Back to Journals » International Journal of General Medicine » Volume 15
A Practical Nomogram for Predicting the Prognosis of Elderly Patients with Gastric Adenocarcinoma After Gastrectomy
Authors Yang H, Ji X, Jin C , Ji K, Jia Z, Wu X, Zhang J, Bu Z
Received 8 October 2021
Accepted for publication 22 December 2021
Published 11 January 2022 Volume 2022:15 Pages 473—488
DOI https://doi.org/10.2147/IJGM.S343306
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Heli Yang,* Xin Ji,* Chenggen Jin,* Ke Ji, Ziyu Jia, Xiaojiang Wu, Ji Zhang, Zhaode Bu
Department of Gastrointestinal Surgery, Key Laboratory of Carcinogenesis and Translational Research (Ministry of Education), Peking University Cancer Hospital and Institute, Beijing, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Heli Yang
Department of Gastrointestinal Surgery, Key Laboratory of Carcinogenesis and Translational Research (Ministry of Education), Peking University Cancer Hospital and Institute, Beijing, 100142, People’s Republic of China
Tel/Fax +86-10-88196970
Email [email protected]
Purpose: To establish a pragmatic prognostic nomogram for predicting the survival of elderly patients undergoing gastrectomy for gastric adenocarcinoma.
Patients and Methods: Data of elderly patients undergoing gastrectomy for gastric adenocarcinoma between 2004 and 2015 were obtained from the Surveillance, Epidemiology, and End Results database. Prognostic factors were identified by the Kaplan–Meier method and the Cox proportional hazards model. Based on these factors, we developed a nomogram to predict the overall survival (OS) and gastric cancer-specific survival (GCSS). Concordance index (C-index) and calibration curve are employed to assess the predictive accuracy of the model. Decision curve analysis (DCA) and receiver operating characteristic curve (ROC) analysis are applied to further appraise the clinical utility of the model.
Results: A total of 8401 cases were incorporated into this research. After univariate and multivariate analyses, nine prognostic factors of OS were identified, including age (P < 0.001), race (P < 0.001), marital status (P < 0.001), tumor site (P < 0.001), tumor size (P = 0.024), differentiation (P < 0.001), T stage (P < 0.001), N stage (P < 0.001), and M stage (P < 0.001); ten prognostic factors of GCSS were identified, including age (P < 0.001), race (P < 0.001), tumor site (P < 0.001), tumor size (P = 0.002), differentiation (P < 0.001), T stage (P < 0.001), N stage (P < 0.001), M stage (P < 0.001), radiotherapy (P < 0.001) and chemotherapy (P < 0.001). The C-index of the constructed nomogram for OS was 0.708 (95% CI: 0.701– 0.715) while for GCSS was 0.745 (95% CI: 0.737– 0.753). The calibration curves of the nomogram predictions and actual observations displayed good agreement for the 3- and 5-year OS and GCSS probabilities. The results of DCA and the area under the curve calculated by ROC analysis showed that the developed model was superior than TNM stage.
Conclusion: The nomogram we established could accurately predict the prognosis of individual elderly patients who underwent gastrectomy for gastric adenocarcinoma.
Keywords: gastric adenocarcinoma, elderly, gastrectomy, survival, nomogram
Introduction
Gastric cancer is a malignant neoplasm occurring in the gastric mucosal epithelium, and its morbidity and mortality are at the forefront of malignant tumors. The latest epidemiological data demonstrate that the mortality of gastric cancer ranks fourth and the incidence ranks fifth among malignant tumors in the world.1 The incidence and mortality rate for gastric cancer are particularly high in East Asia regions containing China, Japan, Korea and Mongolia, but low in North America, Northern Europe and Africa.1 Owing to a large population in China, more than 478,508 new gastric cancer cases and 373,789 cancer-related deaths occur every year.2 Approximately 26,259 people are diagnosed with gastric cancer, and approximately 11,413 will die from it in the United States each year.3
Even though the prevalence of gastric cancer has been steadily declined because of the promotion of Helicobacter pylori (H. pylori) detection and eradication and the popularity of refrigerators, the incidence rate of gastric cancer climbs with age, especially among elderly people.4–6 With the extension of people’s average life expectancy and the aging of the global population, the absolute number of elderly gastric cancer patients is increasing annually.7 Gastrectomy and standard lymphadenectomy are still the most effective treatment methods for gastric cancer. However, the efficacy of gastrectomy for elderly gastric cancer patients is not very clear at present, and few reports have investigated the long-term prognosis of elderly patients underwent gastrectomy.8–10 Therefore, it is necessary to conduct a comprehensive investigation and in-depth analysis of the prognosis of these patients, which is helpful for treatment selection and the improvement of efficacy.
