Back to Journals » Clinical Ophthalmology » Volume 16
A Pilot Study of Subclinical Non-Capillary Peripapillary Perfusion Changes in Thyroid-Related Orbitopathy Detected Using Optical Coherence Tomography Angiography
Authors Pinhas A , Andrade Romo JS, Lynch G, Zhou DB , Castanos Toral MV , Tenzel PA, Otero-Marquez O, Yakubova S, Barash A, Della Rocca D, Della Rocca R, Chui TYP, Rosen RB, Reddy HS
Received 31 December 2021
Accepted for publication 25 February 2022
Published 20 March 2022 Volume 2022:16 Pages 867—875
DOI https://doi.org/10.2147/OPTH.S356631
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Alexander Pinhas,1,2 Jorge S Andrade Romo,1 Giselle Lynch,1,3 Davis B Zhou,1,3 Maria V Castanos Toral,1 Phillip A Tenzel,1 Oscar Otero-Marquez,1 Shoshana Yakubova,1,4 Alexander Barash,1 David Della Rocca,1 Robert Della Rocca,1 Toco YP Chui,1 Richard B Rosen,1 Harsha S Reddy1
1Department of Ophthalmology, New York Eye and Ear Infirmary of Mount Sinai, New York, NY, USA; 2Department of Ophthalmology, State University of New York Downstate Medical Center, Brooklyn, NY, USA; 3Department of Ophthalmology, Icahn School of Medicine at Mount Sinai, New York, NY, USA; 4Department of Biology, Macaulay Honors College at City University of New York Queens College, Flushing, NY, USA
Correspondence: Harsha S Reddy, Department of Ophthalmology, New York Eye and Ear Infirmary of Mount Sinai, 310 E 14th St, New York, NY, 10003, USA, Tel +1 212-979-4284, Fax +1 212-966-6295, Email [email protected]
Purpose: Hemodynamic changes surrounding the optic nerve head are known to occur in thyroid-related orbitopathy (TRO). This pilot study explores the capillary and non-capillary peripapillary perfusion changes of the retina in TRO eyes without dysthyroid optic neuropathy (DON) using optical coherence tomography angiography (OCT-A).
Methods: Non-capillary and capillary peripapillary perfusion densities were calculated using single 4.5 × 4.5mm en face “RPC layer” OCT-A scans of 8 TRO patients without DON (8 eyes, mean age 40.6 years, range 23– 69 years). Results were compared to a previously published dataset of 133 healthy controls (133 eyes, mean 41.5 years, range 11– 83 years). The strength of association was measured between OCT-A perfusion densities and clinical measures of TRO.
Results: Non-capillary peripapillary perfusion density in TRO eyes was found to be significantly decreased compared to healthy controls (TRO group 15.4 ± 2.9% vs controls 21.5 ± 3.1%; p < 0.0001). Capillary peripapillary perfusion densities showed no significant difference (TRO group 42.5 ± 1.8% vs controls 42.5 ± 1.5%; p = 1.0). Clinical measures of disease did not correlate well with OCT-A perfusion densities (p> 0.05).
Conclusion: These findings may represent decreased blood flow and subclinical ischemia to the optic nerve. We discuss possible pathogenic mechanisms of thyroid-related vasculopathy, including vessel wall thickening due to immunologically-induced media enlargement.
Keywords: thyroid-related orbitopathy, optical coherence tomography angiography, peripapillary microvasculature, thyroid-related vasculopathy
A Letter to the Editor has been published for this article.
