Back to Journals » Cancer Management and Research » Volume 14
A Pilot Study of Prognostic Value of Metastatic Lymph Node Count and Size in Patients with Different Stages of Gastric Carcinoma
Authors Gao Y, Wang K, Tang XX, Niu JL, Wang J
Received 3 December 2021
Accepted for publication 13 June 2022
Published 21 June 2022 Volume 2022:14 Pages 2055—2064
DOI https://doi.org/10.2147/CMAR.S352334
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Lu-Zhe Sun
Yong Gao,1,2,* Kun Wang,3,* Xiao-Xian Tang,2 Jin-Liang Niu,4 Jun Wang1
1Department of Medical Imaging, Shanxi Medical University, Taiyuan, 030001, People’s Republic of China; 2Department of Radiology, Shanxi Provincial People’s Hospital, Taiyuan, 030012, People’s Republic of China; 3Department of Hepatopathy, Third People Hospital of Taiyuan City, Taiyuan, 030001, People’s Republic of China; 4Department of Radiology, 2nd Hospital, Shanxi Medical University, Taiyuan, 030001, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Jun Wang, Department of Medical Imaging, Shanxi Medical University, Taiyuan, 030001, People’s Republic of China, Tel +86-351-488-5199, Fax +86-351-496-0092, Email [email protected]
Background: The correlation between the preoperative lymph node count (LNC) on multidetector computed tomography (MDCT) and the prognosis of gastric carcinoma (GC) remains to be defined. This research aims to evaluate the prognostic value of LNC on MDCT in GC patients based on tumor-node-metastasis (TNM) staging, using different size criteria for counting.
Methods: The clinical data of 126 patients with gastric adenocarcinoma undergoing gastrectomy were retrospectively analyzed. Lymph nodes greater than 8mm and 5mm on MDCT were counted and recorded. The prognostic implications of LNC on MDCT for patient survival were analyzed according to different size criteria for counting and tumor TNM staging.
Results: When 8mm was used as the counting criterion, LNC on MDCT had no significant effect on the overall survival (OS) of the entire cohort. In addition, the OS of T1–T2 GC patients with LNC on MDCT ≥ 1 was significantly worse than that of patients with LNC on MDCT < 1. When 5mm was used as the counting criterion, LNC on MDCT was found to be significantly associated with the OS of the entire cohort. In the subgroup analysis, patients with relatively advanced (T3-T4, N+ and III) GC with LNC on MDCT > 7 showed a significantly worse OS than those with LNC on MDCT ≤ 7. LNC on MDCT > 7 with 5mm as the counting criterion and Stage III were independent risk factors for adverse prognosis.
Conclusion: The prognostic value of LNC on MDCT based on different size criteria varies in patients with different stages of GC. LNC of a smaller size (5mm) on MDCT may be a prognostic factor for patients with relatively advanced GC.
Keywords: gastric carcinoma, patient survival, lymph nodes, computed tomography
Introduction
Gastric carcinoma (GC) is a serious global public-health challenge, with statistics indicating 1.09 million new cases of GC and 770,000 associated deaths worldwide.1 GC is asymptomatic in the early stage, but lymph node metastasis (LNM), one of the most common clinical manifestations of GC, is usually present when the patient is diagnosed.2,3 Therefore, lymph node (LN) status is closely associated with the prognosis of GC patients.4,5 The 5-year postoperative survival rate of patients with N0 GC is 72.5%, while it decreases significantly in those with N1 (57.0%), N2 (41.6%) and N3 (23.3%) GC.6 Thus, assessing the LN status perioperatively can help determine the optimal therapeutic regimen.5,7,8
At present, a variety of imaging modalities, such as endoscopic ultrasonography (US), superparamagnetic iron oxide-enhanced magnetic resonance imaging (SPIO-MRI), multidetector computed tomography (MDCT), and positron emission tomography (PET), are used to assess LN status.9–11 Among them, MDCT has been extensively applied for the assessment of LN status such as LN involvement and count in GC patients in virtue of convenience and efficiency. A short axis diameter greater than 8mm is the most common criterion for CT diagnosis of specific metastatic LNs.11,12 However, this size-dependent criterion has its inherent limitations. Since normal-size LNs may present microscopic tumor involvement, the size of metastatic LNs is not always greater than the size defined by the MDCT lower limit. As a result, MDCT detected significantly fewer metastatic LNs than histologic examination, which may lead to an underestimation of N staging.13,14
Recently, multirow-detector CT and CT cine viewing have made it possible to find smaller LNs.15 Lymph node count (LNC) on MDCT is an easily determined parameter that is more accurate than nodal metastasis assessment.16 Previous studies have indicated that independent of LN involvement, the number of LNs removed during gastrectomy is related to the prognosis of GC patients,17,18 which led us to speculate that LNC on MDCT could provide important information about patient survival regardless of LNM. Several reports demonstrated that the greater the number of LNs on MDCT, the worse the prognosis of esophageal and rectal cancers.15,16 Therefore, LNC on MDCT may be a prognostic factor in GC patients.
