Back to Journals » Clinical Ophthalmology » Volume 17
A Pharmacovigilance Study of Drug-Induced Glaucoma Utilizing the Japanese Adverse Event Reporting System
Authors Kozaru M, Iida T, Hosohata K
Received 8 September 2023
Accepted for publication 15 November 2023
Published 29 November 2023 Volume 2023:17 Pages 3645—3653
DOI https://doi.org/10.2147/OPTH.S439255
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Mariko Kozaru, Tatsuya Iida, Keiko Hosohata
Education and Research Center for Clinical Pharmacy, Faculty of Pharmacy, Osaka Medical and Pharmaceutical University, Takatsuki, Osaka, 569-1094, Japan
Correspondence: Keiko Hosohata, Education and Research Center for Clinical Pharmacy, Faculty of Pharmacy, Osaka Medical and Pharmaceutical University, 4-20-1 Nasahara, Takatsuki, Osaka, 569-1094, Japan, Tel +81-72-690-1271, Fax +81-72-690-362, Email [email protected]
Purpose: Clinically, glaucoma is a serious problem because it is asymptomatic until a relatively late stage in most cases, which can lead to delays in the diagnosis and treatment of the disease. The purpose of this study was to clarify the rank-order of the association of glaucoma with the causative drugs using a spontaneous reporting system database.
Methods: Data were extracted from the Japanese Adverse Drug Event Report database of the Pharmaceuticals and Medical Devices Agency (Japan). Based on reports of glaucoma caused by all drugs, we calculated the reporting odds ratio (ROR) and 95% confidence interval (CI) for glaucoma.
Results: Among 609 reports of adverse events corresponding to glaucoma (46%, women), the most frequently implicated drug were steroids (prednisolone, betamethasone sodium phosphate, triamcinolone acetonide, and fluorometholone), pregabalin, ranibizumab, crizotinib, tacrolimus hydrate, darbepoetin alfa, and foscarnet sodium hydrate. Among 207 reports involved in angle-closure glaucoma (86%, women), anticholinergic drug and antidepressants ranked high and showed signals. Signals were also detected in bromazepam (ROR, 69.7; 95% CI, 30.9– 157.5), oral brotizolam (ROR, 16.6; 95% CI, 6.18– 44.8), and oral milnacipran hydrochloride (ROR, 22.8; 95% CI, 8.46– 61.4) for angle-closure glaucoma.
Conclusion: A national pharmacovigilance database enabled us to identify the drugs that frequently induce glaucoma. The likelihood of the reporting of glaucoma varied among the drugs, which should be used carefully in clinical practice to avoid it.
Keywords: glaucoma, pharmacovigilance, adverse drug reactions, reporting odds ratio, spontaneous reporting system
Introduction
Glaucoma is one of the leading causes of irreversible blindness worldwide,1 and is a disease of great social importance. The proportion of glaucoma is known to increase with age and the global prevalence of those was approximately 3–5% for people aged 40–80 years.2 Glaucoma is broadly divided into 2 categories: open-angle glaucoma and angle-closure glaucoma. The common feature for all forms of glaucoma is loss of retinal ganglion cells, thinning of the retinal nerve fiber layer, and cupping of the optic disc.3 Glaucoma can be mostly caused by elevated intraocular pressure,4 and also secondarily by trauma or medications.5 Drug-induced glaucoma occurs when the drugs interfere with the drainage of the aqueous humor that fills the eye, causing an abnormal increase in intraocular pressure. This elevated pressure without optic nerve damage is referred to as “ocular hypertension”. The most common subjective symptoms of glaucoma are the appearance of areas that cannot be seen or a narrowing of the range of vision. However, we see with both eyes in daily life and the disease progresses slowly in most cases. Most people are almost unaware of visual field disturbances in the early stages of the disease of glaucoma, leading to irreversible blindness. Therefore, it is important to know which drugs more often cause glaucoma in clinical settings.
