Back to Journals » Clinical Ophthalmology » Volume 16
A Plant-Derived Antioxidant Supplement Prevents the Loss of Retinal Ganglion Cells in the Retinas of NMDA-Injured Mice
Authors Maekawa S, Sato K, Kokubun T, Himori N , Yabana T, Ohno-Oishi M, Shi G, Omodaka K, Nakazawa T
Received 20 December 2021
Accepted for publication 2 March 2022
Published 18 March 2022 Volume 2022:16 Pages 823—832
DOI https://doi.org/10.2147/OPTH.S354958
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
Shigeto Maekawa,1 Kota Sato,1,2 Taiki Kokubun,1 Noriko Himori,1,3 Takeshi Yabana,1 Michiko Ohno-Oishi,1 Ge Shi,1 Kazuko Omodaka,1,4 Toru Nakazawa1,2,4– 6
1Department of Ophthalmology, Tohoku University Graduate School of Medicine, Sendai, Miyagi, Japan; 2Department of Advanced Ophthalmic Medicine, Tohoku University Graduate School of Medicine, Sendai, Miyagi, Japan; 3Department of Aging Vision Healthcare, Tohoku University Graduate School of Biomedical Engineering, Sendai, Miyagi, Japan; 4Department of Ophthalmic Imaging and Information Analytics, Tohoku University Graduate School of Medicine, Sendai, Miyagi, Japan; 5Collaborative Program for Ophthalmic Drug Discovery, Tohoku University Graduate School of Medicine, Sendai, Miyagi, Japan; 6Department of Retinal Disease Control, Tohoku University Graduate School of Medicine, Sendai, Miyagi, Japan
Correspondence: Toru Nakazawa, Department of Ophthalmology, Tohoku University Graduate School of Medicine, 1-1 Seiryo, Aoba, Sendai, Miyagi, 980-8574, Japan, Tel +81-22-717-7294, Fax +81-22-717-7298, Email [email protected]
Purpose: To investigate the effect of plant-derived antioxidant compounds, identified with primary culture screening, on retinal ganglion cell (RGC) survival in mice under excitotoxic conditions. Additionally, to determine the effect of these compounds on the involvement of calpain inactivation.
Materials and Methods: Plant-derived antioxidant compounds including hesperidin, crocetin, and Tamarindus indica were administrated orally to C57BL/6J mice. The levels of lipid oxidation and calpain activation were assessed with a TBARS assay and western blotting. RGC survival was evaluated with a TUNEL assay and RBPMS immunostaining after intravitreal injection of NMDA.
Results: Plant-derived antioxidant compounds significantly ameliorated the increase in the level of MDA in the retinas after NMDA injury. Cleaved α-fodrin fragments were detected in the NMDA-injured retinas, and these fragments were significantly lower in mice that received the plant-derived antioxidant compounds. The plant-derived antioxidants also ameliorated increases in TUNEL-positive cells and RGC death after NMDA injection.
Conclusion: These results indicate that oral administration of plant-derived antioxidant compounds such as hesperidin, crocetin, and Tamarindus indica suppressed RGC death. This oral supplementation decreased lipid oxidation and excessive calpain activation in NMDA-injured retinas. Thus, our newly developed antioxidant supplement has a potential role in neuroprotective treatment for retinal diseases, such as glaucoma.
Keywords: plant-derived antioxidant compounds, oxidative stress, retinal ganglion cells, glaucoma
Introduction
Glaucoma is an optic neuropathy that is a common cause of visual impairment and blindness worldwide.1 Glaucoma is characterized by the progressive death of retinal ganglion cell (RGC) axons and the irreversible loss of vision.2 It is well known that elevated intraocular pressure (IOP) is the most significant risk factor for glaucoma. However, in some patients the speed of progression does not depend on IOP, suggesting that IOP-independent factors also influence glaucoma progression.3,4 It was previously reported that oxidative stress-associated compounds increase in the eyes and bodily fluids, such as the peripheral blood, urine, and aqueous humor of human glaucoma patients.5–8 Studies of various retinal-injury animal models have shown that oxidative stress is associated with RGC death in mice,9–12 and that treatment to decrease oxidative stress prevents RGC death after optic nerve injury.13,14 These reports show that oxidative stress plays a significant role in RGC death, and suggest that antioxidant therapy may be a promising treatment approach.