LNC on MDCT differs according to the size criterion used for counting. The novelty of this study lies in its attempt to clarify whether LNC on MDCT is predictive of overall survival (OS) in patients with GC based on tumor-node-metastasis (TNM) staging, using different size criteria for counting, which may provide new options and insights into prognosis prediction of GC patients with different stages. We hereby present the following article following the guidelines laid down in the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting checklist.
Materials and Methods
Participants
This study enrolled 126 patients who underwent surgical resection for gastric adenocarcinoma at Shanxi Provincial People’s Hospital between December 2013 and November 2016. Patients were excluded if the interval between MDCT examination and surgery was more than one month, or if they received neoadjuvant chemotherapy or palliative care. All the 126 patients underwent curative gastrectomy with D1+ or D2 lymphadenectomy. Of the 126 patients, 76 patients (60.3%) received postoperative chemotherapy. Pathological staging was evaluated based on the American Joint Committee on Cancer (AJCC) TNM classification, 8th edition.19 Tumor sites were defined as either upper-, middle-, or lower-third of the stomach, and tumors were classified as well, moderately, and poorly differentiated types. Patient demographics, clinicopathological features and OS were analyzed. All patients received postoperative follow-up (mean: 45 months), which was conducted once every 3 months mainly through telephone interviews, medical record inquiries, and visits. The time from pretreatment MDCT examination to death from GC or the last follow-up was calculated as patient survival time.20 This is a retrospective study, which has been approved by the Institutional Review Board of the Shanxi Provincial People’s Hospital and was conducted in strict accordance with the Declaration of Helsinki. All patient data were kept confidential. Due to the retrospective nature, the need for informed consent of patients was waived.
MDCT Protocol
After overnight fasting, all patients underwent relevant examinations using Siemens Somatom Sensation 64-MDCT scanner (Erlangen, Germany) and 256-MDCT scanner (Philips Brilliance iCT, Philips, Cleveland, USA). Before scanning, 800–1000mL water was administered orally. First, pre-contrast imaging was performed on patients in a supine position. Then, 100mL iohexol (Omnipaque, 350, GE Healthcare), a non-ionic iodinated contrast medium, was administered through the antecubital vein at a flow rate of 3 mL/s using a pump injector (MEDRAD-Stellant, MEDRAD Company, Germany). Images were acquired 30, 75, and 180 seconds after contrast medium injection, corresponding to the arterial, portal-venous and equilibrium phase, respectively. The scanning range was from the diaphragm dome to the bilateral anterior superior iliac spine. Scanning parameters: tube voltage, 120 kV; rotation time, 0.5 s; current: automatically determined by the CT automatic exposure control system (Siemens Somatom Sensation 64-MDCT) or 300 mA (Philips Brilliance 256-MDCT); slice thickness for axial image reconstruction: 1.5 mm.