There is a growing consensus that a detailed evaluation of the information through pharmacovigilance activities is important for all drugs to ensure their safe use.6,7 Of note, pharmacovigilance practices can improve information feedback to medical staff and their patients in a timely manner, thereby reducing the overall risk to patients. Certainly, drugs are approved for clinical use on the basis of indicating a satisfactory balance between benefits and risks. However, the safety profile of drugs can change over time as their use expands with patient characteristics and an increase in the number of patients exposed. Spontaneous reporting systems represent a primary source of information to detect safety signals. The Japanese Adverse Drug Event Report (JADER) database is a published large database managed by the Pharmaceuticals and Medical Devices Agency (PMDA) for the pharmacovigilance approach. The objective of this study was to identify the drugs reported most frequently reported to be associated with glaucoma using a spontaneous reporting system database.
Methods
The JADER reports were downloaded from the PMDA website (http://www.pmda.go.jp/safety/info-services/drugs/adr-info/suspected-adr/0003.html), which contains data on the adverse effects of medications and patient information in Japan since April 1, 2004. We used 378,533 cases of data from JADER between April 2004 and January 2017. JADER consists of 4 datasets: patient demographic information (DEMO), drug information (DRUG), adverse events (REAC), and medical history (HISTO). Details of this database have been described previously.8–16 For the current analyses, we only extracted cases that were classified as “suspected medicine” since a “suspected medicine” is defined as a pharmaceutical product with which an adverse event is suspected to be associated and analyzed the reports of suspected drugs and adverse drug reactions (ADRs).
The classification and standardization of adverse drug reactions in JADER data is referred to the Medical Dictionary for Regulatory Activities (MedDRA). In the JADER database, each report is coded using Preferred Terms (PTs) from MedDRA. In MedDRA, a given PT can be assigned to a specific High-level Term (HLT), High-level Group Term (HLGT), and System Organ Class (SOC) level, but each HLT, HLGT, and SOC often contains multiple PT. Based on JADER database, information concerning glaucoma in PT Name coded in MedDRA (version 20.1) was collected. Subsequently, we excluded reports without information of sex (n = 29,976) and age (n = 49,713) from the data (n = 1,984,122). After exclusion, data were available 1,904,433 reports.
We compiled a cross-tabulation table based on two classifications: the presence or absence of glaucoma and the presence or absence of the suspected medicine. Then, we calculated the reporting odds ratio (ROR) by the following formula.
a: the number of patients with a target event when they received a target drug.
b: the number of patients with non-target adverse events when they received a target drug.
c: the number of patients with a target event when they received non-target drugs.
d: the number of patients with non-target adverse events when they received non-target drugs.
Generally, ROR is used with the spontaneous reporting database as an index of the relative risk of drug-associated adverse events. A signal is considered to be present when the lower limit of the 95% confidence interval (CI) of the ROR is greater than one. In this database, age, height, and weight information are indicated as follows: age in decades, height in centimeters, and weight in kilograms. These data are not given as continuous variables because of privacy considerations. Because the prevalence of glaucoma is high at older ages and among men,17 ROR was adjusted for age (< 60s and ≥ 60s) and sex. Statistical significance was set at p < 0.05. All analyses were performed with SPSS for Windows software (ver. 19.0; SPSS Inc., Tokyo, Japan).
Results
During the study period, a total number of 1,904,433 ADR reports including both age and sex information were obtained. Among cases represented by glaucoma, we obtained glaucoma (609 reports), angle-closure glaucoma (207 reports), normal-tension glaucoma (17 reports), open-angle glaucoma (9 reports), and exfoliation glaucoma (1 report). Reports of glaucoma and angle-closure glaucoma accounted for the majority of cases, so further analysis was conducted focused on them. The patients’ characteristics are shown in Table 1. Glaucoma was almost equally reported in men (54%) and women (46%); whereas angle-closure glaucoma was reported more frequently in women (86%). According to the age distribution of the study population, glaucoma occurred frequently in their 60s (19.7%) and angle-closure glaucoma did in their 70s (38.6%). Of note, the rate of remission from drug-induced glaucoma was high (20.5%); whereas the rate of recovery from angle-closure glaucoma was high (34.3%).
