Back to Journals » Journal of Hepatocellular Carcinoma » Volume 4
A Phase I trial using local regional treatment, nonlethal irradiation, intratumoral and systemic polyinosinic-polycytidylic acid polylysine carboxymethylcellulose to treat liver cancer: in search of the abscopal effect
Authors de la Torre AN, Contractor S, Castaneda I, Cathcart CS, Razdan D, Klyde D, Kisza P, Gonzales SF, Salazar AM
Received 10 March 2017
Accepted for publication 16 June 2017
Published 7 August 2017 Volume 2017:4 Pages 111—121
DOI https://doi.org/10.2147/JHC.S136652
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Ahmed Kaseb
Andrew N de la Torre,1,2 Sohail Contractor,3 Ismael Castaneda,1 Charles S Cathcart,4 Dolly Razdan,5 David Klyde,3 Piotr Kisza,3 Sharon F Gonzales,3 Andres M Salazar6
1Department of Surgery, St Joseph’s Regional Medical Center, Paterson, 2Department of Surgery, Rutgers New Jersey Medical School-University Hospital, 3Department of Interventional Radiology, Rutgers New Jersey Medical School-University Hospital, 4Department of Radiation Oncology, Beth Israel Medical Center, Newark, 5Department of Radiation Oncology, Clara Maas Hospital, Belleville, NJ, 6Oncovir, Washington, DC, USA
Purpose: To determine the safety of an approach to immunologically enhance local treatment of hepatocellular cancer (HCC) by combining nonlethal radiation, local regional therapy with intratumoral injection, and systemic administration of a potent Toll-like receptor (TLR) immune adjuvant.
Methods: Patients with HCC not eligible for liver transplant or surgery were subject to: 1) 3 fractions of 2-Gy focal nonlethal radiation to increase tumor antigen expression, 2) intra-/peri-tumoral (IT) injection of the TLR3 agonist, polyinosinic-polycytidylic acid polylysine carboxymethylcellulose (poly-ICLC), to induce an immunologic “danger” response in the tumor microenvironment with local regional therapy, and 3) systemic boosting of immunity with intramuscular poly-ICLC. Primary end points were safety and tolerability; secondary end points were progression-free survival (PFS) and overall survival (OS) at 6 months, 1 year, and 2 years.
Results: Eighteen patients with HCC not eligible for surgery or liver transplant were treated. Aside from 1 embolization-related severe adverse event, all events were ≤grade II. PFS was 66% at 6 months, 39% at 12 months, and 28% at 24 months. Overall 1-year survival was 69%, and 2-year survival 38%. In patients <60 years old, 2-year survival was 62.5% vs. 11.1% in patients aged >60 years (P<0.05). Several patients had prolonged PFS and OS.
Conclusion: Intra-tumoral injection of the TLR3 agonist poly-ICLC in patients with HCC is safe and tolerable when combined with local nonlethal radiation and local regional treatment. Further work is in progress to evaluate if this approach improves survival compared to local regional treatment alone and characterize changes in anticancer immunity.
Keywords: immunotherapy, autologous vaccine, liver cancer, human trial, Toll-like receptor
Introduction
HCC is the third leading cause of cancer-related death worldwide, with a global incidence of over 500,000 cases a year.1 In the US, rates of HCC continue to rise.2 Despite a small survival advantage with the use of the targeted agent sorafenib, there has been little progress with the use of systemic therapy for HCC.3 Local regional therapy, such as TAE, remains a main modality for HCC treatment. Immunologically, TAE has been shown to decrease systemic T-Reg, and it has been found that the proportion of T-Reg assessed 1 month after TACE was related to prognosis.4,5 TAE-induced necrosis has been shown to result in HLA-DR-restricted alpha-fetal protein-derived CD4 T cell epitopes such that CD4 T cells recognize these epitopes, and produce Th1, IFN, and tumor necrosis factor, but not Th2, IL-4, and Th2-associated cytokines.6 Reduction in tumor size after direct tumor ablation or TAE reduces tumor-promoting factors, such as VEGF in patients with HCC.7 Thus, in addition to tumor necrosis, TAE and TACE have immune-modulatory function, but clearly not enough.
Most patients with HCC have cirrhosis that impairs immunity and adversely impacts outcome.8 Cancers produce factors and cell surface proteins that decrease immunologic ability to recognize, target, and eliminate malignant tumors.9 Tolerogenic T-Regs are increased in HCC patients compared to cirrhotic and non-cirrhotic patients without HCC.10 The liver is also involved in suppression of autoimmunity, which can reduce immune responsiveness against HCC.11 Age-related immunosenescence, T-cell exhaustion from chronic infection, and anergy from malnutrition add to immunosuppression in cancer patients and further sway immunoediting away from “elimination” toward “escape”.12 Immune tolerance is believed to facilitate the initiation and progression of cancer as a result of increased populations of myeloid-derived suppressor cells, increased T-Reg, a paucity of IL-17-responsive T cells (Th17), and cytokine dysregulation.13,14 However, the hepatic microenvironment contains numerous immunomodulatory cells and is the body’s largest reservoir of antigen-presenting cells.15 The liver may provide an environment where mounting an immune response against cancer can occur. Thus, it may be possible to modulate the hepatic microenvironment to alter anticancer immunity away from “escape” back to “elimination”.