Imaging Analysis
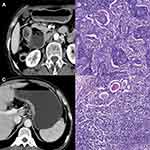
LNs were evaluated using the axial CT images acquired during the venous phase, with a window level and width of 60 and 300, respectively. The location and maximum short-axial diameter of the regional LNs were determined by consensus of two radiologists. Nodes at No. 1–12 stations on MDCT were counted and recorded by referring to the Japanese Gastric Cancer Association (JGCA), with a counting criterion of greater than 8mm and 5mm, respectively, regardless of contrast uptake (Figure 1). Diameters <5mm were rounded to 0mm in the current study. The reasons are as follows: 1. LNs smaller than 5mm can be easily misdiagnosed as vessels and other organs. 2. The larger the LN, the less the contrast loss.21 3. A maximum short axis diameter greater than 8mm is the most commonly used CT criterion for the diagnosis of specific metastatic LNs.11,12 Both radiologists were blinded to the histopathological results.
Clinicopathological Data
We divided patients with different LNC on MDCT into two groups, based on the cut-off of LNC on MDCT determined by the X-tile program (http://www.tissuearray.org/rimmlab/) – a bio-informatics tool for outcome-based cut-point optimization.22–24 Then, LNC on MDCT was converted from a continuous variable into a categorical variable in order to assess its effect on patient survival.
Statistical Analysis
Categorical variables were presented as absolute counts (percentages), and the differences were assessed using the Fisher exact test or chi-square test. Patient age was described as means (standard deviation, SD) and analyzed using the independent t-test. The number of LNs, represented by median and interquartile range (IQR), was compared using the Mann–Whitney’s U-test. Univariate and multivariate survival analyses were performed using the Kaplan–Meier method and Cox proportional hazards model, respectively, while the Log rank test was used for comparison. Significance was assumed at a two-sided p<0.05. SPSS v18.0 (SPSS Inc., Chicago, IL, USA) was used for statistical analysis.
Results
With 8mm as the counting criterion, the cut-off value of LNC on MDCT for grouping GC patients by the X-tile program was identified to be 1. Among the 126 patients enrolled, 84 patients (66.7%) had LNC on MDCT < 1, and 42 patients (33.3%) had LNC on MDCT ≥ 1. When 5mm was used as the counting criterion, the cut-off value of LNC on MDCT for grouping GC patients was determined as 7. Among the 126 patients with GC, 106 patients (84.1%) had LNC on MDCT ≤ 7, and 20 patients (15.9%) had LNC on MDCT > 7. Patient demographics, clinicopathological features and comparison between subgroups are shown in Tables 1–2. There were no differences between subgroups in demographics, tumor locations, tumor differentiation, and type of operation. There were more histologically positive LNs in patients with LNC on MDCT ≥ 1 (8mm as the counting criterion) or LNC on MDCT > 7 (5mm as the counting criterion).
 |
Table 1 Demographics, Clinicopathological Features of Patients with Gastric Carcinoma and Comparison of the Subgroups |
 |
Table 2 LNC in Patients with Gastric Carcinoma |
With 8mm was the counting criterion, LNC on MDCT had no significant effect on the OS of all the enrolled GC patients (p=0.079) (Figure 2). In a further subgroup analysis stratified by T staging, the OS of T1–T2 GC patients with LNC on MDCT ≥ 1 was significantly worse than that of those with LNC on MDCT < 1 (p=0.002). However, LNC on MDCT showed no significant effect on the OS of patients with stage T3–T4 GC (p=0.662). In a subgroup analysis based on N staging, LNC on MDCT had no impact on the OS of N0 or N+ GC patients (N0, p=0.412; N+, p=0.567). Similarly, LNC on MDCT had no impact on the OS of stage I-II or III GC patients in a subgroup analysis based on TNM staging (stage I-II, p=0.922; stage III, p=0.692) (Figure 3).