Introduction
Thyroid-related orbitopathy (TRO) is part of a complex systemic immunological process associated primarily with Graves’ hyperthyroidism, but can be present in hypothyroid and euthyroid states, as well as in other thyroid diseases.1 TRO involves cross-reacting immunoglobulins that can cause inflammation and stimulate orbital fibroblasts, inducing glycosaminoglycan deposition, extraocular muscle enlargement, orbital fat proliferation, accumulation of intra- and extra-cellular fluid, and fibrosis.2
Dysthyroid optic neuropathy (DON), occurring in about 4–8% of TRO cases, is the most serious vision-threatening complication,3 and is preventable and potentially reversible if detected early.4 A number of possible mechanisms have been proposed in DON. Anatomic crowding from muscle enlargement at the annulus of Zinn in the orbital apex may lead to compression of the optic nerve,5 causing axoplasmic stasis and decreased axonal functionality. Orbital compartment syndrome may also lead to vascular compression, causing optic nerve ischemia. Other potential causes of optic nerve injury may include stretching of the optic nerve by proptosis, and direct inflammation-mediated injury to the nerve.6
Recent studies have sought to understand the pathogenesis of early DON to identify patients most at risk of vision loss. Radiographic indices of apical extraocular muscle enlargement and relative muscle position to the optic nerve have been proven to be predictive of DON development.7 TRO patients without clinically evident DON (meaning no changes to visual acuity, color vision, visual field, optic nerve head appearance or retinal nerve fiber layer thickness) demonstrate loss of contrast sensitivity,8 changes in visual evoked potentials (VEPs),9 reduced P50 amplitudes on pattern electroretinography (pERG),10 and have decreased diffusivities and increased fractional anisotropy on MRI diffusion-tensor imaging (DFI).11 These findings suggest the presence of a subclinical optic neuropathy in TRO.
Although DON pathogenesis is thought to involve hemodynamic changes, limitations in technology have prevented easy and reproducible imaging of blood flow through the optic nerve and around the optic nerve head. Color Doppler ultrasound has revealed delayed forward flow in the superior ophthalmic vein (SOV) in TRO, which is considered a surrogate for altered flow through the optic nerve, predictive of DON development.12 Recent advances in retinal imaging, namely optical coherence tomography angiography (OCT-A), have allowed in vivo visualization and quantification of the peripapillary blood flow in humans at a level previously achievable only with histology.13
OCT-A uses moving red blood cells as intrinsic “contrast” as they course through blood vessels.14 Using a variety of decorrelation algorithms, OCT-A is able to accurately and non-invasively depict the perfusion of blood vessels within discrete retinal layers using the en face perspective.15 Zhang et al recently reported that TRO eyes without DON demonstrated significantly decreased peripapillary OCT-A perfused vessel density compared to healthy control eyes.16 The authors found a further significant decrease in peripapillary perfused vessel density in TRO eyes with DON and concluded that the perfused vessel density decrease in TRO without DON may be an early predictor of progression to DON. Notably, this study did not separate non-capillary (arteriole and venule) blood vessels from capillaries in its analysis.
Despite their numerous similarities, including a shared endothelial cell layer and involvement in metabolic exchange with surrounding tissues, retinal capillary and non-capillary blood vessels have some important differences. Retinal non-capillary blood vessels are generally larger, ranging in size from approximately 20 to 200 microns. In addition to an intimal layer composed of endothelial cells, non-capillary blood vessels have a tunica media composed of smooth muscle cells, and a tunica externa composed of loose fibrous connective tissue. The smooth muscle cells contract or relax in response to metabolic signals from local tissues, thus regulating blood flow to and from the capillary bed. Retinal capillary blood vessels, on the other hand, lack smooth muscle cells and are generally smaller, ranging in size from approximately 5 to 10 microns. Their endothelial cell layer is connected by tight junctions surrounded by pericyte cells, which have contractile properties similar to that of smooth muscle cells.
In order to better understand the pathogenesis and role of perfusion changes surrounding the optic nerve in disease, it is important to separate these two classes of blood vessels. Using algorithms to analyze non-capillary and capillary peripapillary perfusion vessel densities separately, recent studies have shown glaucomatous17 and ischemic optic neuropathies,18 as well as Alzheimers’ dementia,19 to preferentially involve the peripapillary capillaries. These studies underscore the importance of separating capillary from non-capillary blood vessels to enhance the sensitivity for detection of changes in peripapillary perfusion.20
The purpose of our pilot study was to explore, for the first time, the retinal capillary and non-capillary peripapillary perfusion changes separately in TRO patients without DON, and compare results to a previously published normative dataset on healthy controls. A commercial OCT-A system and a previously developed in-house algorithm were used.21,22
Methods
Study Population
This study was conducted at the New York Eye and Ear Infirmary of Mount Sinai. It followed the tenets of the Declaration of Helsinki, was HIPAA compliant, and was approved by the Institutional Review Board of New York Eye and Ear Infirmary of Mount Sinai. Written informed consent was obtained from the subjects after explanation of the nature and risks of the study.