Development of cellular immunity, at a minimum, depends on antigen dose, dendritic cell state, the status of the surrounding microenvironment, and timing of cytokine exposure. For example, although IL-2 can expand cytotoxic T cells in the context of infection, in the absence of a “danger” signal within the tumor microenvironment, IL-2 expands populations of tolerizing T-Reg.16 Attempts to break immune tolerance have been applied to melanoma, renal cancer, and others.17,18 Cytokines such as IL-2 and granulocyte/monocyte colony-stimulating factor alone, or in combination, or proceeding tumor antigens have shown limited and unreliable effectiveness.19,20 Checkpoint blockade-modulating antibodies resulting in enhancement of T cell function have been approved to treat melanoma and lung cancer: anti-CTLA-4 (ipilimumab) and anti-PD-1 (nivolumab, pembrolizumab).21 The therapeutic benefit can be significant, but associated with autoimmune AEs. Though responses are infrequent, they can be durable.22 These molecules are believed to reduce tumor tolerance, and some cancer patients develop specific antitumor immunity.23 Though most recently, nivolumab failed to show a survival advantage as first-line treatment in lung cancer patients compared to conventional chemotherapy.24
Given the multiple immune defects in cancer patients, targeting a single immune defect may not be enough to induce robust anticancer immunity. Modulation of multiple immune pathways may be needed to restore and enhance cytotoxic anticancer immunity. Use of local regional treatment combined with immunomodulatory agents may provide a way to augment anticancer responsiveness in the liver systemically. The TLR3 agonist poly-ICLC is a double-stranded RNA viral mimic that strongly induces multiple IFN.25 TLR activation enables the body to sense “danger” within tissue and generate potent immune responses, including dendritic cell activation, antigen presentation, and accessory signaling.26 Multiple animal studies have shown that use of TLR agonists enhances anticancer immunity.27 Systemic poly-ICLC in malignant glioma patients is safe and has shown a clinical benefit.28 Sequential intratumoral and IM poly-ICLC led to significant tumor regression in a case of advanced facial embryonal rhabdomyosarcoma.29 Several closely interrelated mechanisms of action, alone or in combination, could explain these clinical findings. The pathogen-associated molecular pattern danger signal appears to be a key step toward a cytotoxic immune response.30
Sublethal irradiation of human cancer cells has been shown to result in enhanced cancer killing by cytotoxic T lymphocytes through increased tumor antigen expression.31 Local cancer irradiation in combination with vaccine and reduction of CD4+ CD25+ suppressor T cells eliminates established cancers in animal models.32 In vitro dendritic cell tumor antigen loading is most effective following cancer cell irradiation.33 Radiotherapy of a cancer has been shown to infrequently result in an abscopal effect, where a remote nonirradiated tumor regresses. Overall, there is sufficient evidence to suggest radiotherapy facilitates immunity against tumors.34,35
We tested the safety of a strategy to immunologically enhance local regional therapy for HCC by combining 1) nonlethal 3-dimensional conformal radiation to the HCC, 2) TAE to induce tumor necrosis, antigen release, and reduction of pro-tumor factors, and 3) IT injection of the TLR3 agonist poly-ICLC to initiate a “danger” response in the local tumor environment where tumor-associated antigens are most likely expressed. Systemic immunologic “boost” using IM poly-ICLC over the following 3 weeks was then performed.
Methods
This trial was reviewed and approved by the Institutional Review Board (IRB) of the Rutgers New Jersey Medical School in Newark, NJ (protocol number 0120070076). All patients were explained the protocol in lay terms. The principal investigator aided by the research coordinator obtained written informed consent from all patients who were involved in this trial. Potential patients were counseled on treatment options, including the clinical trial. Enrollment was initiated only after the investigation team was assured the patient understood the risks and benefits of the trial and written informed consent was signed. This trial is registered with clinicaltrials.gov NCT00553683. This manuscript will be used as a final report to clinicaltrials.gov.
Inclusion criteria
The inclusion criteria included the following: 1) ineligibility for liver transplant due to social or health reasons, or beyond Milan listing criteria (single lesion >5 cm, >3 tumors of >3 cm, extrahepatic spread, or macrovascular invasion); 2) at least 18 years of age; 3) biopsy-confirmed HCC or Liver Imaging Reporting and Data System 4 or 5 imaging; 4) radiologically measurable HCC confined to the liver; 4) a Karnofsky PS ≥60%, that is, the patient must be able to care for himself/herself with occasional help from others); 5) acceptable laboratory limits: platelets >50,000/mm3, creatinine ≤1.7 mg/dl, total bilirubin ≤1.5 mg/dl, transaminases ≤5 times above the institutional normal, and international normalized ratio <1.5; and 6) a negative pregnancy test.