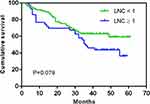 |
Figure 2 Overall survival of gastric carcinoma patients according to LNC on MDCT when 8mm was used as the counting criterion. Abbreviation: LNC, lymph node count. |
When 5mm was used as the counting criterion, patients with LNC on MDCT > 7 had a significantly worse OS than those with LNC on MDCT ≤ 7 (p=0.005) (Figure 4). In a further subgroup analysis stratified by T staging, LNC on MDCT was found to have no impact on the OS of patients with stage T1–T2 GC (p=0.693). However, the OS of T3–T4 patients with LNC on MDCT > 7 was significantly worse than that of patients with LNC on MDCT ≤ 7 (p=0.019). In subgroup analysis based on N staging, LNC on MDCT exerted little impact on the OS of N0 GC patients (p=0.258). However, N+ patients with LNC on MDCT > 7 had a significantly worse OS than those with LNC on MDCT ≤ 7 (p=0.042). In a subgroup analysis based on TNM staging, LNC on MDCT had no impact on the OS of stage I-II GC patients (p=0.511). However, the OS of stage III patients with LNC on MDCT > 7 was significantly worse compared with those with LNC on MDCT ≤ 7 (p=0.048) (Figure 5).
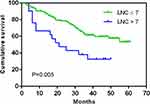 |
Figure 4 Overall survival of gastric carcinoma patients according to LNC on MDCT when 5mm was used as the counting criterion. |
A univariate analysis revealed that LNC on MDCT with 5mm as the counting criterion, TNM stage, tumor differentiation, and type of operation were associated with the OS of patients. The Cox proportional hazards model demonstrated that LNC on MDCT > 7 with 5mm was the counting criterion (HR = 2.153, p=0.019) and stage III (HR = 5.056, p<0.001) were independently associated with unfavorable prognosis (Table 3).
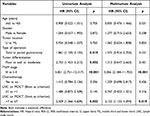 |
Table 3 The Univariate and Multivariate Analysis of Overall Survival in Patients with Gastric Carcinoma |
Discussion
This paper demonstrated that according to different size criteria for counting, LNC on MDCT had different effects on the prognosis of GC patients with different TNM stages. When 8mm was used as the counting criterion, LNC on MDCT had no impact on GC patients’ OS. Among the entire cohort of 126 GC patients, the LNs that were greater than 8mm were not detected by MDCT in 84 patients (66.7%). Moreover, among the 90 GC patients who had metastatic LNs, LNs that were greater than 8mm were undetected by MDCT in 52 patients (57.8%). Although there are varying LN size criteria for diagnosing metastatic LNs, a maximum short axis diameter greater than 8mm is the most commonly used CT criterion.11,12 Kawaguchi et al reported that MDCT detected significantly fewer metastatic LNs than histologic examination, which may lead to an underestimation of N staging.13,14 Indeed, one patient of our study cohort had 49 metastatic nodes, but CT failed to detect any nodes greater than 8mm. The inconsistency between CT findings and histologic examination may explain why LNC on MDCT has no effect on the OS of patients with GC.
In the subgroup analysis based on TNM staging, T1–T2 GC patients with LNC on MDCT ≥ 1 had a significantly worse OS than those with LNC on MDCT < 1. However, LNC on MDCT showed no significant effect on the OS of GC patients in other subgroups. Previous studies have shown a clear association between LN size and metastasis.14,25 In our study, LNM was confirmed in all the three cases with T2 GC and LNC on MDCT ≥ 1, two of which had more than 10 metastatic LNs. Therefore, radiologists should bear in mind that there may be extensive LNM in relatively early-stage GC patients with LNs greater than 8mm on MDCT.