Oxidative stress is an important factor not only in glaucoma, but also in other neurodegenerative diseases, including Alzheimer’s disease, Parkinson’s disease, and amyotrophic lateral sclerosis.15 In the retina, oxidative stress is believed to be an important risk factor for age-related macular degeneration (ARMD) and diabetic retinopathy (DR).16–18 Therefore, inhibiting oxidative stress has potential as a neuroprotective treatment in all these diseases, not only glaucoma. Past studies have found that supplementation with antioxidants has neuroprotective effects. For example, a landmark study, the Age-Related Eye Disease Study (AREDS), showed that antioxidant supplements had a beneficial effect in these diseases and could slow progression.
Our previous work demonstrated that several plant-derived compounds enhanced cell viability in retinal primary cultures under oxidative stress. In particular, hesperidin, a plant-derived bioflavonoid, suppressed oxidative stress and excessive calpain activation, preventing RGC death after N-methyl-D-aspartate (NMDA) injury in vivo. In the current study, we investigate the neuroprotective effect of plant-derived compounds that we identified through retinal primary culture screening.19
Materials and Methods
Animals
Male, 8–12-week-old C57BL/6J mice (SLC Co., Shizuoka, Japan) were used in this study. The mice were treated according to the principles presented in the guidelines of the Declaration of Helsinki and its guiding principles in the care and use of animals. The Ethics Committee for Animal Experiments at Tohoku University Graduate School of Medicine approved all experimental procedures, in accord with the Association for Research in Vision and Ophthalmology (approval #2017-229).
NMDA-Induced Retinal Injury
NMDA injury in the animals was induced as described in previous publications.19,20 Briefly, a 15 mM solution of NMDA (Sigma-Aldrich, St. Louis, MO, USA) in phosphate-buffered saline (PBS) was injected intravitreally (2 µL/eye). Animals were excluded if they had lens injuries or vitreous hemorrhage. Three mice in total were excluded from the experiments due to lens injury or vitreous hemorrhage. Anesthesia was induced with 10% pentobarbital in PBS (0.77 mg/kg).
Oral Supplementation
In a previous study,19 we reported 12 candidate neuroprotective compounds. Based on these results, we developed a supplement containing three of these candidate compounds: hesperidin, crocetin, and Tamarindus indica. Low-dose and high-dose versions of the supplement were prepared, from Wakamoto Pharmaceutical Co., Ltd, with total concentrations of 16 mg/kg and 160 mg/kg, respectively, in sterilized PBS (the composition in Table 1). The mice received supplementation with either a vehicle control (PBS) or one of the supplements for one week orally via gavage. Intravitreal injection of NMDA was then performed, and three hours later, the mice received a final dose of the supplement.
 |
Table 1 The Composition of the Oral Supplement. The Units are All in mg. PBS Was Used as Vehicle, and the Mice of Control Group Were Taken Same Volume of PBS |
2-Thiobarbituric Acid Reactive Substances (TBARS) Assay
To determine lipid peroxidation as an indirect marker of oxidative stress, we measured malondialdehyde (MDA) in retinas extracted from the mice 6 hours after the intravitreal injection of NMDA, and performed a TBARS assay, as described previously.19,21 In brief, a retinal homogenate containing 0.5 mM butylated hydroxytoluene was incubated with a reaction mixture (0.81% SDS, 0.36% thiobarbituric acid, and 9% acetic acid). After heating and centrifugation, the supernatant was collected and its fluorescence was measured at 530 nm excitation and 590 nm emission with a fluorescence microplate reader (SpectraMax Gemini; Molecular Devices LLC, Sunnyvale, CA). The results were normalized with protein concentration, which was measured with the bicinchoninic acid protein (BCA) assay kit (Thermo Fisher Scientific, MA, USA).
Immunoblotting
Retinal protein extraction, SDS-PAGE and an immunoblot analysis were performed as described previously.22 Briefly, membranes were incubated in a blocking buffer containing rabbit anti-α-fodrin antibody (Abcam 1:2000) at room temperature for 1 h. The membranes were then incubated with HRP-conjugated anti-rabbit IgG (dilution 1:5000; Sigma), immunoreactive bands were developed with ECL prime (GE Healthcare, Life Sciences) and the bands were examined with ChemiDoc XRS+ (Bio-rad). As an internal control, membranes were incubated with rabbit anti-β-actin antibody (dilution 1:1000; Sigma) at 4° C overnight. The density of the immunoreactive bands was then determined with a digital scanner and Image J software.