This was a cross-sectional pilot study. A chart review was performed to identify TRO patients for OCT-A imaging. TRO patients without DON and a clinical activity score (CAS) of ≤ 3 were chosen. Comprehensive history and physicals were performed on the TRO patients. History intake included age, sex, past medical and ocular history, past and current medicine and eye drops, thyroid status at time of imaging, history of smoking, and duration of TRO. Eye exams included best-corrected visual acuity (BCVA), intraocular pressure (IOP) measured by Goldman applanation, dilated fundus examination, cup-to-disc ratio, and proptosis measured by Hertel exophthalmometry.
Color fundus photography (Topcon Swept Source DRI OCT Triton, Topcon Medical Systems Inc., Oakland, New Jersey), OCT circumferential retinal nerve fiber layer (cRNFL) thickness measurements (Zeiss Cirrus HD-OCT, Carl Zeiss Meditec Inc, Dublin, California; Heidelberg Spectralis HRA+OCT, Heidelberg Engineering Inc, Heidelberg, Germany), and Humphrey visual fields (HVFs) 24–2 were performed.
Image Acquisition
A commercial spectral-domain OCT-A system (Avanti RTVue-XR, Optovue, Fremont, California) was used to obtain 4.5×4.5mm en face peripapillary scans using a wavelength of 840nm and axial line rate of 70kHz. The system’s split-spectrum amplitude decorrelation angiography (SSADA) algorithm was used to map the perfused vessels for each scan.15
Image Analysis
OCT-A “RPC layer” scans were used, defined as the layer between the inner limiting membrane and the posterior boundary of the RNFL. Default axial length measurements of the system were utilized. The image processing procedures in this study were similar to previously published procedures.21,22 In brief, an original OCT-A 4.5×4.5mm “RPC layer” peripapillary scan (Figure 1A) was used for global thresholding, creating a binary image of non-capillary blood vessels (Figure 1B). Next, after the removal of the non-capillary vessel mask from the original OCT-A image (Figure 1C), local thresholding was performed to achieve capillary segmentation (Figure 1D). Next, the original OCT-A “Choroid layer” peripapillary scan was used to manually estimate the optic nerve head margin with a 1.95-mm diameter circle (Figure 1E). The algorithm then automatically drew a 3.45-mm concentric circle surrounding the 1.95-mm circle, creating a 0.75-mm-wide annular region of interest (ROI) (Figure 1F). The ROI was used for quantitative capillary and non-capillary peripapillary perfused vessel density analysis (Figure 1G and H, respectively). Capillary peripapillary perfused vessel density (%) was calculated as the capillary pixel area divided by the difference between total annular ROI pixel area and non-capillary pixel area. Non-capillary peripapillary perfused vessel density (%) was calculated as non-capillary pixel area divided by the total annular ROI pixel area.
Control Data
Previously published OCT-A data on 133 healthy controls (133 eyes, mean 41.5 years, range 11–83 years) were used for comparison.22 The healthy controls had a self-reported negative past medical history, including absence of cardiovascular disease, hypertension or diabetes mellitus. The healthy controls underwent comprehensive eye exams. Controls had to demonstrate clear media without retinal or optic nerve head pathology, confirmed by fundus photography, and OCT cRNFL and macula scans.
Statistical Analysis
Results were presented as average ± standard deviation (SD). Unpaired two-tailed t-testing was performed to detect differences in perfusion densities between TRO without DON eyes and healthy control eyes. Pearson correlation testing was performed to measure the strength of association between OCT-A perfusion vessel densities (capillary and non-capillary) with clinical measures of disease, including BCVA, IOP, cup-to-disc ratio, proptosis, OCT cRNFL, and HVF mean deviation and pattern standard deviation. P values of less than 0.05 were considered statistically significant.