However, with 5mm as the counting criterion, LNC on MDCT was identified to be an independent risk factor associated with the survival of GC patients. LNs greater than 5mm were detected in 119 of all the 126 cases (94.5%) enrolled and 87 of 90 cases (96.7%) who had metastatic nodes. Yan et al reported that, in fact, nodal size criteria are inversely proportional to the sensitivity of nodal involvement,26 which indicates that there may be more metastatic nodes detected with 5mm than with 8mm as the counting criterion. Noda et al recommended all LNs ≥5mm in size should be examined histologically to avoid downstaging.27 In addition, patients with LNC on MDCT > 7 were found to have a significantly worse OS than those with LNC on MDCT ≤ 7. Cox regression analysis demonstrated that LNC on MDCT > 7 was an independent risk factor affecting the survival of patients with GC. Similar results were obtained by Chi et al when they evaluated the prognostic value of LNC on MDCT in patients with rectal cancer. Most normal LNs are not detectable because of partial volume effects or lower attenuation. In contrast, LNs are easily detected by MDCT when they become greater in size or higher in density as a result of inflammation or metastasis. Thus, LNC on MDCT is an indirect measure of tumor invasiveness.15 Indeed, we found that patients with LNC on MDCT > 7 had more LNs harvested and histologically-positive. Therefore, overall, LNC on MDCT is of predictive value for the OS of GC patients, when 5mm was used as the counting criterion.
In the subgroup analysis based on TNM staging, LNC on MDCT showed no significant prognostic effect on patients with relatively early stage (stage T1– T2, N0, and I–II) GC. However, for patients with relatively advanced (T3–T4, N+, and III) GC, those with LNC on MDCT > 7 had a significantly worse OS compared with those with LNC on MDCT ≤ 7. One possible explanation is that lymphadenectomy may be closer to a radical cure for those with relatively early stage of GC, irrespective of LNC on MDCT. On the contrary, LNs are prone to metastasize in advanced GC.28,29 LNC on MDCT > 7 may indicate extensive LNM or more occult metastasis. Therefore, removal of all macroscopic and microscopic lesions is far from sufficient for patients with relatively advanced GC. Another possibility is that the incidence of LNC on MDCT > 7 was low in patients with relatively early-stage GC. In this study, LNC on MDCT > 7 was detected in 1 of 24 T1-T2 GC patients (4.2%), 3 of 36 N- GC patients (8.3%), and 4 of 49 I–II GC patients (8.2%). The absence of significant difference in OS between subgroups may be due to the limited number of cases included, which means that a large cohort study is needed for further investigation. Based on our results, advanced (T3–T4, N+, and III) GC patients with LNC on MDCT > 7 had a significantly worse OS. We recommend that LNC on MDCT of a smaller size (5mm) be used as a counting criterion to assess the prognosis of patients with relatively advanced stages of GC.
This study still shows some room for improvement. First, not all visible LNs, such as those less than 5mm, were counted. This is because our study design is based on the accuracy of LNC and clinical feasibility. Nonetheless, the results obtained in this research might be sufficient to validate the prognostic significance of LNC on MDCT in GC patients. Second, the design of a single-center retrospective study will inevitably lead to some biases, which warrants a large cohort multicenter study to validate our conclusions. Third, we did not use 3mm as the counting criterion to evaluate the prognosis of GC patients. If the analysis from this perspective can be supplemented, the prognostic efficacy of metastatic LNs detected by MDCT for GC prognosis prediction can be further improved. Fourth, our study was based solely on morphology. Radiomics has broad application prospects in predicting the prognosis of diseases, so building radiomics signature-based nomograms to better predict the prognosis of GC is also one of the directions of our follow-up research.
In conclusion, the prognostic value of LNC on MDCT based on different size criteria varies in patients with GC according to tumor stages. LNC on MDCT with a smaller size (5mm) as the counting criterion may be a prognostic factor for patients with relatively advanced stages of GC.
Ethical Statement
This retrospective observational study was conducted in accordance with the ethical standards of the Declaration of Helsinki and relevant guidelines and regulations. The study was approved by the Institutional Ethics Committee of Shanxi Provincial People’s Hospital as exempted research without registration number and the need for informed consent was waived due to the retrospective nature of the study. All data obtained in the course of the study were reserved confidential and used only for this study.
Author Contributions
All authors have reviewed and approved submission of the final version of the manuscript. All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
All authors have completed the ICMJE uniform disclosure form. The authors have no conflicts of interest to declare.