Cell Counting with a TUNEL Assay
Apoptotic cells were identified with TdT-mediated dUTP nick end-labeling (TUNEL) as previously described.23 Briefly, 24 hrs after NMDA injection, the retinas were fixed, cryoprotected, and mounted onto slides. After washes with PBS, the sections were incubated with TdT enzyme. Then, the sections were incubated with rhodamine-conjugated anti-digoxigenin antibody. Slides containing the sections were shielded with Vectashield mounting medium with DAPI (Vector Laboratories). Photomicrographs of whole retinal sections were taken with a microscope (BZ-9000; Keyence). In detail, we took pictures of four sections from each eye and chose one section that passed through the optic nerve while keeping the retinal structure. Cell counting of TUNEL-positive cells in the inner nuclear layer (INL) was then performed. The number of immunopositive cells was normalized and expressed as the average for each 1-mm length as our previous method.20
Immunohistochemistry and Cell Counting
To identify RGCs, we used an antibody-recognizing RNA-binding protein with multiple splicing (RBPMS), which is a member of the RNA recognition motif family of RNA-binding proteins and is known as a selective marker of retinal ganglion cells.24,25 Immunohistochemical analysis was performed as previously described.19 Cryosections were made and blocked with blocking buffer (10% donkey serum in Tw-PBS) at room temperature for 1 h. The sections were then incubated with a primary antibody against RBPMS (Abcam, 1:200) at 4° C overnight. The sections were washed with Tw-PBS and incubated with Alexa Fluor 488 conjugated goat anti-rabbit IgG antibody (Invitrogen, 1:500) in blocking buffer at room temperature for 1 h. The sections were mounted on Vectashield mounting media with DAPI (Vector Laboratories, Burlingame, CA) and photographs of whole retinal sections were taken with a microscope (BZ-9000; Keyence). Cell counting of RPBMS-positive RGCs in the ganglion cell layer (GCL) was performed in whole retinal sections. The number of immunopositive cells was normalized and expressed as the average for each 1-mm length.
Statistical Analysis
All statistical analyses used JMP Pro 12 software for Windows (SAS Institute Inc.). All continuous variables were expressed as means ± standard deviation. Comparisons used a one-way ANOVA followed by the Student’s t-test or Dunnett’s test, with Bonferroni correction for multiple comparisons. P < 0.05 was considered statistically significant (*).
Results
Oral Supplementation Attenuated NMDA-Induced Lipid Peroxidation in the Retina
To determine whether our newly developed antioxidant supplement could ameliorate NMDA-induced oxidative stress, we performed TBARS assays of retinas extracted from experimental and control mice that had undergone the intravitreal administration of NMDA after receiving a diet including or not including our oral supplement. The TBARS assay allowed us to quantify the amount of MDA in the retinas. We found that the NMDA-injured retinas that received the high- or low-dose supplemented diet showed a lower level of MDA than the controls (controls: 1.26 ± 0.26 nmol/mg protein, low-dose supplement: 0.02 ± 0.03 nmol/mg protein, and high-dose supplement: 0.26 ± 0.21 nmol/mg protein; Figure 1). Thus, oral supplementation attenuated NMDA-induced oxidative stress.
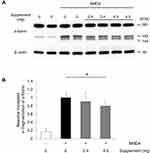
Oral Supplementation Inhibited Calpain Activation After NMDA Injury in the Retina
Previous studies have shown that RGC death after NMDA injury results from excess calpain activation and oxidative stress, and that the intravitreous injection of hesperidin can suppress these effects.19 To determine whether oral supplementation could also suppress excess calpain activation, we used an immunoblot analysis to measure the fragmentation of α-fodrin, an endogenous substrate of calpain, in the retinas of NMDA-injured mice that received a low- or high-dose supplement or a non-supplemented diet. We found that 6 hours after NMDA injection, the level of cleaved α-fodrin (both the 145 kDa and 150 kDa fragments) was not significantly lower in the animals that received the low-dose supplement than in those that received the non-supplemented diet, but was about 20% lower in the animals that received the high-dose supplement (Figure 2A, and B). These findings show that oral supplementation contributed to the suppression of calpain activation after NMDA injury in the mouse retinas.
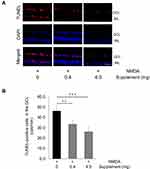
Oral Supplementation Prevented the Loss of RBPMS-Positive Cells After NMDA Treatment in the Retina
To determine whether oral supplementation prevented apoptosis after NMDA injury in the retina, we performed a TUNEL assay on retinal sections taken from NMDA-injured mice that received the supplemented diets. We found that 24 hours after NMDA injury, the number of TUNEL-positive cells in the GCL layer was higher in the non-supplemented diet group than the other groups (non-supplemented group: 46.2 ± 5.2 cells/mm, low-dose supplement group: 33.6 ± 4.2 cells/mm, high-dose supplement group: 26.1 ± 5.4 cells/mm). Thus, the increase in TUNEL-positive cells after NMDA injury was attenuated in animals that received the supplemented diet (Figure 3A and B).