 |
Table 1 Characteristics of Study Patients |
In our analysis, 186 and 106 different drugs were identified as “suspected medicine” which were involved in glaucoma and angle-closure glaucoma, respectively. Of these, the 10 most frequently reported drugs inducing glaucoma were listed in Tables 2 and 3. As shown in Table 2, the most frequently reported drugs were prednisolone (61 reports), pregabalin (37 reports), and ranibizumab (31 reports). Of those, we analyzed ROR of each drug with most frequent route of administration. As shown in Table 4, 10 medications yielded positive signals, with lower CI of ROR of greater than 1. Of note, the association with glaucoma was more noteworthy for fluorometholone eye-drop (ROR, 227.8; 95% CI, 126.1–411.4), triamcinolone acetonide eye-drop (ROR, 201.6; 95% CI, 114.4–355.1), and intravenous foscarnet sodium hydrate (ROR, 57.5; 95% CI, 30.6–107.9).
 |
Table 2 Most Frequently Reported Drugs That Induce Glaucoma |
 |
Table 3 Most Frequently Reported Drugs That Induce Angle-Closure Glaucoma |
 |
Table 4 Most Frequently Reported Drugs That Induce Glaucoma, focusing on the administration route |
As for angle-closure glaucoma, the most frequently reported drugs were atropine sulfate hydrate, followed by scopolamine butylbromide, fluvoxamine maleate, and bromazepam (Table 3). Of those, we analyzed ROR of each drug with most frequent route of administration (Table 5). Angle-closure glaucoma was strongly associated with intravenous atropine sulfate hydrate (ROR, 225.1; 95% CI, 105.1–482.5), inhalational tiotropium bromide hydrate (ROR, 97.4; 95% CI, 36.0–263.5), and oral bromazepam (ROR, 69.7; 95% CI, 30.9–157.5). Even the same reports, there were variety of strength of association of angle-closure glaucoma with inhalational tiotropium bromide hydrate (ROR, 97.4; 95% CI, 36.0–263.5) and oral aripiprazole (ROR, 4.12; 95 CI, 1.53–11.1).
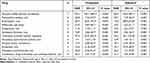 |
Table 5 Most Frequently Reported Drugs That Induce Angle-Closure Glaucoma, focusing on the administration route |
Discussion
This is the first pharmacovigilance study to provide a comprehensive overview of the occurrence and characteristics of patients with drug-induced glaucoma, based on information from the JADER database. In our results, glaucoma and angle-closure glaucoma tended to show a peak age of onset in patients in their 60s and 70s, respectively. Glaucoma was almost equally reported in men and women; whereas, angle-closure glaucoma occurred more often in women. The present study first demonstrated that oral crizotinib, intravenous darbepoetin alfa, and intravenous foscarnet sodium hydrate were associated with glaucoma, and that oral bromazepam, oral brotizolam, and oral milnacipran hydrochloride were associated with angle-closure glaucoma.
In our analysis, it is noteworthy that many of the patients with glaucoma and angle-closure glaucoma were elderly (most frequently in their 60s). These results are consistent with self-reported glaucoma prevalence in elderly patients.18 Elderly patients often have a significant increase in visual impairment with age. Especially in the elderly, the aqueous humour flow is often reduced, resulting in increased intraocular pressure. Age may also play a role in neuroinflammation balance and in retinal environment.19
In the present study, sensitive and quantitative method based on the disproportional reporting rate, such as ROR, has been utilized to capture the drug-related risk for a particular ADR. Our findings that steroids such as prednisolone, betamethasone sodium phosphate, and triamcinolone acetonide, and fluorometholone were associated with glaucoma are consistent with previous studies.20–22 Steroids induce eye pressure with an open angle systematically, topically, or intravitreally.5,23 In our database, it is possible that reports as “glaucoma” may include “open-angle glaucoma”. Our results revealed that pregabalin induced glaucoma. This is consistent with a nested case-control study that pregabalin use is associated with incidence of glaucoma.24 Animal experiments demonstrated that pregabalin reduce intraocular pressure, and underlying mechanisms are thought to be that pregabalin binds to CACNA2D1, which causes the α1 pore to close, leading to a decrease in Ca2+ influx into cells and a resultant decrease in free cytosolic Ca2+. Consequently, the cells relax and aqueous humor inflow may be reduced and/or outflow may be increased, leading to a reduction in intraocular pressure.25 As for anti-VEGF agents (bevacizumab, ranibizumab, and aflibercept), our