Exclusion criteria
The exclusion criteria included the following: 1) surgically resectable disease, 2) left ventricular ejection fraction <50%, 3) pregnancy or breastfeeding, and 4) serious concurrent infection or medical illness.
Treatment cycle
The treatment cycle followed was 1) 3 fractions of 2-Gy focal nonlethal radiation to a selected tumor, 2) local regional treatment (HAE, HACE, or RFA) with concurrent IT injection of poly-ICLC, and 3) IM poly-ICLC. A detailed overview of the treatment cycle is shown in Figure 1.
Administration of 3D-CRT
The radiation dose used, 22.5 Gy, for 3D-CRT is below the dose associated with a 5% risk of radiation-induced liver disease.36 In each of 3 intended protocol cycles, 3D-CRT was administered for 3 consecutive days prior to TAE/TACE, directed at the selected target lesion. Patients received 1 fraction per day at 2.5 Gy per fraction using a 6-MV linear accelerator (Varian 21-EX; Varian Medical Systems Inc., Palo Alto, CA, USA). Patients were to receive a total of 9 fractionated radiation treatments throughout the vaccination protocol at a dose of 22.5 Gy.
TAE or TACE
Initially, TAE was used for local regional treatment. Once it was determined that TAE was safe and tolerable, patients underwent TACE for local regional treatment to determine if it was safe and tolerable. To perform the procedure, a full diagnostic celiac/hepatic/superior mesenteric angiogram was performed to evaluate for the normal vasculature as well as identify the feeding vessels to the tumor or tumors. Assessment of portal vein patency was also made with delayed-phase imaging. Following the identification and super-selective catheterization of the feeding vessels to the selected tumor with iodinated contrast, embolization particles (100–300 µm) were administered until stasis within the main artery feeding the tumor was approximately 90% reduced in flow. For patients receiving TACE, prior to stasis, mitomycin C 10 mg and adriamycin 30 mg were administered intra-arterially. Patients undergoing TAE or TACE were monitored for heart rate, blood pressure, and oxygen saturation during the procedure and in the postanesthesia care unit for 6 hours after the procedure. Patients receiving initial TAE or TACE were admitted for observation overnight. Antiemetics, hydration, antihistamines, and antibiotics were given routinely to minimize post-embolization syndrome and infectious complications.
Polyinosinic-polycytidylic acid stabilized with polylysine and carboxymethylcellulose
IT injection
Poly-ICLC was supplied in 1-ml vials at 2 mg/ml and stored at 40°F. A poly-ICLC dose of 0.25 or 1 mg was diluted in 5 ml of buffered 0.9 N normal saline (NaCl). Patients were administered poly-ICLC within and at the periphery of the tumor by the interventional radiologist. Under ultrasound guidance, the tip of the needle was positioned within the peripheral region of the tumor and aspirated, and poly-ICLC was injected. The needle was repositioned at the peri-tumoral interface and aspirated, and poly-ICLC was again injected. After the injection was completed, the needle was flushed with 0.5 cm3 of buffered 0.9 N NaCl. Heart rate, blood pressure, and oxygen saturation were monitored during poly-ICLC administration and during the following 6 hours. The first 5 patients enrolled received an IT dose of 0.25 mg poly-ICLC. After the 0.25 mg IT dose was deemed safe and tolerable, subsequent patients received an IT dose of 1 mg poly-ICLC.
IM injection of poly-ICLC for systemic boosting
Patients received 20 µg/kg IM poly-ICLC twice weekly for 4 weeks following the intratumoral injection. The posterior loin was chosen as an injection site based on scintography data showing drainage near perihepatic lymphatics.37
Nutrition and vitamin supplements
Once consented, all patients in this trial were counseled on the importance of nutrition, and instructed to drink a supplement and to take a multivitamin and vitamin D 1000 IU daily. Vitamin D was added because it has been shown to modulate immunity and decrease AEs in patients with liver disease.38,39 Patients with a weight loss >15 pounds were given the option of using appetite stimulants.
Outcome
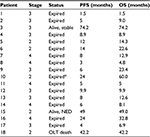
The primary end points were safety and tolerance of the regimen. Blood testing was performed a week following IT injection of poly ICLC, and then every 2 weeks between cycles. Secondary end point included PFS assessed by multiple-phase CAT or MRI scans using mRECIST criteria 4–6 weeks following each cycle and then every 3 months thereafter.40 OS was reported according to direct clinical observation and follow-up phone calls by a research coordinator. Table 1 provides an overview of tumor stage, PFS, and OS.
Results
Demographics
Table 2 provides a description of patient demographic data. Of 18 patients enrolled, the mean age was 56.6 (range 25–80) years. There were 14 males and 4 females which included 14 Caucasians (2 Latinos) and 4 African Americans. Ten patients had HCV-related cirrhosis. All liver cancer patients were Childs–Pugh A. PS of 5 patients was 0, 12 patients 1, and 1 patient 2.
  | Table 2 Patient demographics, liver disease etiology, and PS Abbreviations: PS, performance status; NASH, nonalcoholic steatohepatitis; HCV, hepatitis C virus; HBV, hepatitis B virus. |
Staging
Burden of disease (Table 3) and staging for hepatoma (Table 1) was determined according to the American Liver Tumor Study Group.41
  | Table 3 Cancer type and burden of disease Abbreviation: PV, portal vein. |
Overview of treatment
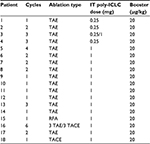
Table 4 provides an overview of the treatment regimen of each patient enrolled.