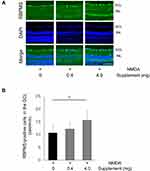
To further evaluate the survival of the RGCs, we performed RBPMS immunostaining. The number of RBPMS-positive RGCs was lower in the non-supplemented diet group and low-dose supplement group than the high-dose supplement group (10.7 ± 3.4, 12.2 ± 2.3, and 15.6 ± 4.0 cells/mm, respectively) (Figure 4A and B). This finding suggests that oral supplementation prevented RGC death after NMDA-induced retinal injury.
Discussion
In the present study, we show that NMDA-induced lipid peroxidation and the number of TUNEL-positive cells in the retina decreased in mice that received a low-dose or high-dose oral supplement containing three plant-derived compounds with a strong antioxidant effect: hesperidin, crocetin, and Tamarindus indica. Furthermore, animals that received a high dose of the supplement also showed an increase in the number of RBPMS-positive cells. These results suggest that our novel oral supplement was effective in suppressing cell death in the retina of mice under conditions of experimentally induced high oxidative stress.
We found that lowering lipid peroxide in retinal cells with our supplement was associated with a neuroprotective effect in these cells. This reinforces other recent studies of the role of oxidative stress in RGC damage, which have shown that oxidative stress-associated damage causes RGC death,26 and is associated with RGC death in mice that were subjected to IOP-dependent retinal injury,27 excitotoxicity-induced retinal injury,10 and axonal degeneration.11 Human trials have also suggested that oxidative stress plays a role in glaucoma pathogenesis. For example, relationships have been observed between the level of oxidative DNA damage, IOP increases, and the severity of visual field defects in glaucoma patients.28 Systemic levels of oxidative stress, represented by skin autofluorescence (SAF), have been shown to be associated with mean deviation in patients with open-angle glaucoma.29 Systemic oxidative stress is also associated with glaucomatous damage in relatively young male patients.30 These results suggest that antioxidants should have a neuroprotective effect. Indeed, in mice with ocular hypertension, Tempol, a multifunctional antioxidant, had anti-inflammatory effects in the retina and optic nerve.31 Additionally, coenzyme Q10 has been shown to inhibit oxidative stress and confer neuroprotection in DBA/2J mice.32 Human trials have shown an association between glaucoma risk and a low intake of green vegetables,33 and extract of Ginkgo biloba, a nitric oxide scavenger, has a neuroprotective effect in some glaucoma patients.34 Therefore, many previous reports suggest that antioxidants may have potential as a therapeutic treatment for glaucoma. Our current study also demonstrates that oral supplementation with antioxidants can reduce retinal lipid oxidation and prevent retinal apoptotic cell death, so we consider that our supplement may also have potential as a therapeutic drug in clinical practice.
In the present study, we found that a reduction in RBPMS-positive cells and excessive calpain activation were not suppressed by a low-dose antioxidant supplement, but were suppressed by a high-dose supplement. Thus, for RGCs, the neuroprotective effect of the high-dose supplement might have acted by suppressing the activation of calpain. Calpain is a member of the Ca2+-activated cysteine protease family, and calpain activation increases the level of intracellular calcium via the Ca2+-gated ion channels. Calpain is associated with fundamental cellular events, including cell motility, differentiation, proliferation, and apoptosis.35 Previous studies have demonstrated that the overactivation of calpain occurs in RGC death.36–40 Therefore, inhibiting calpain overactivation might contribute to protecting the RGCs. In the current study, a low dose of the supplement appeared to produce a greater antioxidant effect than a high dose, but only the high dose induced a protective effect in the RGCs. This finding suggests that antioxidant activity is not the main neuroprotective mechanism. One possibility is that inflammation and endoplasmic reticulum (ER) stress may be involved. In our past study, we found that anti-inflammation and ER-stress blockade had a synergistic neuroprotective effect against NMDA injury in mice.20 Hesperidin suppressed the expression of inflammatory cytokines, such as TNFα, IL-1b, and IL-6, and MCP-1 after NMDA-induced excitotoxicity in the retinas of mice.20 Crocetin also suppressed ER-stress related proteins and prevented retinal cell damage.41 These studies suggest that our antioxidant supplement may also have a neuroprotective effect that acts via anti-neuroinflammatory and anti-ER stress signaling. In addition, recent studies have demonstrated that NMDA-induced injury is associated with nitrosative stress42 and that hesperidin can ameliorate aluminum-induced neurotoxicity by suppressing nitrosative stress.43 This neuroprotective mechanism may also have contributed to the effects we observed with our supplement treatment. Moreover, hesperidin shows an affinity for the NMDA receptor and prevents pentylenetetrazole-induced convulsions.44 Citrus aurantium extracts, including hesperidin, reduce glutamate binding with NMDA receptors.45 These findings suggest that hesperidin acts as an antagonist to the NMDA receptors and directly blocks the downstream signal. These neuroprotective mechanisms may also have contributed to RGC protection in our model.
