with AICVD, aiming to know the characteristics of SSR in patients with AICVD and their correlations with clinical presentations of patients, and to assess the practical value of SSR in evaluating the condition and predicting long-term prognosis in patients with AICVD. Our study makes a point of SSR changes in the upper limbs of patients with AICVD, and the correlation between SSR changes and NIHSS score, ADL score, ESRS score etc. SSR tests of 30 non-AICVD patients in the control group all had complete waveform, which were mainly negative-positive-negative (NPN) and negative-positive (N-P) types. No significant difference was observed between left and right upper limbs in terms of SSR latency and amplitude in the control group; and the amplitude was found to be more variable than the latency. Among the patients with AICVD, 46 cases (69.70%) had SSR abnormalities, including 33 cases (50%) with prolonged latency, 21 cases (31.82%) with reduced amplitude, and 14 cases (21.21%) with disappeared waveform. It indicated that the function of sympathetic nervous system was inhibited in the acute phase of cerebral ischemia. This result is in line with previous studies such as Ping Liu19 and Tuncay.20 The overall abnormality rate and the abnormality rate of latency, however, were slightly higher than those reported in the other Chinese patients, which might be related to subtypes of ischemic strokes, sample sizes, criteria for patient recruitment, and detailed SSR procedures. Studies such as Ellaway et al4 and Muslumanoglu et al21 found that ANS is functionally plastic, and the results of SSR test may vary at different stages of disease or with different lifestyles of the patients. Among the 46 patients with SSR abnormalities, 41 cases (89.13%) had bilateral lesions, ie, 11 cases (55.00%) in the TIA group and 30 cases (65.22%) in the AIS group. It indicated that the SSR abnormalities of patients with AICVD were mostly bilateral, which is in line with the study by Linden et al22 and it’s also consistent with the clinical manifestation of lacunar stroke is milder than that of large vessel stroke.23 Some scholars24 proposed that the ascending reticular activating system (ARAS) pathways or the descending cortical reticular pathways may be impaired after cortical lesions, and these pathways are projected onto the bilateral reticular formations through the cortex on one side. The cortex damage on one side can therefore lead to functional changes in the bilateral reticular formations and functional decreases of the bilateral sympathetic nervous systems, which is manifested as inhibitory changes in bilateral SSRs. At present, there is no definitive conclusion about the relationship of the functional state of sympathetic nerves in AICVD patients with the NIHSS and BI scores, and there are also few reports on its relationship with the ESRS score. This study showed that among patients with AICVD, the higher the abnormality rate of SSR was, the higher the NIHSS and ESRS scores were, and the lower the BI score was. Specifically, the abnormality rate of SSR was positively correlated with the degree of neurologic impairment and the risk of stroke recurrence (r=0.347, p=0.004; r=0.437, p=0.000), and was negatively correlated with the ADL (r=−0.346, p=0.004). This result is in line with previous research findings.19,21 Among the 46 AICVD patients with SSR abnormalities, 33 cases (71.74.0%) had prolonged latency, 21 cases (45.65%) had reduced amplitude, and 14 cases (30.43%) had disappeared waveform. Further statistical analysis showed that the prolonged latency and reduced amplitude of SSR were positively correlated with the NIHSS score (r=0.371, p=0.005; r=0.341, p=0.012) and negatively correlated with the BI score (r=−0.426, p=0.001; r=−0.316, p=0.02). This result is not in line with the study by Ping Liu et al,19 but it was probably due to the selection of study subjects and the size of samples. Both the prolonged latency and disappeared waveform of SSR were positively correlated with the ESRS score (p=0.001, r=0.433; p=0.015, r=0.299), but the specific mechanism is not yet clear and needs further study in the future.