References
1. Zhou L, Lu H, Zeng F, et al. Constructing a new prognostic signature of gastric cancer based on multiple data sets. Bioengineered. 2021;12(1):2820–2835. doi:10.1080/21655979.2021.1940030
2. Chen S, Yu Y, Li T, et al. A novel DNA methylation signature associated with lymph node metastasis status in early gastric cancer. Clin Epigenetics. 2022;14(1):18. doi:10.1186/s13148-021-01219-x
3. Du D, Han Z, Lian D, Amin B, Yan W, Zhang N. The value of preoperative lymphocytes-to-monocytes ratio in predicting lymph node metastasis in gastric cancer. Transl Cancer Res. 2019;8(5):2053–2058. doi:10.21037/tcr.2019.09.17
4. Sun Z, Li J, Wang T, Xie Z, Jin L, Hu S. Predicting perigastric lymph node metastasis in gastric cancer with CT perfusion imaging: a prospective analysis. Eur J Radiol. 2020;122:108753. doi:10.1016/j.ejrad.2019.108753
5. Gao X, Ma T, Cui J, et al. A radiomics-based model for prediction of lymph node metastasis in gastric cancer. Eur J Radiol. 2020;129:109069. doi:10.1016/j.ejrad.2020.109069
6. Zhang L, Zang L, Sun J, et al. Long-term outcomes of laparoscopy-assisted gastrectomy for T4a advanced gastric cancer: a single-center retrospective study. Surg Laparosc Endosc Percutan Tech. 2019;29(6):476–482. doi:10.1097/SLE.0000000000000684
7. Jiang Y, Wang W, Chen C, et al. Radiomics signature on computed tomography imaging: association with lymph node metastasis in patients with gastric cancer. Front Oncol. 2019;9:340. doi:10.3389/fonc.2019.00340
8. Li J, Dong D, Fang M, et al. Dual-energy CT-based deep learning radiomics can improve lymph node metastasis risk prediction for gastric cancer. Eur Radiol. 2020;30(4):2324–2333. doi:10.1007/s00330-019-06621-x
9. Oh SY, Lee JH, Lee HJ, et al. Natural history of gastric cancer: observational study of gastric cancer patients not treated during follow-up. Ann Surg Oncol. 2019;26(9):2905–2911. doi:10.1245/s10434-019-07455-z
10. Tan J, Sun W, Lu L, et al. I6P7 peptide modified superparamagnetic iron oxide nanoparticles for magnetic resonance imaging detection of low-grade brain gliomas. J Mater Chem B. 2019;7(40):6139–6147. doi:10.1039/C9TB01563A
11. Xu SH, Feng LL, Chen YM, Zhang R. [Study on the sensitivity of multi-slice spiral CT in diagnosis of lymph node metastasis in different lymph node stations of gastric cancer]. Zhonghua Wei Chang Wai Ke Za Zhi. 2019;22(10):984–989. Chinese. doi:10.3760/cma.j.issn.1671-0274.2019.10.015
12. You JM, Kim TU, Kim S, et al. Preoperative N stage evaluation in advanced gastric cancer patients using multidetector CT: can the sum of the diameters of metastatic LNs be used for N stage evaluation? Clin Radiol. 2019;74(10):782–789. doi:10.1016/j.crad.2019.06.030
13. Kumamoto T, Shindoh J, Mita H, et al. Optimal diagnostic method using multidetector-row computed tomography for predicting lymph node metastasis in colorectal cancer. World J Surg Oncol. 2019;17(1):39. doi:10.1186/s12957-019-1583-y
14. Bai H, Deng J, Zhang N, et al. Predictive values of multidetector-row computed tomography combined with serum tumor biomarkers in preoperative lymph node metastasis of gastric cancer. Chin J Cancer Res. 2019;31(3):453–462. doi:10.21147/j.issn.1000-9604.2019.03.07
15. Chi YK, Zhang XP, Li J, Sun YS. To be or not to be: significance of lymph nodes on pretreatment CT in predicting survival of rectal cancer patients. Eur J Radiol. 2011;77(3):473–477. doi:10.1016/j.ejrad.2009.09.016
16. Zhang XY, Yan WP, Sun Y, et al. CT signs can predict treatment response and long-term survival: a study in locally advanced esophageal cancer with preoperative chemotherapy. Ann Surg Oncol. 2015;22(Suppl 3):S1380–1387. doi:10.1245/s10434-015-4531-2
17. Zhang M, Ding C, Xu L, et al. Comparison of a tumor-ratio-metastasis staging system and the 8th AJCC TNM staging system for gastric cancer. Front Oncol. 2021;11:595421. doi:10.3389/fonc.2021.595421
18. Wang S, Feng C, Dong D, et al. Preoperative computed tomography-guided disease-free survival prediction in gastric cancer: a multicenter radiomics study. Med Phys. 2020;47(10):4862–4871. doi:10.1002/mp.14350
19. Lin T, Yu J, Hu Y, et al. [Preliminary experience of dual-port laparoscopic distal gastrectomy for gastric cancer]. Zhonghua Wei Chang Wai Ke Za Zhi. 2019;22(1):35–42. Chinese.