AE
AE were classified according to Common Terminology Criteria for Adverse Events volume 4.0. Table 5 provides an overview of AEs. There was 1 severe AE early in the trial, deemed due to overembolization, as the main right hepatic artery was entirely embolized and the patient experienced dramatic hepatic dysfunction immediately following the procedure. Henceforth, patients only underwent selective embolization. No subsequent patient demonstrated such an effect following selective HAE or HACE. There were no AEs of IT poly-ICLC injection. IM poly-ICLC caused its expected transient flu-like side effects as previously described.42 The most common AEs were as follows: fatigue in 11 patients, anorexia in 8, fever in 5, injection site-related AE in 7, and diarrhea in 2.
  | Table 5 Summary of AE Abbreviation: AE, adverse event. |
Patient outcomes
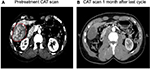
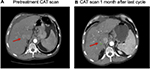
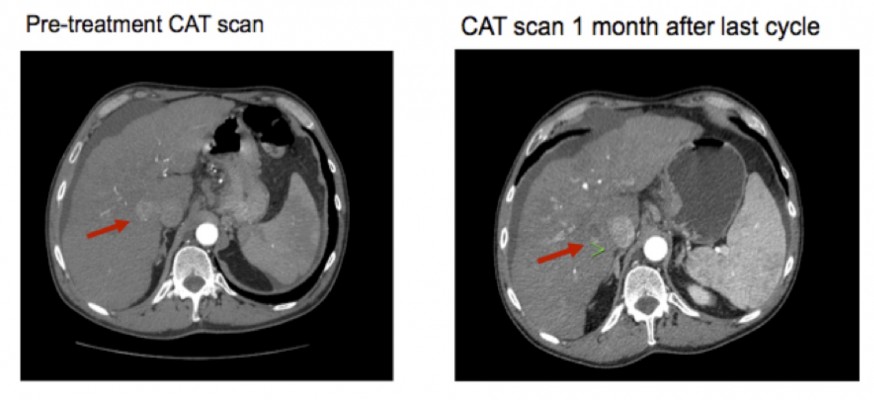
Table 1 provides an overview of patient survival. Depending on the presence of residual tumor on imaging 4–6 weeks following the end of a given cycle indicating a complete response, some patients received <3 cycles; however, several patients received >3 cycles as shown in Table 4. All patients with the exception of patient 16 received 7.5 Gy to the targeted tumor. Patients 1, 2, 3, and 4 received 0.25 mg IT poly-ICLC, bland embolization, and IM poly-ICLC. Patients 5–18 received 1.0 mg IT poly-ICLC, bland embolization, and IM poly-ICLC. Given patients received concurrent intratumoral poly-ICLC and embolization therapy, response rates were hard to assess. Several patients exhibited prolonged stabilization of their HCC. Patient 3 had biopsy-proven multifocal well-differentiated HCC in the setting of portal vein agenesis.43 He presented with a 15-pound weight loss prior to initiating treatment. He was prescribed dronabinol 3 times a day, and vitamin D 1000 IU and a multivitamin daily. After a month, he gained 6 pounds. He underwent 3 full cycles of treatment to his dominant tumor that was 10 cm in its greatest diameter exophytic off of segment 6, as well as 2 additional chemoembolizations to lesions in segments 8 and 5. Figure 2 shows the response of his dominant lesions before and after 3 cycles of treatment. He was alive 6 years following his initial treatment with no evidence of progressive HCC. Patient 5 had HCC with Childs A HCV cirrhosis. He presented with 5 lesions (1–2 cm) and a 10-pound weight loss. He was prescribed dronabinol, vitamin D, and a multivitamin daily. He completed 4 cycles with IT poly-ICLC, bland HAE, and systemic poly-ICLC. None of his tumors progressed, and a tumor that was not embolized or injected with poly-ICLC lost its enhancement on multiphase CAT scan imaging, that is, “remote effect” (Figure 3). He unfortunately presented with strangulated bowel from an incarcerated inguinal hernia and died from sepsis following surgery. He had stable disease for almost 9 months at the time of his death and was to be referred for liver transplant evaluation. Patient 12 with Laennec’s cirrhosis and lupus had 4 enhancing lesions consistent with HCC and was outside Milan criteria. She underwent 2 TACE procedures prior to trial enrollment. She underwent 1 cycle with nonlethal radiation, IT poly-ICLC, bland HAE, and systemic poly-ICLC. Further cycles were held as she had borderline renal function. Despite a 6-month interval with no cancer progression, she was turned down for liver transplant evaluation locally, but ultimately was accepted for liver transplant evaluation at another institution. During liver transplant evaluation, she developed worsening ascites, hepatorenal syndrome, sepsis, and liver failure and died. She had no new enhancing lesions consistent with HCC at the time of her death. Patient 15 had a localized 3-cm hepatoma with well-compensated hepatitis B virus cirrhosis and was on tenofovir. She underwent laparoscopic RFA and concurrent intraoperative IT injection of poly-ICLC. She was not a transplant candidate due to immigration status. She was alive without evidence of HCC 4 years following treatment. Patient 16 presented with multifocal large (12 cm) rapidly progressing biopsy-proven moderately differentiated hepatomas with portal vein and extrahepatic lymph node invasion (Figure 4). He progressed on sorafenib and was sent to hospice just prior to evaluation. He was 5′7″, weighed 118 pounds, and had a PS of 3 on initial