A nomogram can intuitively and accurately display the complex long-term prognosis prediction formula with multiple variables and could be utilized to calculate the individual survival probability of cancerous person.11 Hence, nomograms have great value in clinical practice and have gradually received increasing attention from doctors and patients. Our goals were to investigate the long-term survival of elderly gastric cancer patients after gastrectomy through large-sample data derived from national Surveillance, Epidemiology, and End Results (SEER) database and to screen out the prognostic factors affecting survival. Based on our findings, we established a practical nomogram prognostic prediction model to individually predict the survival of elderly gastric cancer cases and to provide a reference for clinical treatment decision-making.
Materials and Methods
Source
Our research data came from the National Cancer Institute SEER database. This database involves 18 registered agencies covering about one third of the US population. It gathers data on the prevalence and mortality of cancer, including tumor characteristics, treatment and population-based variables.12 Because the SEER database is available for the public and provides anonymous patient information, the approved consent was exempt from local ethics committee.
Study Population
We extracted data from 2004 to 2015 through SEER*Stat software. The eligibility criteria were as follows: patients aged 65 years or older; patients only suffered from gastric cancer or had two or more primary cancers but gastric cancer was the first; underwent gastrectomy; pathologically diagnosed with gastric adenocarcinoma; and complete clinicopathological and follow-up data. The exclusion criteria included the following: patients younger than 65 years; patients not only suffered from gastric cancer or gastric cancer was not the first of 2 or more primary cancers; the pathological diagnosis was non-adenocarcinoma (including squamous cell carcinoma, adenosquamous carcinoma, neuroendocrine carcinoma and so on); patients did not undergo surgery or only underwent local excision (including endoscopic mucosal resection, endoscopic submucosal dissection and so on); and patients without complete information on marital status, race, tumor site, tumor size, differentiation, TNM stage, surgical type, survival state, death reason and those with follow-up ≤1 month.
Informations obtained from SEER database included the demographics of the patients (sex, age, race, marital status), clinicopathological characteristics of cancer (tumor size, tumor site, differentiation, TNM stage, radiotherapy and chemotherapy status) and survival data (survival months, survival status, death reason). Age was partitioned into three groups: 65–74, 75–84, and > 85, which representing young-old, old-old, and oldest-old.13 Race was categorized into three groups: black, white, and others (Asian Americans, Pacific Islanders, Alaska native, American Indian, and so on). Marital status was divided into three groups: married, divorced, and others (single, unmarried, widowed, or domestic partner). The primary tumor site was classified into the following groups: cardia, gastric fundus, gastric body, antrum, pylorus, gastric lesser and greater curvatures, and overlapping lesion. Tumor size was classified as three groups: ≤2 cm, 2–5 cm, and > 5 cm. Tumor differentiation was divided into well, moderately, poorly, and undifferentiated. TNM stage was defined according to the 6th stage version of the American Joint Committee on Cancer (AJCC).
OS was defined as the time from operation to death for any reason. GCSS referred to the time from operation to death from gastric cancer.
Statistical Analysis
We used IBM SPSS Statistics ver. 22.0 software (IBM Corporation, Armonk, NY) to analyze the data. Kaplan–Meier method and Log rank test was used for survival analysis. Cox proportional hazards regression model was used to make univariate and multivariate analyses to distinguish prognostic factors. R software (version 3.4.4) was employed to develop a nomogram.14 The maximum score was set to 100 for each factor. Concordance index (C-index) was used to evaluate the accuracy of nomogram. Calibration curves were drawn to compare the nomogram predicted and actual observed survival probabilities. The result was tested using 1000 bootstrap resamples. Decision curve analysis (DCA) was used to assess the clinical utility of the nomogram by quantifying the net benefit under different threshold probabilities. The receiver operating characteristic (ROC) curve and the area under the curve (AUC) were calculated and analyzed with MedCalc (version 19.1.7). A P value <0.05 for two-sided was identified as statistically significant.