study revealed that ranibizumab was associated with glaucoma, which is consistent with the results of several studies.26–29 For example, a retrospective cohort study involving nondiabetic patients without pre-existing glaucoma revealed that the incidence of intraocular pressure increase was higher among bevacizumab and ranibizumab users compared with aflibercept users.27 On the other hand, one retrospective and nationwide cohort study showed that no significant differences in the risk of major arterial thromboembolic events and glaucoma were found between ranibizumab and aflibercept.28 Another retrospective chart review of patients receiving intravitreal ranibizumab (0.5 mg) and/or bevacizumab (1.25 mg) injection showed that incidence of delayed and sustained ocular hypertension is low after their single or multiple intravitreal injections.29 Several studies reported that aflibercept is less risk of glaucoma, and our results did not include aflibercept in the list of most frequently reported drugs inducing glaucoma. Among VEGF inhibitors, aflibercept binds to VEGF-B and PlGF in addition to VEGF-A (the target of bevacizumab and ranibizumab), which differentiate its pharmacodynamic effects from bevacizumab and ranibizumab. It is known that VEGF inhibitors can facilitate to up-regulate other growth factors such as PlGF, which acts to sustain pathological angiogenesis and inflammation and is not involved in physiological angiogenic processes.30 Repeated intravitreal injections of ranibizumab and bevacizumab may promote an inflammatory response by increase in PlGF levels in the eye, which could lead to treated eyes being at a higher risk of inflammatory-related intraocular pressure increase than aflibercept. As for tacrolimus hydrate, our findings that it was associated with glaucoma; however, it is not agreement with a retrospective longitudinal study that there was no significant difference in incidence of primary open-angle glaucoma between FK506-treated patients (15%) and non-FK506 group (22%).31 Interestingly, our results revealed that oral crizotinib, intravenous darbepoetin alfa, and intravenous foscarnet sodium hydrate were significantly associated with glaucoma. There have been few studies of them involving with glaucoma (Table 6).
 |
Table 6 Comparison of Our Study with Previous Studies Regarding Glaucoma |
As for drug-induced angle-closure glaucoma, we found frequently in women (86.0%) and over 60 years, which is line with previous studies showing high prevalence of women and older age in drug-induced angle-closure glaucoma.32,33 In our results, anticholinergic drugs (atropine and tiotropium), antidepressants (fluvoxamine, bromazepam, aripiprazole, quetiapine, paroxetine, brotizolam, and milnacipran), VEGF inhibitors (aflibercept), and fentanyl ranked in top-10 drugs which is associated with angle-closure glaucoma. This observation is in accordance with previous studies that the main causes of drug-induced angle-closure glaucoma were anticholinergic drug,34,35 antidepressants,36–39 VEGF inhibitors,40 and fentanyl.41 Interestingly, there were few studies showing the association of oral bromazepam, brotizolam, milnacipran hydrochloride with angle-closure glaucoma (Table 7). We found that signals were detected in oral bromazepam (ROR, 69.7; 95% CI, 30.9–157.5), oral brotizolam (ROR, 16.6; 95% CI, 6.18–44.8), and oral milnacipran hydrochloride (ROR, 22.8; 95% CI, 8.46–61.4) for angle-closure glaucoma. To the best of our knowledge, this is the first study to show the association of oral bromazepam, oral bromazepam, and oral milnacipran hydrochloride with angle-closure glaucoma.
 |
Table 7 Comparison of Our Study with Previous Studies Regarding Angle-Closure Glaucoma |
The JADER database is considered a useful tool to screen potential associations in drug-induced glaucoma; however, several limitations inherent to spontaneous reporting are included. First, the JADER database has various biases, such as the lack of a denominator that indicates the total number of patients who received the drugs of interest, as well as missing data and confounding factors. Second, the ROR does not provide a robust indication of the signal strength. In this kind of study, the ROR corresponds to the risk of spontaneous notification of an ADR and not the risk of glaucoma occurrence per se. Finally, the present method did not provide us with detailed clinical information on the patients’ clinical status such as on unknown etiology of underlying disease and the possible role of disease in the reported adverse event.