Results
Eight TRO patients (8 eyes, mean age 40.6 years, range 23–69 years) were studied (Table 1). The 8 TRO patients had a self-reported negative past medical history for systemic cardiovascular disease such as hypertension or diabetes mellitus. The patients did not have a history of thyroid radioactive iodine ablation or surgery. Thyroid function tests (TSH, free T4) showed a euthyroid state in all patients. Imaged eyes had a negative history of any ocular or orbital surgery including orbital decompression, eye muscle surgery, or eyelid surgery. If both eyes fit imaging criteria in a patient, the eye chosen for imaging was the more proptotic eye.
 |
Table 1 Patient Demographics. #, Number; Yo, Years Old; M, Male; F, Female; Yrs, Years; s/p, Status Post; ppd, Packs per Day |
The 8 TRO eyes were phakic with BCVA of at least 20/25, and IOP ranged from 15 to 26 mmHg. None of the TRO eyes showed a relative afferent pupillary defect. Color fundus photography confirmed healthy-appearing retinas and optic nerve heads in all TRO eyes. OCT cRNFL was within normal limits for all TRO eyes and showed no thinning. The HVF 24–2 exams were full or showed only minimal nonspecific defects.
Capillary and non-capillary peripapillary perfusion density data per TRO eye are presented in Table 2. Group average ± SD values are shown in Figure 2 and are compared to that of healthy controls. Capillary peripapillary perfusion densities showed no significant difference (TRO group 42.5±1.8% vs controls 42.5±1.5%; p=1.0). Non-capillary peripapillary perfusion density in TRO eyes was found to be significantly decreased compared to healthy controls (TRO group 15.4±2.9% vs controls 21.5±3.1%; p<0.0001). Pearson correlation testing showed no significant correlation between OCT-A perfusion vessel densities (capillary or non-capillary) and the clinical measures of disease (p>0.05).
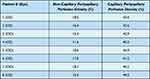 |
Table 2 Non-Capillary and Capillary Peripapillary Perfusion Density Data per Patient Eye. #, Number |
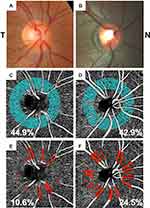
Figure 3 shows a comparison of the right eye of Patient 5 compared to that of a 24-year-old male healthy control (age-, sex- and laterality-matched). Color photos revealed normal appearing optic nerve heads in both participants (Figures 3A and B). The capillary peripapillary perfusion densities were similar between the two eyes, measuring 44.9% and 42.9%, respectively (Figures 3C and D). The non-capillary peripapillary perfusion density, however, showed a notable difference: 10.6% in the TRO eye and 24.5% in the healthy control eye (Figures 3E and F).
Discussion
In our pilot study, TRO eyes without clinically evident DON showed subclinical alterations in peripapillary hemodynamics, as measured by our OCT-A “RPC layer” analysis. Specifically, non-capillary peripapillary perfusion densities were lower in TRO eyes without DON compared to that of healthy control eyes. Notably, the observed hemodynamic changes in TRO eyes did not involve the associated peripapillary capillary beds, which showed very similar perfusion densities to healthy control eyes. Traditional clinical measures of disease did not correlate with OCT-A perfusion densities. Our results indicate the importance of separating capillary from non-capillary peripapillary vessels during analysis. Our results suggest that OCT-A may be a sensitive marker of subclinical hemodynamic alterations surrounding the optic nerve head.
A possible explanation for decreased non-capillary peripapillary perfusion densities may be due to decreased vessel diameter from intra-orbital compression of the central retinal blood vessels. However, compression near the annulus of Zinn in the setting of enlarged extraocular muscles would also likely produce visual field changes.23 Moreover, intra-orbital pressure would need to exceed intra-arterial pressure before arterial compression would occur. Therefore, we do not believe that arterial compression can fully explain our findings in a study population that had full visual fields and relatively mild proptosis. Another unlikely explanation is the stretching or pulling of the optic nerve and the central retinal blood vessels in the setting of severe proptosis. The intra-orbital optic nerve and associated vessels have considerable slack protecting them from becoming taut with mild to moderate proptosis.
A decreased non-capillary peripapillary perfusion density could result from thickening of the vessel wall. Hypothyroidism has been associated with a worse cardiovascular risk factor profile, including hypertension, hyperlipidemia, and hyper-homocysteinemia, leading to accelerated progression of atherosclerosis. Subclinical hypothyroidism has been shown to be associated with higher carotid intima-media thickness, and is thought to be secondary to subclinical atherosclerosis.24 Hyperthyroidism has been associated with atrial fibrillation and cardioembolic disease, as well as acute cerebral venous thrombosis, Moyamoya disease, and giant cell arteritis.25 Some degree of contribution from these factors to the observed vasculopathy in our patients is possible. Still, these factors do not explain the sparing of the associated capillary bed.