Result
Demographic Characteristics
8401 patients were finally included in our study in line with the inclusion and exclusion criteria. Details of the study flow are provided in Figure 1. Patients characteristics of demography is displayed in Table 1. Most patients were white (5515; 65.6%), male (5113; 60.9%), and married (5164; 65.6%). Of those, 51.1% (4294) were between the ages of 65 and 74 years, 38.7% (3251) were between the ages of 75 and 84 years, and 10.2% (856) were older than 85 years. The most frequently affected site was the gastric antrum (2705; 32.2%), followed by the cardia (2014; 23.9%), lesser curvature of the stomach (1099; 13.1%), body of the stomach (858; 10.2%), overlapping lesion of the stomach (613; 7.5%), greater curvature of the stomach (456; 5.4%), pylorus (381; 4.5%), and fundus of the stomach (275; 3.2%). Most patients (3753; 44.7%) had tumors between 2 and 5 cm in diameter, 2989 (35.6%) patients had tumors greater than 5 cm and 1659 patients (19.7%) had tumors smaller than or equal to 2 cm. Most cases were poorly differentiated (5090; 60.6%), followed by moderately differentiated (2656; 31.6%), well differentiated (488; 5.8%), and undifferentiated (167; 2%). With regard to T stage, T2 was the most prominent (4034; 48%), followed by T1 (2003; 23.8%), T3 (1839; 21.9%), and T4 (525; 6.3%). For N stage, N0 had the highest number of patients (3547; 42.2%), followed by N1 (3144; 37.4%), N2 (1233; 14.7%), and N3 (477; 5.7%). Majority of the patients were classified as M0 (7724; 91.9%). A total of 2296 (27.3%) and 3428 (40.8%) patients received radiation therapy and chemotherapy, respectively.
 |
Table 1 Patient Characteristics for 3-Year and 5-Year OS and GCSS of Elderly Patients with Gastric Adenocarcinoma After Gastrectomy |
 |
Figure 1 The flow diagram of the selection process for elderly patients with gastric adenocarcinoma undergoing radical gastrectomy. |
Survival Analysis
The follow-up time scope was 2 to 155 months. The mean follow-up was 40 months. A total of 5367 deaths occurred, including 3973 deaths from gastric cancer. After surgery, the 5-year OS and GCSS rates were 38.7% and 48.5% (Figure 2).
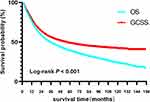 |
Figure 2 OS and GCSS curves of all elderly patients with gastric adenocarcinoma undergoing radical gastrectomy. Abbreviations: OS, overall survival; GCSS, gastric cancer-specific survival. |
Regarding OS, univariate analysis showed that age, race, marital status, tumor site, tumor size, differentiation, T stage, N stage, and M stage were prognostic factors for OS (all P<0.001); while multivariate analyses showed that age (P<0.001), race (P<0.001), marital status (P<0.001), tumor site (P<0.001), tumor size (P=0.024), differentiation (P<0.001), T stage (P<0.001), N stage (P<0.001), and M stage (P<0.001) were still independent prognostic factors for OS. The detailed results are listed in Table 2.
 |
Table 2 Univariate and Multivariate Cox Regression Analyses Estimating the Risk Factors for OS of Elderly Patients with Gastric Adenocarcinoma After Gastrectomy |
In terms of GCSS, univariate analysis showed that age, race, marital status, tumor site, tumor size, differentiation, T stage, N stage, M stage, radiotherapy, and chemotherapy were prognostic factors (all P<0.001); while multivariate analyses showed that age (P<0.001), race (P<0.001), tumor site (P<0.001), tumor size (P=0.002), differentiation (P<0.001), T stage (P<0.001), N stage (P<0.001), M stage (P<0.001), radiotherapy (P<0.001), and chemotherapy (P<0.001) were still independent prognostic factors for GCSS, except for marital status (P=0.052). The detailed results are listed in Table 3.