Past studies have shown that the number of RGCs and amacrine cells declines in the ganglion cell layer after NMDA injury.46 Here, we chose to examine the effects of oral supplementation with three compounds (hesperidin, crocetin, and Tamarindus indica) that have been found to improve cell viability in retinal cells in vitro.19 Each of these compounds has been reported to have distinct pharmacological activities. First, hesperidin acts as an antioxidant,47 and has been shown to lower intracellular calcium (II) and reduce ROS level.48 Our previous work also showed that intravitreal injection of hesperidin attenuated lipid peroxidation after NMDA injury.19 Second, crocetin has been shown to possess the pharmacological action of countering oxidative stress by directly scavenging ROS,49 and can suppress atherosclerosis.50 Finally, Tamarindus indica has also been reported to have antioxidant potential, and to enhance antioxidant enzyme activities in HepG2 cells.51 Thus, past studies suggest that each compound acts as an antioxidant via different pathways. In the current study, we only investigated the effect of supplementation against oxidative stress by measuring MDA, and obtained results that we have previously shown for hesperidin alone.19 Determining if the three compounds in our new supplement have a synergistic neuroprotective effect will require further experiments.
In conclusion, the results of this study indicate that our newly developed oral supplement can prevent the death of retinal cells, including the RGCs, after the induction of excitotoxicity with NMDA in mice. We obtained evidence that the mechanism of this protective effect involves the suppression of ROS generation and the inhibition of calpain activation. Excitotoxic damage is believed to be one of the causes of glaucomatous neuropathy. In fact, elevated glutamate levels have been found in the vitreous body of eyes with glaucoma in humans and monkeys.52 Moreover, glutamate metabolism is involved with several ocular pathologies, such as diabetic retinopathy.53 Recently, we performed a clinical study that revealed that an antioxidant supplement including hesperidin, crocetin, and Tamarindus indica improved antioxidant levels in the blood of glaucoma patients with high oxidative stress.54 Therefore, dietary supplementation with plant containing hesperidin, crocetin, or Tamarindus indica has potential as a new therapeutic approach to protect against retinal damage associated with excitotoxic injury in diseases such as glaucoma and diabetic retinopathy.
Acknowledgment
The authors thank Tim Hilts for reviewing and editing the language of the manuscript, Junko Sato, Kanako Sakai, Mayumi Suda, Eriko Kamii and Amane Fujioka for their technical assistance. We also thank the Biomedical Research Unit of Tohoku University Hospital and the Biomedical Research Core of Tohoku University Graduate School of Medicine for technical support. This study was supported in part by JSPS KAKENHI Grants-in-Aid for Scientific Research (K.S. 26893019).
Disclosure
The authors declare that they have no competing interests in this work.
References
1. Quigley HA. Number of people with glaucoma worldwide. Br J Ophthalmol. 1996;80(5):389–393. doi:10.1136/bjo.80.5.389
2. Weinreb RN, Tee Khaw P. Primary open-angle glaucoma. Lancet. 2004;363(9422):1711–1720. doi:10.1016/S0140-6736(04)16257-0
3. Yokoyama Y, Maruyama K, Konno H, et al. Characteristics of patients with primary open angle glaucoma and normal tension glaucoma at a university hospital: a cross-sectional retrospective study. BMC Res Notes. 2015;8(1):1–8. doi:10.1186/s13104-015-1339-x
4. Nakazawa T. Ocular blood flow and influencing factors for glaucoma. Asia Pacific J Ophthalmol. 2016;5(1):38–44. doi:10.1097/APO.0000000000000183
5. Tanito M, Kaidzu S, Takai Y, Ohira A. Status of Systemic Oxidative Stresses in Patients with Primary Open-Angle Glaucoma and Pseudoexfoliation Syndrome. PLoS One. 2012;7(11):1–7. doi:10.1371/journal.pone.0049680
6. Himori N, Kunikata H, Shiga Y, et al. The association between systemic oxidative stress and ocular blood flow in patients with normal-tension glaucoma. Graefe’s Arch Clin Exp Ophthalmol. 2016;254(2):333–341. doi:10.1007/s00417-015-3203-z
7. Alvarado J, Murphy C, Polansky J, Juster R. Age-related changes in trabecular meshwork cellularity. Investig Ophthalmol Vis Sci. 1981;21(5):714–727.