Conclusion
In conclusion, the rank-orders of the suspected drugs associated with glaucoma and angle-closure glaucoma were determined using a nationwide pharmacovigilance database. Especially, oral crizotinib, intravenous darbepoetin alfa, and intravenous foscarnet sodium hydrate were associated with glaucoma, and that oral bromazepam, oral brotizolam, and oral milnacipran hydrochloride were associated with angle-closure glaucoma. These data strongly suggest that physicians can be alerted to take precautions against drugs inducing glaucoma and angle-closure glaucoma, select appropriate therapeutic medicine, and potentially avoid glaucoma.
Abbreviations
CI, confidence interval; ROR, reporting odds ratio.
Data Sharing Statement
The datasets during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Funding
K. Hosohata received research support from the Science Research Promotion Fund.
Disclosure
The authors report no conflicts of interest in this work.
References
1. GBD 2019 Blindness and Vision Impairement Collaborators. Causes of blindness and vision impairment in 2020 and trends over 30 years, and prevalence of avoidable blindness in relation to vision 2020: the right to sight: an analysis for the global burden of disease study. Lancet Glob Health. 2021;9:e144–e160. doi:10.1016/S2214-109X(20)30489-7
2. Tham YC, Li X, Wong TY, Quigley HA, Aung T, Cheng CY. Global prevalence of glaucoma and projections of glaucoma burden through 2040: a systematic review and meta-analysis. Ophthalmology. 2014;121:2081–2090. doi:10.1016/j.ophtha.2014.05.013
3. Jonas JB, Aung T, Bourne RR, Bron AM, Ritch R, Panda-Jonas S. Glaucoma. Lancet. 2017;390:2183–2193. doi:10.1016/S0140-6736(17)31469-1
4. The AGIS Investigators. The advanced glaucoma intervention study (AGIS): 7. The relationship between control of intraocular pressure and visual field deterioration. Am J Ophthalmol. 2000;130:429–440. doi:10.1016/S0002-9394(00)00538-9
5. Razeghinejad MR, Myers JS, Katz LJ. Iatrogenic glaucoma secondary to medications. Am J Med. 2011;124:20–25. doi:10.1016/j.amjmed.2010.08.011
6. World Health Organization. The Safety of Medicines in Public Health Programmes: Pharmacovigilance an Essential Tool. Geneva, Switzerland: WHO Press, World Health Organization; 2006.
7. Ramirez E, Carcas AJ, Borobia AM, et al. A pharmacovigilance program from laboratory signals for the detection and reporting of serious adverse drug reactions in hospitalized patients. Clin Pharmacol Ther. 2010;87:74–86. doi:10.1038/clpt.2009.185
8. Kambara H, Oyama S, Inada A, Niinomi I, Wakabayashi T, Hosohata K. Current status of adverse event profile of tacrolimus in patients with solid organ transplantation from a pharmacovigilance study. Int J Clin Pharmacol Ther. 2021;59:753–759. doi:10.5414/CP204016
9. Wakabayashi T, Ueno S, Nakatsuji T, et al. Safety profiles of new xanthine oxidase inhibitors: a post-marketing study. Int J Clin Pharmacol Ther. 2021;59:372–377. doi:10.5414/CP203898
10. Kambara H, Hosohata K, Nakatsuji T, et al. Safety profile of vonoprazan compared with proton pump inhibitors: insight from a pharmacovigilance study. Die Pharmazie. 2020;75:527–530. doi:10.1691/ph.2020.0604
11. Niinomi I, Hosohata K, Oyama S, Inada A, Hirai T, Iwanaga K. Drug-induced thrombotic microangiopathy using the Japanese pharmacovigilance database. Int J Clin Pharmacol Ther. 2020;58:543–549. doi:10.5414/CP203724