 |
Table 3 Univariate and Multivariate Cox Regression Analyses Estimating the Risk Factors for GCSS of Elderly Patients with Gastric Adenocarcinoma After Gastrectomy |
Nomogram Construction and Validation
Based on the above identified independent prognostic factors, we constructed a nomogram to predict survival for elderly patients undergoing gastrectomy for gastric adenocarcinoma (Figure 3). In essence, the nomogram is a visualization of the Cox regression equation. On the top of the nomogram, the point axis were rated scale from 0 to 100. The identified independent prognostic factors had their own evaluation criteria according to the weights of their regression coefficients. First, a line perpendicular to the point axis should be made at the point value of each independent variable. The intersection point represents the score under the value of the independent variable. Then, the corresponding individual scores of all prognostic factors can be summed to obtain the total score, and the corresponding points can be found on the total point score axis. Finally, a vertical line can be drawn on the value point of the total score axis directly to the survival probability axis, and the intersection point is the patient’s 3-year or 5-year OS or GCSS probability. The C-indices of our nomogram were 0.708 (95% CI: 0.701–0.715) for OS and 0.745 (95% CI: 0.737–0.753) for GCSS. The calibration curves for the 3- and 5-year OS and GCSS probabilities showed good agreement between the nomogram predictions and the actual observations, which are shown in Figures 4 and 5. To further evaluate the clinical utility of the nomogram, DCA curves showed the established nomogram performed well compared with traditional TNM staging system, which are shown in Figure 6. The results of AUC calculated by ROC analysis showed that the developed model was better than the TNM stage, which are shown in Figure 7 and Table 4.
 |
Table 4 Comparison of AUC Between the Nomogram and TNM Stage |
 |
Figure 6 Decision curve analysis (DCA) curves of the two nomograms for elderly patients undergoing radical gastrectomy. (A) DCA for overall OS; (B) DCA for GCSS. |
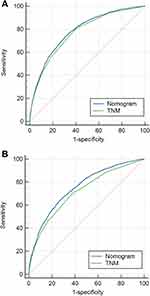 |
Figure 7 Receiver operating characteristic (ROC) curves of the two nomograms for elderly patients undergoing radical gastrectomy. (A) ROC curve for OS; (B) ROC curve for GCSS. |
Discussion
Gastric cancer remains one of the most important tumors worldwide with high mortality.1 Gastrectomy is still the main modality for the treatment of gastric cancer.7 Recently, with the ageing population and increasing life expectancy, the incidence and absolute number of gastric cancer in the elderly population continues to increase.15 It is an urgent task to investigate the long-term survival of elderly gastric cancer patients underwent gastrectomy and to establish relevant prognosis prediction models. However, large sample studies concentrated on on these issues are still lacking. Relevant prognosis prediction models are even rare. Hence, we searched the SEER database to screen out 8401 elderly gastric cancer cases data and built a pragmatic nomogram to predict the survival rates of elderly cases undergoing gastrectomy.
The TNM stage, which is created to codify the anatomic extent of cancer to assist in making treatment decisions and predicting survival, is by far the most commonly used classification for gastric patients.16 However, it should be pointed out that the survival prediction based on TNM staging is calculated for the patient population but not for individual cancer patients. Actually, gastric cancer is a highly heterogeneous malignant tumor, and the prognosis of gastric cancer patients in the same TNM stage scope is not exactly the same or may even vary greatly in clinical practice. In the context of precision medicine, more accurate individualized prognosis predictions of gastric cancer have more important clinical guiding significance.17 Therefore, prognosis prediction for an individual gastric cancer patient cannot be precisely determined by TNM stage grouping alone and should include additional prognostic factors, such as clinical information about the patient, pathological information related to the tumor or even the molecular biological characteristics of the tumor, to better guide the prognosis evaluation and treatment choice. In this study, through multivariate Cox survival analysis and statistical modeling, we established a nomogram combining TNM stage with patient demographic variables (age, race, and marital status) and clinicopathological variables (tumor site, tumor size, differentiation, radiation therapy and chemotherapy) that could provide more precise prognostic prediction for individual elderly patients receiving gastrectomy for gastric adenocarcinoma.