8. Ferreira SM, Lerner SF, Brunzini R, Evelson PA, Llesuy SF. Oxidative stress markers in aqueous humor of glaucoma patients. Am J Ophthalmol. 2004;137(1):62–69. doi:10.1016/S0002-9394(03)00788-8
9. Harada T, Harada C, Nakamura K, et al. The potential role of glutamate transporters in the pathogenesis of normal tension glaucoma. J Clin Invest. 2007;117(7):1763–1770. doi:10.1172/JCI30178
10. Inokuchi Y, Imai S, Nakajima Y, et al. Edaravone, a free radical scavenger, protects against retinal damage in vitro and in vivo. J Pharmacol Exp Ther. 2009;329(2):687–698. doi:10.1124/jpet.108.148676
11. Noro T, Namekata K, Kimura A, et al. Spermidine promotes retinal ganglion cell survival and optic nerve regeneration in adult mice following optic nerve injury. Cell Death Dis. 2015;6(4):1–9. doi:10.1038/cddis.2015.93
12. Kanamori A, Catrinescu MM, Mahammed A, Gross Z, Levin LA. Neuroprotection against superoxide anion radical by metallocorroles in cellular and murine models of optic neuropathy. J Neurochem. 2010;114(2):488–498. doi:10.1111/j.1471-4159.2010.06781.x
13. Levkovitch-Verbin H, Harris-Cerruti C, Groner Y, Wheeler LA, Schwartz M, Yoles E. RGC death in mice after optic nerve crush injury: oxidative stress and neuroprotection. Investig Ophthalmol Vis Sci. 2000;41(13):4169–4174.
14. Himori N, Yamamoto K, Maruyama K, et al. Critical role of Nrf2 in oxidative stress-induced retinal ganglion cell death. J Neurochem. 2013;127(5):669–680. doi:10.1111/jnc.12325
15. Coyle JT, Puttfarcken P. Puttfarcken JTC and P. Oxidative Stress, Glutamate, and Neurodegenerative Disorders. Science. 1993;262(5134):689–695. doi:10.1126/science.7901908
16. Yasuda M, Shimura M, Kunikata H, et al. Relationship of skin autofluorescence to severity of retinopathy in type 2 diabetes. Curr Eye Res. 2015;40(3):338–345. doi:10.3109/02713683.2014.918152
17. Hashimoto K, Kunikata H, Yasuda M, et al. The relationship between advanced glycation end products and ocular circulation in type 2 diabetes. J Diabetes Complications. 2016;30(7):1371–1377. doi:10.1016/j.jdiacomp.2016.04.024
18. Smith W, Mitchell P, Webb K, Leeder SR. Dietary antioxidants and age-related maculopathy: the Blue Mountains Eye Study. Ophthalmology. 1999;106(4):761–767. doi:10.1016/S0161-6420(99)90164-1
19. Maekawa S, Sato K, Fujita K, et al. The neuroprotective effect of hesperidin in NMDA-induced retinal injury acts by suppressing oxidative stress and excessive calpain activation. Sci Rep. 2017;7(1):1–13. doi:10.1038/s41598-017-06969-4
20. Sato K, Sato T, Ohno-Oishi M, et al. CHOP Deletion and Anti-Neuroinflammation Treatment With Hesperidin Synergistically Attenuate NMDA Retinal Injury in Mice. Exp Eye Res. 2021;213((February):108826):108826. doi:10.1016/j.exer.2021.108826
21. Yamamoto K, Maruyama K, Himori N, et al. The novel Rho kinase (ROCK) inhibitor K-115: a new candidate drug for neuroprotective treatment in glaucoma. Invest Ophthalmol Vis Sci. 2014;55(11):7126–7136. doi:10.1167/iovs.13-13842
22. Sato K, Li S, Gordon WC, et al. Receptor interacting protein kinase-mediated necrosis contributes to cone and rod photoreceptor degeneration in the retina lacking interphotoreceptor retinoid-binding protein. J Neurosci. 2013;33(44):17458–17468. doi:10.1523/JNEUROSCI.1380-13.2013
23. Sato K, Shiga Y, Nakagawa Y, et al. Ecel1 knockdown with an AAV2-mediated CRISPR/Cas9 system promotes optic nerve damage-induced RGC death in the mouse retina. Investig Ophthalmol Vis Sci. 2018;59(10):3943–3951. doi:10.1167/iovs.18-23784