12. Niinomi I, Hosohata K, Oyama S, Inada A, Wakabayashi T, Iwanaga K. Pharmacovigilance assessment of drug-induced acute pancreatitis using a spontaneous reporting database. Int J Toxicol. 2019;38:487–492. doi:10.1177/1091581819870717
13. Inada A, Oyama S, Niinomi I, Wakabayashi T, Iwanaga K, Hosohata K. Association of Stevens-Johnson syndrome and toxic epidermal necrolysis with antiepileptic drugs in pediatric patients: subgroup analysis based on a Japanese spontaneous database. Clin Pharm Ther. 2019;44:775–779. doi:10.1111/jcpt.13001
14. Hosohata K, Oyama S, Niinomi I, Wakabayashi T, Inada A, Iwanaga K. Comparison of safety profiles of new oral anticoagulants with warfarin using the Japanese spontaneous reporting database. Clin Drug Invest. 2019;39:665–670. doi:10.1007/s40261-019-00788-3
15. Hosohata K, Inada A, Oyama S, Niinomi I, Wakabayashi T, Iwanaga K. Adverse cutaneous drug reactions associated with old- and new- generation antiepileptic drugs using the Japanese pharmacovigilance database. Clin Drug Invest. 2019;39:363–368. doi:10.1007/s40261-019-00754-z
16. Hosohata K, Inada A, Oyama S, Furushima D, Yamada H, Iwanaga K. Surveillance of drugs that most frequently induce acute kidney injury: a pharmacovigilance approach. Clin Pharm Ther. 2019;44:49–53. doi:10.1111/jcpt.12748
17. Hashemi H, Khabazkhoob M, Nabovati P, et al. The prevalence of age-related eye disease in an elderly population. Ophthalmic Epidemiol. 2017;24:222–228. doi:10.1080/09286586.2016.1270335
18. Castellanos-Perilla N, Garcia-Cifuentes E, Pineda-Ortega J, et al. Self-reported glaucoma prevalence and related factors, contribution to reported visual impairment, and functional burden in a cross-sectional study in Colombia. Int Ophthalmol. 2023;43:2447–2455. doi:10.1007/s10792-023-02643-z
19. Bongaarts J. Human population growth and the demographic transition. Philos Trans R Soc Lond B Biol Sci. 2009;364:2985–2990. doi:10.1098/rstb.2009.0137
20. Chen YH, Gepstein R, Sharief L, et al. Outcome and risk of ocular complications of managing children with chronic anterior uveitis with topical rimexolone 1. Int Ophthalmol. 2020;40:1061–1068. doi:10.1007/s10792-020-01358-9
21. Shokoohi-Rad S, Daneshvar R, Jafarian-Shahri M, Rajaee P. Comparison between betamethasone, fluorometholone and loteprednol etabonate on intraocular pressure in patients after keratorefractive surgery. J Curr Ophthalmol. 2018;30(2):130–135. doi:10.1016/j.joco.2017.11.008
22. Kuley B, Storey PP, Pancholy M, et al. Ocular hypertension following 40 mg sub-tenon triamcinolone versus 0.7 mg dexamethasone implant versus 2 mg intravitreal triamcinolone. Can J Ophthalmol. 2020;55:480–485. doi:10.1016/j.jcjo.2020.06.021
23. Tripathi RC, Tripathi BJ, Haggerty C. Drug-induced glaucomas: mechanism and management. Drug Safety. 2003;26:749–767. doi:10.2165/00002018-200326110-00002
24. Browne MJ, Zakrzewski H, Carleton B, Etminan M, Mikelberg FS. Association of gabapentin or pregabalin use and incidence of acute angle-closure glaucoma. J Glaucoma. 2019;28:777–779. doi:10.1097/IJG.0000000000001330
25. Chintalapudi SR, Maria D, Di Wang X, Bailey JNC, Consortium N, et al.; International Glaucoma Genetics c. Systems genetics identifies a role for cacna2d1 regulation in elevated intraocular pressure and glaucoma susceptibility. Nat Commun. 2017;8:1755. doi:10.1038/s41467-017-00837-5