Age and race were independent prognostic factors for elderly patients with gastric cancer undergoing gastrectomy. Our study showed that older age at diagnosis and white and black ethnic backgrounds were associated with poor OS and GCSS. Song et al, identifying 10,092 patients in the SEER database, analyzed the effect of age on the survival outcome after gastrectomy, and the results showed that compared to young patients, elderly patients had a lower survival rate after gastrectomy, and the risk of death increased with increasing age.6 Alshehri et al analysed the prognosis of 2005 gastric cancer cases following surgical treatment between 2002 and 2007 and drawn the conclusion that older age is a adverse prognostic factor for both OS and relapse-free survival.18 The unfavourable prognosis of elderly patients may be explained by poor tolerance to radical surgery and inability to complete perioperative chemotherapy.19 Furthermore, elderly patients could be incline to die of non-gastric cancer diseases, for instance of cerebrovascular disease, cardiovascular disease and other chronic comorbidity diseases.20 Our study also found that the prognosis of gastric cancer patients in the Asian population was better than that of white and black populations, which is in line with the reports of some previous researches.21,22 Employing the National Cancer Database, Tee’s study showed that the five-year OS for Asians was significantly better than that for whites and blacks, with rates of 49.5%, 30.8% and 32.5%, respectively.21 The possible reason is that Asian American patients have a greater number of retrieved lymph nodes (17, 15 and 16), a decreased lymph node ratio (0.03, 0.05 and 0.09) and a lower 90-day postoperative mortality rate (4.2%, 8.6% and 7.3%) than whites and blacks.21 Jin et al analyzed the 5-year OS of patients with gastric cancer in SEER from 2000 to 2012 by race and found that all Asian populations, including Chinese, Japanese, Korean, Vietnamese, Filipino, South Asian, had higher rates than non-Hispanic whites (42.2%, 45.4%, 43.4%, 36.8%, 36.4%, 35.7% and 29.8%, respectively).22 The favorable survival of Asian patients is possibly attributable to sociodemographic factors, such as higher socioeconomic status, better insurance, younger age composition and tumor characteristics, such as early stage at diagnosis and better anatomic location. However, the real reason for the better prognosis of Asian American gastric cancer patients remains elusive, and further fundamental researches are needed to elucidate the potential biological mechanisms involved in this result.
Tumor size and site could affect the survival of gastric cancer. We found that the the prognosis of gastric cancer patients gradually deteriorates with the increase of tumor diameter, which validated the clinical perception. Zhou et al showed that the 5-year OS of small tumor was significantly better than that of large, with a cutoff of 4.9 cm (44.96% and 30.63%, respectively, P <0.05).23 This is most likely because larger tumor size indicate the larger the tumor burden and the greater the chances of infiltrative growth and metastasis to lymphatic channels and vessels. In fact, in many solid tumors, such as lung cancer, liver cancer, and breast cancer, tumor size is the criterion for T staging due to its effect on prognosis. Liang et al pointed out that tumor size can also affect the prognosis of hollow organ tumors such as gastric cancer and should be incorporated into T staging to better predict prognosis.24 Furthermore, our study displayed that the survival of cardiac cancer was worse than that of non-cardiac cancer, which suggested that the site of gastric cancer was related to prognosis. Xue et al conducted a meta-analysis to compare the prognosis of proximal and distal gastric cancer patients who underwent gastrectomy, and the results showed that the prognosis of proximal gastric cancer patients was inferior than those of distal gastric cancer patients.25 Although distal gastric cancer is still the main site of gastric cancer, it is clear that the incidence of cardiac cancer is increasing year by year.26 Compared with adenocarcinoma of the gastric antrum, cardiac cancer is more aggressive in biological behavior, which suggests that the heterogeneity and clonal evolution process may be different from those of cancers originating from other parts of the stomach.27
Tumor differentiation is an important prognostic factor of gastric cancer. Our results suggest that tumor differentiation affects not only OS but also GCSS in elderly patients with gastric cancer. The differentiation of tumors reflects the malignant degree of the biological behavior of the tumor itself. In effect, tumor differentiation is an independent prognostic factor in many malignant neoplasms, such as lung cancer, breast cancer, and esophageal cancer.28–30 Even in some tumors, tumor differentiation has been included as a reference factor for staging. For example, tumor differentiation, which is more important than the tumor site and only second to the TNM factor, has been incorporated into the 8th edition of the TNM staging of esophageal cancer as a key factor for the substaging of stage 0-II.30
It is widely agreed that surgery combined with chemotherapy and/or radiotherapy should be adopted for gastric cancer patients staged IB disease or above to improve the long-term survival rate. Multiple RCT studies have demonstrated that multiple therapies significantly improve survival over surgery alone, including adjuvant chemoradiotherapy (used in two US intergroup trials, INT 0116 and CALGB 80101),31,32 perioperative chemotherapy (preoperative + postoperative chemotherapy; MAGIC and FLOT trials),33,34 and adjuvant chemotherapy alone (used in East Asia, ACTS-GC and CLASSIC trials).35,36 However, our study shows that chemotherapy and radiotherapy can improve GCSS but not OS. We suppose that the possible reasons were that early gastric cancer cases were mixed in the cohort, the follow-up time was relatively short, and the overall survival of elderly patients was affected by preoperative comorbidity and postoperative complications.