With the wide application of SSR, SEP, MEP and other neuroelectrophysiological technologies, brain dysfunction caused by ischemic injury and small or subclinical lesions that cannot be found by imaging examination have gradually attracted people’s attention. In fact, due to the limitations of neuroimaging techniques, sparse pathological studies and the lack of effective experimental models, there has been a lack of effective experimental indicators for the assessment of cerebral function status and short-term and long-term prognosis of patients with ischemic cerebrovascular disease. Our study found that the sympathetic reflex activity was inhibited in patients with AICVD, and the abnormal rate of SSR was correlated with the clinical manifestations of patients with AICVD, suggesting that SSR is expected to be used in the study on clinical characteristics and prognosis of different subtypes of acute ischemic stroke, especially in the aspects of small vascular diseases23 such as lacunar infarction with no lesions detected by imaging.
Conclusions
There may be inhibition of sympathetic reflex activity in patients with AICVD, SSR abnormality rate in patients with AICVD may be correlated with the degree of neurological impairment and long-term prognosis.
Limitations of This Study
Due to the small sample size, the case group failed to strictly distinguish stroke subtypes, and the exclusion criteria, in both the healthy control group and the case group, were mainly based on relatively subjective clinical data inquiry, which may lead to bias in the results.
Ethics Approval and Consent
We confirm our study complies with the Declaration of Helsinki. All participants were fully informed about the research project and gave written consent. Our study was approved and agreed by the ethics committees of Maternal and Child Health Hospital of Hubei Province, Tongji Medical College, Huazhong University of Science and Union Hospital, Tongji Medical College, Huazhong University of Science and Technology.
Author Contributions
All authors contributed to data analysis, drafting or revising the article, have agreed on the journal to which the article will be submitted, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
There was no conflict of interest in our study.
References
1. On AY, Tanigor G, Baydar DA. Relationships of autonomic dysfunction with disease severity and neuropathic pain features in fibromyalgia: is it really a sympathetically maintained neuropathic pain? Korean J Pain. 2022;35(3):327–335. doi:10.3344/kjp.2022.35.3.327
2. Nojszewska M, Potulska-Chromik A, Jamrozik Z, Janik P, Zakrzewska-Pniewska B. Electrophysiological and clinical assessment of dysautonomia in multiple system atrophy (MSA) and progressive supranuclear palsy (PSP): a comparative study. Neurol Neurochir Pol. 2019;53(1):26–33. doi:10.5603/PJNNS.a2019.0005
3. Emmer A, Mangalo S, Kornhuber ME. Augmentation of the sympathetic skin response after electrical train stimuli. Front Neurol. 2012;3(152):1–3. doi:10.3389/fneur.2012.00152
4. Ellaway PH, Kuppuswamy A, Nicotra A, Mathias CJ. Sweat production and the sympathetic skin response: improving the clinical assessment of autonomic function. Auton Neurosci. 2010;155:109–114. doi:10.1016/j.autneu.2010.01.008
5. Cavaliere S, Bertini G, Cossu C. Maria bastianelli, comparison of Sympathetic Skin Response (SSR) between electrical and acoustic stimuli in a healthy pediatric population. Pediatr Rep. 2021;13:520–529. doi:10.3390/pediatric13030060
6. Emad MR, Farpour HR, Ahmed F, et al. Is there any sympathetic skin response abnormality in raynaud phenomenon? Sultan Qaboos Univ Med J. 2022;22(2):274–279.
7. Jeffrey J, Goldberger MD, Rishi Arora MD, et al. Autonomic nervous system dysfunction. J Am Coll Cardiol. 2019;73(10):1189–1206.