evaluation. His sorafenib was stopped, and he was prescribed dronabinol, vitamin D, and a multivitamin daily. After a month, his weight increased to 124 pounds with a PS of 2, before entering the protocol. After 2 cycles, his weight increased to 136 pounds, and he recovered to a 0 PS. His disease remained stable for a year and a half, but recurred after he stopped treatment and went to Belize for 6 months. Upon return, he underwent 3 cycles of chemoembolization (mitomycin C/adriamycin), and IT and IM poly-ICLC. He survived 33 months. Patient 18 underwent post-laparoscopic left lobe liver resection for a 4-cm moderately differentiated hepatoma in the setting of well-compensated HCV cirrhosis. On follow-up imaging after 4 months, he had 1.2-cm enhancing lesion in segment 7, which was treated with ultrasound-guided RFA. Within several months, he developed a new second 2-cm enhancing lesion in segment 5. He was referred for liver transplant, but was turned down due to the short interval over which he developed new lesions. He received 1 cycle of nonlethal radiation, IT poly-ICLC, chemoembolization, and systemic boost. He had no radiologic evidence of recurrent cancer for a year and was reevaluated for a liver transplant. After waiting for 2 years, he received a liver transplant. He died of complications following liver transplant. His liver transplant explant showed a cirrhotic liver with several sub-centimeter hepatomas and 2 lymph nodes positive for tumor.
Survival
PFS was 66% at 6 months, 39% at 12 months, and 28% at 24 months on multiphase imaging. The mean PFS was 16.9 months. The mean survival was 22 months (range 1.5–74 months). One-year survival was 69%, and 2-year survival was 38%. Patients 3 and 15 were alive at 74 and 49 months from study entry (Table 1). Two-year survival of HCC patients aged <60 vs. ≥60 years was compared. Using a 2-tailed Fisher’s exact test, survival was calculated as 62.5% in patients aged <60 years vs. 11.1% in patients aged ≥60 years (P<0.05) (Table 6).
  | Table 6 Two-year survival vs. age >60 years in HCC patients Note: Fisher’s exact test, 2-tailed analysis, P<0.05. Abbreviation: HCC, hepatocellular cancer. |
Discussion
As long-term graph tolerance is the “Holy Grail” in transplant immunology, so is vaccinating patients against their tumor in cancer immunotherapy. The process of immunologic elimination of a cancer has many obstacles. Intuitively, overcoming tolerance within the tumor microenvironment is the first step needed to initiate processes leading to cytotoxic tumor antigen recognition. Additional steps are then needed to improve tumor recognition, activate dendritic cells, and reduce tumor-promoting factors to induce long-term immunity.
Local regional treatments such as TAE or TACE of tumors in the liver provide an opportunity to modulate the tumor microenvironment. Although a meta-analysis of trials comparing TACE with radiotherapy vs. TACE alone showed a significant OS advantage in the combined treatment group, no mention is made regarding a possible mechanism.44 One possibility is enhanced local activation of immunity associated with damage-associated molecular pattern.45 Modulation of a HCC within the hepatic microenvironment may be particularly suited for interventional radiologic techniques combined with radiation and targeted immunomodulation.
This study shows intratumoral injection of a potent immune adjuvant, the TLR3 agonist, poly-ICLC, is tolerable and safe in HCC. Intra-tumoral poly-ICLC can also be safely combined with nonlethal radiation and TAE. The rational to use intratumoral poly-ICLC, nonlethal radiation, and TAE stems from their reported abilities to modulate immunity. Although the study size was too small to determine if this approach significantly improves survival, several patients exhibited prolonged cancer stabilization and survival following immune-enhanced TAE, as shown in Table 1.
Patient 3, with biopsy-confirmed multifocal HCC, had a complete response and did not relapse for over 5 years. Patients 5, 12, and 18 all had near-complete response. The death of patient 5 was not related to his HCC. Patient 12 died with minimal cancer due to complications of liver failure and lupus-related renal insufficiency. Both were being evaluated for transplant. Patient 16, with advanced liver cancer, had extended stabilization and survived 33 months. Patient 18 was ultimately transplanted, but died following surgical complications of liver transplant.