In addition, we found that marital status can affect OS but not GCSS for elderly patients underwent gastrectomy for gastric cancer. Zhang et al analyzed 16,910 gastric cancer patients using SEER data, the results showed that unmarried patients had worse 5-year OS (24.61% vs 32.09%, P<0.001) and GCSS rates (32.79% vs 37.74%, P<0.001) than married patients.37 Marital status can also affect survival in other malignancies, such as lung cancer, breast cancer, and colorectal cancer.38–40 The possible reason is that married patients with cancer have better psychosocial support, have less tension and anxiety, are more likely to be suitable for surgery, chemotherapy and radiotherapy, and can complete the planned course of treatment.
It should be pointed out that our research has some shortcomings. First, the SEER database cannot offer more detailed data about patients’ comorbidities, performance status, nutrition score, and cardiopulmonary function, which are often associated with prognosis. Second, specific information on treatment, such as the mode of surgical resection (open or laparoscopic; proximal gastrectomy, distal gastrectomy, or total gastrectomy), dissection range of lymph nodes (including D0, D1 and D2), radiotherapy target, technique, dosage, and methods for segmentation, and chemotherapeutic drugs (including dose, cycles, and regimens), cannot be obtained, and this information is related to the prognosis. Third, the patient’s recurrence information and the treatment after recurrence were not available to provide a complete picture of the whole course of disease, so a more comprehensive and detailed survival analysis could not be carried out. Finally, our research is a retrospective study with patient selection bias and lacks external validation, so the findings need to be confirmed by prospective data.
Conclusion
We found that for elderly patients undergoing gastrectomy for gastric adenocarcinoma, age, race, tumor site, tumor size, differentiation, T stage, N stage, and M stage were independent prognostic factors for both OS and GCSS, while marital status only affected OS and radiotherapy and chemotherapy only affected GCSS. The nomogram developed based on these factors performed well in forecasting the 3- and 5-year OS and GCSS probabilities of individual patients.
Data Sharing Statement
The datasets generated and analysed during the current study are available in the homepage of the Surveillance, Epidemiology, and End Results (SEER) Program.
Acknowledgments
We sincerely acknowledge the efforts of the Surveillance, Epidemiology, and End Results (SEER) Program tumor registries in the creation of the SEER database.
Author Contributions
Conception and design: HL Yang, X Ji. Analysis and interpretation of data: HL Yang, X Ji, CG Jin. Drafting of article: HL Yang, X Ji, CG Jin. Critical revision for intellectual content: HL Yang, X Ji, CG Jin, K Ji, ZY Jia, XJ Wu, J Zhang, ZD BU. Final approval: HL Yang, X Ji, CG Jin, K Ji, ZY Jia, XJ Wu, J Zhang, ZD BU. All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was supported by the Research Incubation Project, Beijing Municipal Administration of Hospitals (PX2019039).
Disclosure
The authors claim no conflicts of interest.
References
1. Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi:10.3322/caac.21660
2. Yang L, Ying X, Liu S, et al. Gastric cancer: epidemiology, risk factors and prevention strategies. Chin J Cancer Res. 2020;32(6):695–704. doi:10.21147/j.issn.1000-9604.2020.06.03
3. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69(1):7–34. doi:10.3322/caac.21551
4. Yan S, Gan Y, Song X, et al. Association between refrigerator use and the risk of gastric cancer: a systematic review and meta-analysis of observational studies. PLoS One. 2018;13(8):e0203120. doi:10.1371/journal.pone.0203120
5. Zhang T, Chen H, Yin X, et al. Changing trends of disease burden of gastric cancer in China from 1990 to 2019 and its predictions: findings from Global Burden of Disease Study. Chin J Cancer Res. 2021;33(1):11–26. doi:10.21147/j.issn.1000-9604.2021.01.02
6. Song P, Wu L, Jiang B, Liu Z, Cao K, Guan W. Age-specific effects on the prognosis after surgery for gastric cancer: a SEER population-based analysis. Oncotarget. 2016;7(30):48614–48624. doi:10.18632/oncotarget.9548
7. Smyth EC, Nilsson M, Grabsch HI, van Grieken NC, Lordick F. Gastric cancer. Lancet. 2020;396(10251):635–648. doi:10.1016/S0140-6736(20)31288-5
8. Biondi A, Cananzi FC, Persiani R, et al. The road to curative surgery in gastric cancer treatment: a different path in the elderly. J Am Coll Surg. 2012;215(6):858–867. doi:10.1016/j.jamcollsurg.2012.08.021
9. Takeshita H, Ichikawa D, Komatsu S, et al. Surgical outcomes of gastrectomy for elderly patients with gastric cancer. World J Surg. 2013;37(12):2891–2898. doi:10.1007/s00268-013-2210-7
10. Wang X, Zhao J, Fairweather M, Yang T, Sun Y, Wang J. Optimal treatment for elderly patients with resectable proximal gastric carcinoma: a real world study based on National Cancer Database. BMC Cancer. 2019;19(1):1079. doi:10.1186/s12885-019-6166-3
11. Balachandran VP, Gonen M, Smith JJ, DeMatteo RP. Nomograms in oncology: more than meets the eye. Lancet Oncol. 2015;16(4):e173–180. doi:10.1016/S1470-2045(14)71116-7
12. National Cancer Institute [homepage on the Internet]. Surveillance, epidemiology, and end results. Available from: http://www.seer.cancer.gov.