26. Gabrielle PH, Nguyen V, Wolff B, et al. Intraocular pressure changes and vascular endothelial growth factor inhibitor use in various retinal diseases: long-term outcomes in routine clinical practice: data from the fight retinal blindness! Registry. Ophthalmology Retina. 2020;4:861–870. doi:10.1016/j.oret.2020.06.020
27. Spini A, Giometto S, Donnini S, et al. Risk of intraocular pressure increase with intravitreal injections of vascular endothelial growth factor inhibitors: a cohort study. Am J Ophthalmol. 2023;248:45–50. doi:10.1016/j.ajo.2022.11.015
28. Chang YH, Chien LN, Chen WT, Lin IC. Comparison of risks of arterial thromboembolic events and glaucoma with ranibizumab and aflibercept intravitreous injection: a nationwide population-based cohort study. PLoS One. 2022;17:e0267088. doi:10.1371/journal.pone.0267088
29. Mansoori T, Agraharam SG, Manwani S, Balakrishna N. Intraocular pressure changes after intravitreal bevacizumab or ranibizumab injection: a retrospective study. J Curr Ophthalmol. 2021;33:6–11. doi:10.4103/JOCO.JOCO_5_20
30. Piette J, Yaniv M. Two different factors bind to the alpha-domain of the polyoma virus enhancer, one of which also interacts with the sv40 and c-fos enhancers. EMBO J. 1987;6:1331–1337. doi:10.1002/j.1460-2075.1987.tb02372.x
31. Reffatto V, Gupta PK, Williams T, Schmitz-Brown ME, Vizzeri G. Fk506 treatment prevents retinal nerve fiber layer thinning in organ-transplanted glaucoma patients: a retrospective longitudinal study. Cureus. 2021;13:e18192. doi:10.7759/cureus.18192
32. Lachkar Y, Bouassida W. Drug-induced acute angle closure glaucoma. Curr Opin Ophthalmol. 2007;18:129–133. doi:10.1097/ICU.0b013e32808738d5
33. Vadot E, Grateau C. [The frequency of acute glaucoma crises. Implications for the detection of the risk of angle-closure]. Bull Soc Ophtalmol Fr. 1989;89:675–677. French.
34. Mandak JS, Minerva P, Wilson TW, Smith EK. Angle closure glaucoma complicating systemic atropine use in the cardiac catheterization laboratory. Catheter Cardiovasc Diag. 1996;39:262–264. doi:10.1002/(SICI)1097-0304(199611)39:3<262::AID-CCD11>3.0.CO;2-H
35. Oksuz H, Tamer C, Akoglu S, Duru M. Acute angle-closure glaucoma precipitated by local tiotropium absorption. Pulm Pharmacol Ther. 2007;20:627–628. doi:10.1016/j.pupt.2006.07.002
36. Jimenez-Jimenez FJ, Orti-Pareja M, Zurdo JM. Aggravation of glaucoma with fluvoxamine. Ann Pharmacother. 2001;35:1565–1566. doi:10.1345/aph.1Z440
37. Shen E, Farukhi S, Schmutz M, Mosaed S. Acute angle-closure glaucoma associated with aripiprazole in the setting of plateau iris configuration. J Glaucoma. 2018;27:e40–e43. doi:10.1097/IJG.0000000000000836
38. Bennett HG, Wyllie AM. Paroxetine and acute angle-closure glaucoma. Eye. 1999;13(5):691–692. doi:10.1038/eye.1999.196
39. Matsuo M, Sano I, Ikeda Y, Fujihara E, Tanito M. Intraoperative floppy-iris syndrome associated with use of antipsychotic drugs. Can J Ophthalmol. 2016;51:294–296. doi:10.1016/j.jcjo.2016.02.008
40. Scott IU, VanVeldhuisen PC, Ip MS, et al. Effect of bevacizumab vs aflibercept on visual acuity among patients with macular edema due to central retinal vein occlusion: the score2 randomized clinical trial. JAMA. 2017;317:2072–2087. doi:10.1001/jama.2017.4568
41. Nitta Y, Kamekura N, Takuma S, Fujisawa T. Acute angle-closure glaucoma after general anesthesia for bone grafting. Anesth Prog. 2014;61:162–164. doi:10.2344/0003-3006-61.4.162
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