A possible explanation for our observations may relate to the smooth muscle cells, since they are present in non-capillary blood vessels but are absent in capillaries. In TRO, orbital fibroblasts upregulate transforming growth factor (TGF)-β, a potent soluble multifunctional cytokine involved in cell differentiation, proliferation, survival and apoptosis.26 TGF-β is well known to induce myofibroblast differentiation and proliferation and to promote hyaluronan synthesis in TRO, leading to extraocular muscle hypertrophy and interstitial edema.2 TGF-β may also be acting on vascular smooth muscle cells in TRO, as it has been shown to be able to convert normal contractile vascular smooth muscle cells to less differentiated, proliferative and migratory cell types. Furthermore, TGF-β has been shown to have the ability to convert vascular endothelial cells, vascular resident stem cells, adventitial fibroblasts and bone marrow cells into smooth muscle cells.27 Additionally, immune-complex and/or glycosaminoglycan deposition may contribute to the wall thickening of non-capillary blood vessels.
Vessel wall thickening from smooth muscle cell involvement would result in decreased perfused luminal areas of non-capillary blood vessels, and would leave the associated capillary bed unaffected, as was observed in our study. Extensive literature review did not yield prior descriptions of such a thyroid-related vasculopathy. We could not find a histological study focusing on intra-orbital vessel walls in TRO. However, we were able to find a histological study of thyroid-related pretibial myxedema that revealed a few lymphocytes within the perivascular spaces, the significance of which is unclear.28
It is possible that the detected non-capillary peripapillary perfusion changes may be responsible for ischemia to the nerve and subclinical optic neuropathy. Following Poiseuille’s law of fluidics, the flow rate through blood vessels is proportional to the vessel radius to the fourth power. Therefore, even a slight decrease in blood vessel radius could produce a significant decrease in forward flow through the non-capillary peripapillary blood vessels. Typically, larger non-capillary peripapillary blood vessels are surrounded by an avascular zone, since the tissue immediately surrounding the vessels receives its oxygen directly from diffusion through the vessel wall. A reduced intraluminal perfusion area, as was noted in our study, would thus result in a larger “avascular zone” between the circulating red blood cells and the surrounding tissues. Combined with a reduced forward flow rate secondary to decreased vessel radius, this could result in some degree of relative hypoxia in the surrounding tissue. To confirm this hypothesis, future studies will need to correlate these OCT-A findings with contrast sensitivity testing, VEP, pERG, DFI, CT, MRI and color Doppler ultrasound imaging, as well as serological markers of TRO.29
It is too early to tell whether these findings can be used to predict development of DON. Future cross-sectional studies will need to apply our OCT-A analysis methodology to TRO with DON eyes as compared to TRO without DON and healthy control eyes. Also, longitudinal studies will be needed to evaluate whether non-capillary peripapillary hypoperfusion progresses with time or is affected by clinical changes in proptosis or inflammation. It would be interesting to see if the capillary bed becomes involved in TRO eyes with DON. Such studies will allow us to determine if non-capillary peripapillary perfusion changes are predictive of DON development prior to changes in clinical measures such as color vision, visual acuity, and visual fields. Ultimately, OCT-A may prove useful in guiding management decisions, perhaps allowing clinicians to intervene earlier prior to the development of irreversible nerve damage.