20. Dong Y, Qiu Y, Deng J, et al. Insufficient examined lymph node count underestimates staging in pN3a patients after curative gastrectomy: a multicenter study with external validation. J Cancer Res Clin Oncol. 2020;146(2):515–528. doi:10.1007/s00432-019-03081-0
21. Vinatha K, Pradeep Kumar D, Ramesh V, et al. Tuberculous mediastinal lymphadenopathy presenting with left vocal cord palsy: a rare entity. Indian J Tuberc. 2020;67(3):400–403. doi:10.1016/j.ijtb.2019.11.013
22. Yang X, Lei P, Huang L, Tang X, Wei B, Wei H. Prognostic value of LRRC4C in colon and gastric cancers correlates with tumour microenvironment immunity. Int J Biol Sci. 2021;17(5):1413–1427. doi:10.7150/ijbs.58876
23. Zhuang W, Chen J, Li Y, Liu W. Valuation of lymph node dissection in localized high-risk renal cell cancer using X-tile software. Int Urol Nephrol. 2020;52(2):253–262. doi:10.1007/s11255-019-02307-x
24. Feng JF, Sheng C, Zhao Q, Chen P. Prognostic value of mean platelet volume/platelet count ratio in patients with resectable esophageal squamous cell carcinoma: a retrospective study. Peer J. 2019;7:e7246. doi:10.7717/peerj.7246
25. Lale A, Yur M, Ozgul H, et al. Predictors of non-sentinel lymph node metastasis in clinical early stage (cT1-2N0) breast cancer patients with 1–2 metastatic sentinel lymph nodes. Asian J Surg. 2020;43(4):538–549. doi:10.1016/j.asjsur.2019.07.019
26. Yan C, Zhu Z, Yan M, et al. Value of multidetector-row computed tomography in the preoperative T and N staging of gastric carcinoma: a large-scale Chinese study. Journal of surgical oncology. 2009;100(3):205-214. doi:10.1002/jso.21316
27. Noda N, Sasako M, Yamaguchi N, et al. Ignoring small lymph nodes can be a major cause of staging error in gastric cancer. The British journal of surgery. 1998;85(6): 831-834. doi:10.1046/j.1365-2168.1998.00691.x
28. Kosuga T, Konishi T, Kubota T, et al. Value of prognostic nutritional index as a predictor of lymph node metastasis in gastric cancer. Anticancer Res. 2019;39(12):6843–6849. doi:10.21873/anticanres.13901
29. Ding P, Gao Z, Zheng C, Chen J, Li K, Gao S. Risk evaluation of splenic hilar or splenic artery lymph node metastasis and survival analysis for patients with proximal gastric cancer after curative gastrectomy: a retrospective study. BMC Cancer. 2019;19(1):905. doi:10.1186/s12885-019-6112-4
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.