24. Kwong JMK, Caprioli J, Piri N. RNA binding protein with multiple splicing: a new marker for retinal ganglion cells. Investig Ophthalmol Vis Sci. 2010;51(2):1052–1058. doi:10.1167/iovs.09-4098
25. Rodriguez AR, de Sevilla Müller LP, Brecha NC. The RNA binding protein RBPMS is a selective marker of ganglion cells in the mammalian retina. J Comp Neurol. 2014;522(6):1411–1443. doi:10.1002/cne.23521
26. Yokoyama Y, Maruyama K, Yamamoto K, et al. The role of calpain in an in vivo model of oxidative stress-induced retinal ganglion cell damage. Biochem Biophys Res Commun. 2014;451(4):510–515. doi:10.1016/j.bbrc.2014.08.009
27. Shareef S, Sawada A, Neufeld AH. Isoforms of nitric oxide synthase in the optic nerves of rat eyes with chronic moderately elevated intraocular pressure. Investig Ophthalmol Vis Sci. 1999;40(12):2884–2891.
28. Izzotti A, Bagnis A, Saccà SC. The role of oxidative stress in glaucoma. Mutat Res. 2006;612(2):105–114. doi:10.1016/j.mrrev.2005.11.001
29. Himori N, Kunikata H, Kawasaki R, et al. The association between skin autofluorescence and mean deviation in patients with open-angle glaucoma. Br J Ophthalmol. 2017;101(2):233–238. doi:10.1136/bjophthalmol-2016-309504
30. Asano Y, Himori N, Kunikata H, et al. Age- and sex-dependency of the association between systemic antioxidant potential and glaucomatous damage. Sci Rep. 2017;7(1):1–8. doi:10.1038/s41598-017-08624-4
31. Yang X, Hondur G, Tezel G. Antioxidant treatment limits neuroinflammation in experimental glaucoma. Investig Ophthalmol Vis Sci. 2016;57(4):2344–2354. doi:10.1167/IOVS.16-19153
32. Lee D, Shim MS, Kim KY, et al. Coenzyme Q10 inhibits glutamate excitotoxicity and oxidative stress-mediated mitochondrial alteration in a mouse model of glaucoma. Investig Ophthalmol Vis Sci. 2014;55(2):993–1005. doi:10.1167/iovs.13-12564
33. Kang JH, Willett WC, Rosner BA, Buys E, Wiggs JL, Pasquale LR. Association of dietary nitrate intake with primary open-angle glaucoma: a prospective analysis from the nurses’ health study and health professionals follow-up study. JAMA Ophthalmol. 2016;134(3):294–303. doi:10.1001/jamaophthalmol.2015.5601
34. Lee J, Sohn SW, Kee C. Effect of ginkgo biloba extract on visual field progression in normal tension glaucoma. J Glaucoma. 2013;22(9):780–784. doi:10.1097/IJG.0b013e3182595075
35. Wu HY, Tomizawa K, Oda Y, et al. Critical Role of Calpain-mediated Cleavage of Calcineurin in Excitotoxic Neurodegeneration. J Biol Chem. 2004;279(6):4929–4940. doi:10.1074/jbc.M309767200
36. Sakamoto Y, Nakajima T, Fukiage C, et al. Involvement of calpain isoforms in ischemia-reperfusion injury in rat retina. Curr Eye Res. 2000;21(1):571–580. doi:10.1076/0271-3683(200007)2111-ZFT571
37. McKernan DP, Guerin MB, O’Brien CJ, Cotter TG. A key role for calpains in retinal ganglion cell death. Investig Ophthalmol Vis Sci. 2007;48(12):5420–5430. doi:10.1167/iovs.07-0287
38. Sanvicens N, Cotter TG. Ceramide is the key mediator of oxidative stress-induced apoptosis in retinal photoreceptor cells. J Neurochem. 2006;98(5):1432–1444. doi:10.1111/j.1471-4159.2006.03977.x
39. Chiu K, Tim TL, Li WWY, Caprioli J, Kwong JMK. Calpain and N-methyl-D-aspartate (NMDA)-induced excitotoxicity in rat retinas. Brain Res. 2005;1046(1–2):207–215. doi:10.1016/j.brainres.2005.04.016