Limitations in this pilot study include the small sample size of TRO patients and the cross-sectional nature of the study. An additional limitation was the reliance on self-reported cardiovascular health histories to evaluate microvascular disease or inflammation. Future studies will need to expand on the sample size, and perform more detailed systemic and laboratory evaluations. A limitation of our methodology was the potentially erroneous identification of poorly perfused non-capillary blood vessels as capillaries in TRO eyes by our algorithm, potentially causing falsely elevated capillary perfusion density readings. OCT-A relies upon moving erythrocytes within blood vessels as intrinsic “contrast,” and does not visualize vessel wall structure directly. Since part of the explanation of our findings involves the possibility of non-capillary blood vessel wall thickening, future studies will need to include imaging modalities such as structural OCT and non-confocal adaptive optics scanning light ophthalmoscopy to directly visualize vessel wall changes occurring in TRO.30
Our pilot study revealed that OCT-A non-capillary peripapillary perfusion density has potential as a surrogate marker for early detection of decreased perfusion surrounding the optic nerve head in TRO, prior to clinical evidence of DON. Advantages of OCT-A in diagnosing activity and severity of TRO versus the clinical scale scoring systems include earlier detection of compromised perfusion to the optic nerve, quantitative versus qualitative assessment, ability to grade severity of disease per eye, and ability to monitor changes after treatment. When compared to pERG, DFI, CT, MRI and color Doppler ultrasound imaging, OCT-A shows advantages in ease of clinical accessibility, quick acquisition time, good reliability, and non-invasiveness.
Acknowledgment
This material was previously presented as a poster presentation at the ARVO Imaging in the Eye Annual Conference, Vancouver, Canada in April 2019.
Funding
NEI/NIH Grant R01EY027301. The Marrus Family Foundation. The Geraldine Violett Foundation for Ophthalmic and Orbital Research.
Disclosure
Richard B. Rosen M.D. is a paid consultant of Optovue, and has received personal fees and non-financial support from Optovue, and non-financial support from Topcon Medical. In addition, Dr. Rosen has a US patent - # WO 2016/109750 Al, 43625.140US01 issued to Optovue. Outside the submitted work, Dr. Rosen is a paid consultant of Guardion Health, and has received personal fees and stock from Boehringer-Ingelheim, non-financial support from Ocusciences, and equipment from CellView. Harsha S. Reddy M.D. is a paid advisory board member for Horizon Therapeutics. The authors report no other conflicts of interest in this work.
References
1. Della Rocca RC. Thyroid-related orbitopathy: concepts and management. Facial Plastic Surgery. 2007;23(3):168–173. doi:10.1055/s-2007-984556
2. Khong JJ, McNab AA, Ebeling PR, et al. Pathogenesis of thyroid eye disease: review and update on molecular mechanisms. Br J Ophthalmol. 2016;100(1):142–150.
3. Bartley GB, Fatourechi V, Kadrmas EF, et al. The incidence of Graves’ ophthalmopathy in Olmsted County, Minnesota. Am J Ophthalmol. 1995;120(4):511–517.
4. Rajabi MT, Ojani M, Riazi Esfahani H, et al. Correlation of peripapillary nerve fiber layer thickness with visual outcomes after decompression surgery in subclinical and clinical thyroid-related compressive optic neuropathy. J Curr Ophthalmol. 2019;31(1):86–91.
5. Blandford AD, Zhang D, Chundury RV, Perry JD. Dysthyroid optic neuropathy: update on pathogenesis, diagnosis, and management. Expert Rev Ophthalmol. 2017;12(2):111–121.
6. Saeed P, Tavakoli Rad S, Bisschop PHLT. Dysthyroid Optic Neuropathy. Ophthalmic Plast Reconstr Surg. 2018;34(4SSuppl 1):S60–S7.
7. Lo C, Ugradar S, Rootman D. Management of graves myopathy: orbital imaging in thyroid-related orbitopathy. J AAPOS. 2018;22(4):
8. Ü B, Kaya S, Yeter V, Erkan D. Contrast sensitivity of thyroid associated ophthalmopathy patients without obvious optic neuropathy. ScientificWorldJournal. 2013;2013:943789.
9. Pérez-Rico C, Rodríguez-González N, Arévalo-Serrano J, Blanco R. Evaluation of multifocal visual evoked potentials in patients with Graves’ orbitopathy and subclinical optic nerve involvement. Doc Ophthalmol. 2012;125(1):11–19.
10. Przemysław P, Janusz M, Alina B, Maria G. Pattern electroretinogram (PERG) in the early diagnosis of optic nerve dysfunction in the course of Graves’ orbitopathy. Klin Oczna. 2013;115(1):9–12.
11. Lee H, Lee YH, Suh SI, et al. Characterizing Intraorbital Optic Nerve Changes on Diffusion Tensor Imaging in Thyroid Eye Disease Before Dysthyroid Optic Neuropathy. J Comput Assist Tomogr. 2018;42(2):293–298.