The incidence of cancer increases and the success of vaccination decreases with advancing age.46,47 It is reasonable to expect tumor vaccination will be more difficult to achieve in older patients. This may be reflected in the difference in mean survival when comparing HCC patients less than and greater than age 60. Patients presenting with severe weight loss are more likely to be anergic. Thus, stabilizing a patient’s weight and restoring an acceptable nutritional status prior to initiating tumor vaccination should be attempted when possible. Another challenge, particularly in cirrhotic patients with chronic inflammation, is their progressive metabolic and immunologic deterioration. Fortunately, new direct-acting antiviral agents are able to halt viral infection in the vast majority of patients, halting chronic inflammation, potential T cell exhaustion, and recovery of liver function.12,48
As discussed, addition of checkpoint blockers or other co-stimulators to the IT poly-ICLC regimen may reduce tumor tolerance. Preclinical studies have shown synergy of PD-1 and PDL-1 with poly-ICLC, virtually clearing established lung and colon cancers in mice, in a CD8-dependent manner.49 Activated hepatic stellate cells have been reported to express increased PDL-1 and are also pivotal in the onset of fibrosis and cirrhosis.50 Thus, PD-1/PDL-1 blockade may be particularly useful to restore immunity prior to cancer vaccination in the liver. However, agents blocking PD-1/PDL-1 may be limited in the absence of increased tumor-associated antigen expression. In this regard, nonlethal or lethal radiation may be useful. Hepatomas produce angiogenic and immunosuppressive factors such as VEGF.7 Thus, methods that enhance tumor antigen expression and release or decrease tumor burden may be helpful in future protocols. We did not attempt to measure immunologic blood tests, as the primary goal was to determine the safety of the combination of treatments. Additionally, widely available reliable immunologic tests that correlate with outcome have yet to be identified. Should further trials demonstrate a survival advantage, resources will then be directed toward immunologic blood testing.
Conclusion
These data show combined nonlethal radiation, TAE, and IT and IM poly-ICLC are safe and tolerable. Further trials are needed to determine if this combination improves survival compared to TAE or TACE alone. Given no attempt to reduce tumor-related tolerance was performed, tolerance-reducing antibodies against PD-1 or PDL-1 (nivolumab, pembrolizumab) should also be investigated. However, what occurs in the tumor microenvironment may not be reflected systemically or vice versa. Clinically, what ultimately counts is quality of life and survival. Further trials are in progress to immunologically enhance local regional therapies such as TACE, RFA, and yttrium 90 hepatic artery infusion as well as measure immunomodulatory parameters.
Abbreviations
3D-CRT, 3-dimensional conformal radiotherapy
AE, adverse event
CD, cluster determinant
CTLA-4, cytotoxic T-lymphocyte antigen-4
HAE, hepatic artery embolization
HACE, hepatic artery chemoembolization
HCC, hepatocellular cancer
HCV, hepatitis C virus
IFN, interferon
IL, interleukin
IM, intramuscular
IRB, institutional review board
IT, intra-/peri-tumoral
OS, overall survival
PD-1, programmed death receptor 1
PDL-1, programmed death receptor ligand 1
PFS, progression-free survival
poly-ICLC, polyinosinic-polycytidylic acid polylysine carboxymethylcellulose
PS, performance status
RFA, radiofrequency ablation
TACE, transhepatic artery chemoembolization
TAE, transhepatic artery embolization
Th1, type-1 T helper cells
Th2, type-2 T helper cells
T-Reg, regulatory T cells
VEGF, vascular endothelial growth factor
Acknowledgments
Theresa Garcia, PA (Department of Interventional Radiology, Rutgers New Jersey Medical School-University Hospital, Newark, NJ), assessed patients during trial to assist with management of AEs related to HAE. Maria Korogodsky, PA (St Joseph’s Regional Medical Center, Paterson, NJ), assessed patients during trial to assist with management of AEs, and coordinated all aspects of scheduling of patients for procedures, acquisition of poly-ICLC for IT and IM injection, patient education, and management of AEs.
Author contributions
AD prepared clinical trial protocol, prepared IRB documents, obtained patient consent, oversaw safety monitoring, managed AEs, and prepared manuscript. IC assisted in preparation of IRB documents for submission, oversaw coordination of protocol, compiled patient data during clinical trial, and registered clinical trial with clinicaltrials.gov. SC, DK, PK, and SFG performed IT injection and embolization of liver cancers. CSC and DR planned and performed sublethal external beam radiation therapy of liver cancers. AMS was integral in conducting this clinical trial and significantly contributed to protocol design and manuscript. All authors contributed toward data analysis, drafting and critically revising the paper and agree to be accountable for all aspects of the work.
Disclosure
AMS is an employee of Oncovir and owns stock in the company. The other authors have no conflicts of interests in this work and not made any financial investment, nor have received direct funds from Oncovir Pharmaceutical.