13. Crews DE, Zavotka S. Aging, disability, and frailty: implications for universal design. J Physiol Anthropol. 2006;25(1):113–118. doi:10.2114/jpa2.25.113
14. Frank EHJ. [homepage on the Internet]. Rms: Regression Modeling Strategies. R Package version 3.4-4. Available from: http://www.rproject.org/.
15. Bray F, Jemal A, Grey N, Ferlay J, Forman D. Global cancer transitions according to the human development index (2008–2030): a population-based study. Lancet Oncol. 2012;13(8):790–801. doi:10.1016/S1470-2045(12)70211-5
16. Egner JR. AJCC cancer staging manual. JAMA. 2010;304(15):1726–1727. doi:10.1001/jama.2010.1525
17. Kattan MW, Hess KR, Amin MB, et al. American Joint Committee on Cancer acceptance criteria for inclusion of risk models for individualized prognosis in the practice of precision medicine. CA Cancer J Clin. 2016;66(5):370–374. doi:10.3322/caac.21339
18. Alshehri A, Alanezi H, Kim BS. Prognosis factors of advanced gastric cancer according to sex and age. World J Clin Cases. 2020;8(9):1608–1619. doi:10.12998/wjcc.v8.i9.1608
19. Nelen SD, Bosscha K, Lemmens V, Hartgrink HH, Verhoeven R, de Wilt J. Morbidity and mortality according to age following gastrectomy for gastric cancer. Br J Surg. 2018;105(9):1163–1170. doi:10.1002/bjs.10836
20. Hashimoto T, Kurokawa Y, Mikami J, et al. Postoperative long-term outcomes in elderly patients with gastric cancer and risk factors for death from other diseases. World J Surg. 2019;43(11):2885–2893. doi:10.1007/s00268-019-05109-5
21. Tee MC, Pirozzi N, Brahmbhatt RD, Raman S, Franko J. Oncologic and surgical outcomes for gastric cancer patients undergoing gastrectomy differ by race in the United States. Eur J Surg Oncol. 2020;46(10Pt A):1941–1947. doi:10.1016/j.ejso.2020.05.014
22. Jin H, Pinheiro PS, Callahan KE, Altekruse SF. Examining the gastric cancer survival gap between Asians and whites in the United States. Gastric Cancer. 2017;20(4):573–582. doi:10.1007/s10120-016-0667-4
23. Zhou L, Li W, Cai S, Yang C, Liu Y, Lin Z. Large tumor size is a poor prognostic factor of gastric cancer with signet ring cell: results from the surveillance, epidemiology, and end results database. Medicine (Baltimore). 2019;98(40):e17367. doi:10.1097/MD.0000000000017367
24. Liang Y, Liu L, Xie X, et al. Tumor size improves the accuracy of the prognostic prediction of lymph node-negative gastric cancer. J Surg Res. 2019;240:89–96. doi:10.1016/j.jss.2019.02.037
25. Xue J, Yang H, Huang S, Zhou T, Zhang X, Zu G. Comparison of the overall survival of proximal and distal gastric cancer after gastrectomy: a systematic review and meta-analysis. World J Surg Oncol. 2021;19(1):17. doi:10.1186/s12957-021-02126-4
26. Arnold M, Ferlay J, van Berge Henegouwen MI, Soerjomataram I. Global burden of oesophageal and gastric cancer by histology and subsite in 2018. Gut. 2020;69(9):1564–1571. doi:10.1136/gutjnl-2020-321600
27. Duan Q, Tang C, Ma Z, et al. Genomic heterogeneity and clonal evolution in gastroesophageal junction cancer revealed by single cell DNA sequencing. Front Oncol. 2021;11:672020. doi:10.3389/fonc.2021.672020
28. Yang H, Li X, Shi J, et al. A nomogram to predict prognosis in patients undergoing sublobar resection for stage IA non-small-cell lung cancer. Cancer Manag Res. 2018;10:6611–6626. doi:10.2147/CMAR.S182458