40. Volbracht C, Chua BT, Ng CP, Bahr BA, Hong W, Li P. The critical role of calpain versus caspase activation in excitotoxic injury induced by nitric oxide. J Neurochem. 2005;93(5):1280–1292. doi:10.1111/j.1471-4159.2005.03122.x
41. Yamauchi M, Tsuruma K, Imai S, et al. Crocetin prevents retinal degeneration induced by oxidative and endoplasmic reticulum stresses via inhibition of caspase activity. Eur J Pharmacol. 2011;650(1):110–119. doi:10.1016/j.ejphar.2010.09.081
42. Mohamad MHN, Abu IF, Fazel MF, et al. Neuroprotection Against NMDA-Induced Retinal Damage by Philanthotoxin-343 Involves Reduced Nitrosative Stress. Front Pharmacol. 2021;12(December):1–11. doi:10.3389/fphar.2021.798794
43. Jangra A, Kasbe P, Pandey SN, et al. Hesperidin and Silibinin Ameliorate Aluminum-Induced Neurotoxicity: modulation of Antioxidants and Inflammatory Cytokines Level in Mice Hippocampus. Biol Trace Elem Res. 2015;168(2):462–471. doi:10.1007/s12011-015-0375-7
44. Sharma P, Kumari S, Sharma J, Purohit R, Singh D. Hesperidin Interacts With CREB-BDNF Signaling Pathway to Suppress Pentylenetetrazole-Induced Convulsions in Zebrafish. Front Pharmacol. 2021;11:
45. Rosa-Falero C, Torres-Rodríguez S, Jordán CC, et al. Modulation of PTZ induced seizures by Citrus aurantium in zebrafish: role of NMDA and metabotropic glutamate receptors. Front Pharmacol. 2014;5:
46. Kuehn S, Rodust C, Stute G, et al. Concentration-Dependent Inner Retina Layer Damage and Optic Nerve Degeneration in a NMDA Model. J Mol Neurosci. 2017;63(3–4):283–299. doi:10.1007/s12031-017-0978-x
47. Menze ET, Tadros MG, Abdel-Tawab AM, Khalifa AE. Potential neuroprotective effects of hesperidin on 3-nitropropionic acid-induced neurotoxicity in rats. Neurotoxicology. 2012;33(5):1265–1275. doi:10.1016/j.neuro.2012.07.007
48. See-Lok H, Poon C-Y, Lin C, Yan T. Inhibition of β-amyloid Aggregation By Albiflorin, Aloeemodin And Neohesperidin And Their Neuroprotective Effect On Primary Hippocampal Cells Against β-amyloid Induced Toxicity. Curr Alzheimer Res. 2015;12(5):424–433. doi:10.2174/1567205012666150504144919
49. Ohba T, Ishisaka M, Tsujii S, et al. Crocetin protects ultraviolet A-induced oxidative stress and cell death in skin in vitro and in vivo. Eur J Pharmacol. 2016;789:244–253. doi:10.1016/j.ejphar.2016.07.036
50. Zheng S, Qian Z, Sheng L, Wen N. Crocetin attenuates atherosclerosis in hyperlipidemic rabbits through inhibition of LDL oxidation. J Cardiovasc Pharmacol. 2006;47(1):70–76. doi:10.1097/01.fjc.0000194686.11712.02
51. Razali N, Aziz AA, Lim CY, Junit SM. Investigation into the effects of antioxidant-rich extract of Tamarindus indica leaf on antioxidant enzyme activities, oxidative stress and gene expression profiles in HepG2 cells. PeerJ. 2015;2015(10):865. doi:10.7717/peerj.1292
52. Dreyer EB, Zurakowski D, Schumer RA, Podos SM, Lipton SA. Elevated glutamate levels in the vitreous body of humans and monkeys with glaucoma. Arch Ophthalmol. 1996;114:299. doi:10.1001/archopht.1996.01100130295012
53. Jin H, Zhu B, Liu X, Jin J, Zou H. Metabolic characterization of diabetic retinopathy: an 1H-NMR-based metabolomic approach using human aqueous humor. J Pharm Biomed Anal. 2019;174:414–421. doi:10.1016/j.jpba.2019.06.013
54. Himori N, Yanagimachi MI, Omodaka K, et al. The effect of dietary antioxidant supplementation in patients with glaucoma. Clin Ophthalmol. 2021;15:2293–2300. doi:10.2147/OPTH.S314288
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.