References
Bosch FX, Ribes J, Cleries R, Diaz M. Epidemiology of hepatocellular carcinoma. Clin Liver Dis. 2005;9(2):191–211. | ||
Kim Y, Ejaz A, Tayal A, et al. Temporal trends in population-based death rates associated with chronic liver disease and liver cancer in the United States over the last 30 years. Cancer. 2014;120(19):3058–3065. | ||
Llovet JM, Ricci S, Mazzaferro V, et al; SHARP Investigators Study Group. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359(4):378–390. | ||
Liao J, Xiao J, Zhou Y, Liu Z, Wang C. Effect of transcatheter arterial chemoembolization on cellular immune function and regulatory T cells in patients with hepatocellular carcinoma. Mol Med Rep. 2015;12(4):6065–6071. | ||
Xiong B, Feng G, Luo S, et al. Changes of CD4(+) CD25 (+) regulatory T cells in peripheral blood in patients with hepatocellular carcinoma before and after TACE. J Huazhong Univ Sci Technol Med Sci. 2008;28(6):645–648. | ||
Ayaru L, Pereira SP, Alisa A, et al. Unmasking of alpha-fetoprotein-specific CD4(+) T cell responses in hepatocellular carcinoma patients undergoing embolization. J Immunol. 2007;178(3):1914–1922. | ||
Xiong ZP, Yang SR, Liang ZY, et al. Association between vascular endothelial growth factor and metastasis after transcatheter arterial chemoembolization in patients with hepatocellular carcinoma. Hepatobiliary Pancreat Dis Int. 2004;3(3):386–390. | ||
Philips CA, Sarin SK. Sepsis in cirrhosis: emerging concepts in pathogenesis, diagnosis and management. Hepatol Int. 2016;10(6):871–882. | ||
Blank CU. The perspective of immunotherapy: new molecules and new mechanisms of action in immune modulation. Curr Opin Oncol. 2014;26(2):204–214. | ||
Ormandy LA, Hillemann T, Wedemeyer H, Manns MP, Greten TF, Korangy F. Increased populations of regulatory T cells in peripheral blood of patients with hepatocellular carcinoma. Cancer Res. 2005;65(6):2457–2464. | ||
Thomson AW, Knolle PA. Antigen-presenting cell function in the tolerogenic liver environment. Nat Rev Immunol. 2010;10(11):753–766. | ||
Crespo J, Sun H, Welling TH, Tian Z, Zou W. T cell anergy, exhaustion, senescence, and stemness in the tumor microenvironment. Curr Opin Immunol. 2013;25(2):214–221. | ||
Wesolowski R, Markowitz J, Carson WE 3rd. Myeloid derived suppressor cells - a new therapeutic target in the treatment of cancer. J Immunother Cancer. 2013;1:10. | ||
Facciabene A, Motz GT, Coukos G. T-regulatory cells: key players in tumor immune escape and angiogenesis. Cancer Res. 2012;72(9):2162–2171. | ||
Knolle PA, Thimme R. Hepatic immune regulation and its involvement in viral hepatitis infection. Gastroenterology. 2014;146(5):1193–1207. | ||
Guo Z, Khattar M, Schroder PM, et al. A dynamic dual role of IL-2 signaling in the two-step differentiation process of adaptive regulatory T cells. J Immunol. 2013;190(7):3153–3162. | ||
Homsi J, Grimm JC, Hwu P. Immunotherapy of melanoma: an update. Surg Oncol Clin N Am. 2011;20(1):145–163. | ||
George S, Pili R, Carducci MA, Kim JJ. Role of immunotherapy for renal cell cancer in 2011. J Natl Compr Canc Netw. 2011;9(9):1011–1018. | ||
Rosenberg SA. IL-2: the first effective immunotherapy for human cancer. J Immunol. 2014;192(12):5451–5458. | ||
Kondo H, Hazama S, Kawaoka T, et al. Adoptive immunotherapy for pancreatic cancer using MUC1 peptide-pulsed dendritic cells and activated T lymphocytes. Anticancer Res. 2008;28(1B):379–387. | ||
Lipson EJ, Drake CG. Ipilimumab: an anti-CTLA-4 antibody for metastatic melanoma. Clin Cancer Res. 2011;17(22):6958–6962. | ||
Davar D, Tarhini AA, Kirkwood JM. Adjuvant immunotherapy of melanoma and development of new approaches using the neoadjuvant approach. Clin Dermatol. 2013;31(3):237–250. | ||
Nastoupil LJ, Neelapu SS. Novel immunologic approaches in lymphoma: unleashing the brakes on the immune system. Curr Oncol Rep. 2015;17(7):30. | ||
Pollack A. Immunotherapy drug fails lung cancer trial. New York Times 2016 Aug 6;Page B1. | ||
Maluish AE, Reid JW, Crisp EA, et al. Immunomodulatory effects of poly(I,C)-LC in cancer patients. J Biol Response Mod. 1985;4(6):656–663. | ||
Akira S, Takeda K, Kaisho T. Toll-like receptors: critical proteins linking innate and acquired immunity. Nat Immunol. 2001;2(8):675–680. | ||
Ammi R, De Waele J, Willemen Y, et al. Poly(I:C) as cancer vaccine adjuvant: knocking on the door of medical breakthroughs. Pharmacol Ther. 2015;146:120–131. | ||