12. Konuk O, Onaran Z, Ozhan Oktar S, et al. Intraocular pressure and superior ophthalmic vein blood flow velocity in Graves’ orbitopathy: relation with the clinical features. Graefes Arch Clin Exp Ophthalmol. 2009;247(11):1555–1559.
13. Mo S, Phillips E, Krawitz BD, et al. Visualization of Radial Peripapillary Capillaries Using Optical Coherence Tomography Angiography: the Effect of Image Averaging. PLoS One. 2017;12(1):e0169385.
14. Spaide RF, Fujimoto JG, Waheed NK, et al. Optical coherence tomography angiography. Prog Retin Eye Res. 2018;64:1–55.
15. Jia Y, Tan O, Tokayer J, et al. Split-spectrum amplitude-decorrelation angiography with optical coherence tomography. Opt Express. 2012;20(4):4710–4725.
16. Zhang T, Xiao W, Ye H, et al. Peripapillary and Macular Vessel Density in Dysthyroid Optic Neuropathy: an Optical Coherence Tomography Angiography Study. Invest Ophthalmol Vis Sci. 2019;60(6):1863–1869.
17. Mansoori T, Sivaswamy J, Gamalapati JS, Balakrishna N. Radial Peripapillary Capillary Density Measurement Using Optical Coherence Tomography Angiography in Early Glaucoma. J Glaucoma. 2017;26(5):438–443.
18. Fard MA, Suwan Y, Moghimi S, et al. Pattern of peripapillary capillary density loss in ischemic optic neuropathy compared to that in primary open-angle glaucoma. PLoS One. 2018;13(1):e0189237.
19. Zhang YS, Zhou N, Knoll BM, et al. Parafoveal vessel loss and correlation between peripapillary vessel density and cognitive performance in amnestic mild cognitive impairment and early Alzheimer’s Disease on optical coherence tomography angiography. PLoS One. 2019;14(4):e0214685.
20. Holló G. Influence of Removing the Large Retinal Vessels-related Effect on Peripapillary Vessel Density Progression Analysis in Glaucoma. J Glaucoma. 2018;27(8):e137–e9.
21. Scripsema NK, Garcia PM, Bavier RD, et al. Optical Coherence Tomography Angiography Analysis of Perfused Peripapillary Capillaries in Primary Open-Angle Glaucoma and Normal-Tension Glaucoma. Invest Ophthalmol Vis Sci. 2016;57(9):OCT611–OCT20.
22. Pinhas A, Linderman R, Mo S, et al. A method for age-matched OCT angiography deviation mapping in the assessment of disease- related changes to the radial peripapillary capillaries. PLoS One. 2018;13(5):e0197062.
23. Choi CJ, Oropesa S, Callahan AB, et al. Patterns of visual field changes in thyroid eye disease. Orbit. 2017;36(4):201–207.
24. Peixoto de Miranda É, Bittencourt MS, Pereira AC, et al. Subclinical hypothyroidism is associated with higher carotid intima-media thickness in cross-sectional analysis of the Brazilian Longitudinal Study of Adult Health (ELSA-Brasil). Nutr Metab Cardiovasc Dis. 2016;26(10):915–921.
25. Squizzato A, Gerdes VE, Brandjes DP, et al. Thyroid diseases and cerebrovascular disease. Stroke. 2005;36(10):2302–2310.
26. Derynck R, Budi EH. Specificity, versatility, and control of TGF-β family signaling. Sci Signal. 2019;12:570.
27. Wang G, Jacquet L, Karamariti E, Xu Q. Origin and differentiation of vascular smooth muscle cells. J Physiol. 2015;593(14):3013–3030.
28. Fatourechi V. Pretibial myxedema: pathophysiology and treatment options. Am J Clin Dermatol. 2005;6(5):295–309.
29. Turck N, Eperon S, De Los Angeles Gracia M, et al. Thyroid-Associated Orbitopathy and Biomarkers: where We Are and What We Can Hope for the Future. Dis Markers. 2018;2018:7010196.
30. Chui TY, Gast TJ, Burns SA. Imaging of vascular wall fine structure in the human retina using adaptive optics scanning laser ophthalmoscopy. Invest Ophthalmol Vis Sci. 2013;54(10):7115–7124.
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.