29. Wu SC, Chiang MC, Lee YG, et al. Long-term survival and prognostic implications of patients with invasive breast cancer in southern Taiwan. Medicine (Baltimore). 2020;99(7):e19122. doi:10.1097/MD.0000000000019122
30. Rice TW, Gress DM, Patil DT, Hofstetter WL, Kelsen DP, Blackstone EH. Cancer of the esophagus and esophagogastric junction-major changes in the American Joint Committee on Cancer eighth edition cancer staging manual. CA Cancer J Clin. 2017;67(4):304–317. doi:10.3322/caac.21399
31. Macdonald JS, Smalley SR, Benedetti J, et al. Chemoradiotherapy after surgery compared with surgery alone for adenocarcinoma of the stomach or gastroesophageal junction. N Engl J Med. 2001;345(10):725–730. doi:10.1056/NEJMoa010187
32. Fuchs CS, Niedzwiecki D, Mamon HJ, et al. Adjuvant chemoradiotherapy with epirubicin, cisplatin, and fluorouracil compared with adjuvant chemoradiotherapy with fluorouracil and leucovorin after curative resection of gastric cancer: results from CALGB 80101 (Alliance). J Clin Oncol. 2017;35(32):3671–3677. doi:10.1200/JCO.2017.74.2130
33. Cunningham D, Allum WH, Stenning SP, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med. 2006;355(1):11–20. doi:10.1056/NEJMoa055531
34. Al-Batran SE, Homann N, Pauligk C, et al. Perioperative chemotherapy with fluorouracil plus leucovorin, oxaliplatin, and docetaxel versus fluorouracil or capecitabine plus cisplatin and epirubicin for locally advanced, resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4): a randomised, Phase 2/3 trial. Lancet. 2019;393(10184):1948–1957. doi:10.1016/S0140-6736(18)32557-1
35. Sasako M, Sakuramoto S, Katai H, et al. Five-year outcomes of a randomized Phase III trial comparing adjuvant chemotherapy with S-1 versus surgery alone in stage II or III gastric cancer. J Clin Oncol. 2011;29(33):4387–4393. doi:10.1200/JCO.2011.36.5908
36. Bang YJ, Kim YW, Yang HK, et al. Adjuvant capecitabine and oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): a Phase 3 open-label, randomised controlled trial. Lancet. 2012;379(9813):315–321. doi:10.1016/S0140-6736(11)61873-4
37. Zhang J, Gan L, Wu Z, Yan S, Liu X, Guo W. The influence of marital status on the stage at diagnosis, treatment, and survival of adult patients with gastric cancer: a population-based study. Oncotarget. 2017;8(14):22385–22405. doi:10.18632/oncotarget.7399
38. Wu Y, Ai Z, Xu G. Marital status and survival in patients with non-small cell lung cancer: an analysis of 70006 patients in the SEER database. Oncotarget. 2017;8(61):103518–103534. doi:10.18632/oncotarget.21568
39. Liu YL, Wang DW, Yang ZC, et al. Marital status is an independent prognostic factor in inflammatory breast cancer patients: an analysis of the surveillance, epidemiology, and end results database. Breast Cancer Res Treat. 2019;178(2):379–388. doi:10.1007/s10549-019-05385-8
40. Alyabsi M, Ramadan M, Algarni M, Alshammari K, Jazieh AR. The effect of marital status on stage at diagnosis and survival in Saudis diagnosed with colorectal cancer: cancer registry analysis. Sci Rep. 2021;11(1):8603. doi:10.1038/s41598-021-88042-9
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.