Salazar AM, Levy HB, Ondra S, et al. Long-term treatment of malignant gliomas with intramuscularly administered polyinosinic-polycytidylic acid stabilized with polylysine and carboxymethylcellulose: an open pilot study. Neurosurgery. 1996;38(6):1096–1103; discussion 1103–1104. | ||
Salazar AM, Erlich RB, Mark A, Bhardwaj N, Herberman RB. Therapeutic in situ autovaccination against solid cancers with intratumoral poly-ICLC: case report, hypothesis, and clinical trial. Cancer Immunol Res. 2014;2(8):720–724. | ||
Gallucci S, Matzinger P. Danger signals: SOS to the immune system. Curr Opin Immunol. 2001;13(1):114–119. | ||
Garnett CT, Palena C, Chakraborty M, Tsang KY, Schlom J, Hodge JW. Sublethal irradiation of human tumor cells modulates phenotype resulting in enhanced killing by cytotoxic T lymphocytes. Cancer Res. 2004;64(21):7985–7994. | ||
Kudo-Saito C, Schlom J, Camphausen K, Coleman CN, Hodge JW. The requirement of multimodal therapy (vaccine, local tumor radiation, and reduction of suppressor cells) to eliminate established tumors. Clin Cancer Res. 2005;11(12):4533–4544. | ||
Strome SE, Voss S, Wilcox R, et al. Strategies for antigen loading of dendritic cells to enhance the antitumor immune response. Cancer Res. 2002;62(6):1884–1889. | ||
Zeng J, Harris TJ, Lim M, Drake CG, Tran PT. Immune modulation and stereotactic radiation: improving local and abscopal responses. Biomed Res Int. 2013;2013:658126. | ||
Formenti SC, Demaria S. Combining radiotherapy and cancer immunotherapy: a paradigm shift. J Natl Cancer Inst. 2013;105(4):256–265. | ||
Cheng JC, Wu JK, Huang CM, et al. Radiation-induced liver disease after three-dimensional conformal radiotherapy for patients with hepatocellular carcinoma: dosimetric analysis and implication. Int J Radiat Oncol Biol Phys. 2002;54(1):156–162. | ||
Uren RF, Howman-Giles R, Thompson JF. Patterns of lymphatic drainage from the skin in patients with melanoma. J Nucl Med. 2003;44(4):570–582. | ||
Wang JB, Abnet CC, Chen W, et al. Association between serum 25(OH) vitamin D, incident liver cancer and chronic liver disease mortality in the Linxian Nutrition Intervention Trials: a nested case-control study. Br J Cancer. 2013;109(7):1997–2004. | ||
Lagishetty V, Liu NQ, Hewison M. Vitamin D metabolism and innate immunity. Mol Cell Endocrinol. 2011;347(1–2):97–105. | ||
Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010;30(1):52–60. | ||
Institute of Medicine. Organ procurement and transplantation: assessing current policies and the potential impact of the DHHS final rule. Washington, DC: The National Academies Press;1999. | ||
Levine AS, Sivulich M, Wiernik PH, Levy HB. Initial clinical trials in cancer patients of polyriboinosinic-polyribocytidylic acid stabilized with poly-l-lysine, in carboxymethylcellulose [poly(ICLC)], a highly effective interferon inducer. Cancer Res. 1979;39(5):1645–1650. | ||
Pichon N, Maisonnette F, Pichon-Lefièvre F, Valleix D, Pillegand B. Hepatocarcinoma with congenital agenesis of the portal vein. Jpn J Clin Oncol. 2003;33(6):314–316. | ||
Huo YR, Eslick GD. Transcatheter arterial chemoembolization plus radiotherapy compared with chemoembolization alone for hepatocellular carcinoma: a systematic review and meta-analysis. JAMA Oncol. 2015;1(6):756–765. | ||
Kohles N, Nagel D, Jüngst D, Stieber P, Holdenrieder S. Predictive value of immunogenic cell death biomarkers HMGB1, sRAGE, and DNase in liver cancer patients receiving transarterial chemoembolization therapy. Tumour Biol. 2012;33(6):2401–2409. | ||
Piano A, Titorenko VI. The intricate interplay between mechanisms underlying aging and cancer. Aging Dis. 2015;6(1):56–75. | ||
Chen WH, Kozlovsky BF, Effros RB, Grubeck-Loebenstein B, Edelman R, Sztein MB. Vaccination in the elderly: an immunological perspective. Trends Immunol. 2009;30(7):351–359. | ||
Mangia A, Piazzolla V. Overall efficacy and safety results of sofosbuvir-based therapies in Phase II and III studies. Dig Liver Dis. 2014;46 Suppl 5:S179–S185. | ||
Nagato T, Lee YR, Harabuchi Y, Celis, E. Combinatorial immunotherapy of polyinosinic-polycytidylic acid and blockade of programmed death-ligand 1 induce effective CD8 T-cell responses against established tumors. Clin Cancer Res. 2014;20(5):1223–1234. | ||
Yu MC, Chen CH, Liang X, et al. Inhibition of T-cell responses by hepatic stellate cells via B7-H1-mediated T-cell apoptosis in mice. Hepatology. 2004;40(6):1312–1321. |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.