8. Audrys G, Thakkar P, Tasic T, et al. GLP1R attenuates sympathetic response to high glucose via carotid body inhibition. Circ Res. 2022;130:694–707. doi:10.1161/CIRCRESAHA.121.319874
9. Gronda E, Dusi V, D’Elia E, et al. Sympathetic activation in heart failure. Eur Heart J Suppl. 2022;24(Supplement E):E4–E11. doi:10.1093/eurheartjsupp/suac030
10. Shubham Debnath TJ, Levy MB, Schwartz RM, et al. A method to quantify autonomic nervous system function in healthy, able-bodied individuals. Bioelectron Med. 2021;7:13. doi:10.1186/s42234-021-00075-7
11. van Dijk AE, van Lien R, van Eijsden M, Gemke RJ, Vrijkotte TG, de Geus EJ. Measuring cardiac Autonomic Nervous System (ANS) activity in children. J Vis Exp. 2013;74(e50073):P2–6.
12. Myles W, Petterson JL, Kimmerly DS, et al. An open-source program to analyze spontaneous sympathetic neurohemodynamic transduction. J Neurophysiol. 2021;125(3):972–976. doi:10.1152/jn.00002.2021
13. De Almei da Chaves Rodrigues AF, De lima IL, Bergamaschi CT, et al. Increased renal sympathetic nerve activity leads to hypertension and renal dysfunction in offspring from diabetic mothers. Am J Physiol Renal Physiol. 2013;304(2):F189–F197. doi:10.1152/ajprenal.00241.2012
14. Chroni E, Argyriou AA, Polychronopoulos P and Sirrou V. The effect of stimulation technique on sympathetic skin responses in healthy subjects. Clin Auton Res. 2006;16(6):396–400. doi:10.1007/s10286-006-0376-x
15. Beissner F, Meissner K, Bär KJ and Napadow V. The autonomic brain: an activation likelihood estimation meta-analysis for central processing of autonomic function. J Neurosci. 2013;33(25):10503–10511. doi:10.1523/JNEUROSCI.1103-13.2013
16. Duan R, Sun K, Fang F, et al. An ischemia-homing bioengineered nano-Scavenger for specifically alleviating multiple pathogeneses in ischemic stroke. J Nanobiotechnology. 2022;20:397. doi:10.1186/s12951-022-01602-7
17. Yang GH, Wang Y, Zeng YX, et al. Rapid health transition in China, 1990–2010: findings from the Global Burden of Disease Study 2010. Lancet. 2013;38l(9882):1987–2015. doi:10.1016/S0140-6736(13)61097-1
18. Feigin VL, Stark BA, Johnson CO, et al. Global, regional, and national burden of stroke and its risk factors, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet Neurol. 2021;20(10):795–820. doi:10.1016/S1474-4422(21)00252-0
19. Liu P, Wang S, Zhang X, et al. Relationship of sympathetic skin response and clinical presentation in acute cerebral infarction. J Apoplexy Nervous Dis. 2009;26(5):592–594.
20. Cakir T, Evcik FD, Subasi V, et al. Investigation of the H reflexes, F waves and sympathetic skin response with electromyography (EMG) in patients with stroke and the determination of the relationship with functional capacity. Acta Neurol Belg. 2015;115:295–301. doi:10.1007/s13760-014-0397-5
21. Muslumanoglu L, Aki S, Turkdoran D, et al. Involvement of sympathetic reflex activity in patients with acute and chronic stroke: a comparison with functional motor capacity. Arch Phys Med Rehabil. 2004;85:470–473. doi:10.1016/j.apmr.2003.03.009
22. Linden D, Berlit P. Sympathetic skin responses (SSRs) in monofocal brain lesions: topographical aspects of central sympathetic pathways. Acta Neurol Scand. 1995;91:372–376. doi:10.1111/j.1600-0404.1995.tb07023.x
23. Rudilosso S, Rodríguez-Vázquez A, Urra X, et al. The potential impact of neuroimaging and translational research on the clinical management of lacunar stroke. Int J Mol Sci. 2022;23:1497. doi:10.3390/ijms23031497
24. Korpelainen JT, Tolonen U, Sotaniemi KA, et al. Suppressed sympathetic skin response in brain infarction. Stroke. 1993;24:1389. doi:10.1161/01.STR.24.9.1389
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
