Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 16
A Novel Variant in the Desmoplakin Gene in One Case of the Rare Carvajal Syndrome with Dilated Cardiomyopathy: A Case Report and Literature Review
Authors Zhao XJ, Bai CY, Li XY, Wang L, Wang RP, Xia Y, Liu G, Zhao HL , Xu HZ
Received 5 July 2023
Accepted for publication 14 September 2023
Published 29 September 2023 Volume 2023:16 Pages 2737—2748
DOI https://doi.org/10.2147/CCID.S429030
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jeffrey Weinberg
Xiu-Jie Zhao,1 Chun-Yu Bai,2 Xiao-Yan Li,1 Lei Wang,2 Ren-Ping Wang,2 Yue Xia,1,2 Gang Liu,1 Hong-Liang Zhao,1,* Hong-Zun Xu2,*
1Department of Cardiology, The First Hospital of Hebei Medical University, Shijiazhuang, 050031, People’s Republic of China; 2Department of Cardiology, Shijiazhuang Great Wall Cardiovascular Hospital, Shijiazhuang, 050035, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Hong-Liang Zhao; Hong-Zun Xu, Email [email protected]; [email protected]
Abstract: Carvajal syndrome is a rare hereditary cardiocutaneous syndrome caused by the variants of the desmoplakin (DSP) gene. In this study, we report a patient of Carvajal syndrome with a novel homozygous missense variant of DSP gene. We diagnosed a 7-year-old female patient with Carvajal syndrome characterized by dilated cardiomyopathy, palmoplantar keratoderma, woolly hair, and dental dysplasia, who disclosed a novel homozygous missense variant c.4597C > T (p.Q1533X) in exon 6 of the DSP gene found for the first time. Both her parents were heterozygous for the identified nonsense variant c.4597C > T (p.Q1533X) in DSP gene but neither showed evidence of Carvajal syndrome, indicating that this novel variant causes the disease in an autosomal recessive manner. Genotypes of Carvajal syndrome are even broader than so far anticipated. When patients with dilated cardiomyopathy, palmoplantar keratoderma, woolly hair, and dental dysplasia are found in clinical practice, Carvajal syndrome should be highly suspected, and family gene sequencing should be actively carried out.
Keywords: Carvajal syndrome, desmoplakin, genotype, variant, novel
Introduction
As a hereditary cardiocutaneous syndrome, Carvajal syndrome is characterized by dilated cardiomyopathy, palmoplantar keratoderma, woolly hair, and dental dysplasia.1 Carvajal syndrome was first identified by Rao et al in 19962 and then was described in detail by Carvajal⁃Huerta in 1998.3 Soon thereafter, in 2000, Norgett et al first found its etiology was mainly the variants of the desmoplakin (DSP) gene.4 Carvajal syndrome tends to develop in childhood and is mainly autosomal recessive, with occasional autosomal dominant inheritance.5 This syndrome is rare, and not many cases have been reported worldwide. Recently, we diagnosed one 7-year-old female with the Carvajal syndrome who visited hospital mainly due to the manifestations of de novo heart failure, which will be reported below with a literature review.
Case Presentation
The 7-year-old female patient was born normally as the first child of no-consanguineous parents of Chinese Han, with yellow-brown woolly hair on her forehead at birth. Before she was 4 years old, she had no health problems. Since then, palmoplantar keratoderma began to present on her palms and soles, and gradually spread to the fingers and toes. Meanwhile, she received irregular external ointment treatment. She developed dysplasia of deciduous teeth at the age of 5 years, with bilateral multiple incisors and molars oligodontia and dentition irregularity. At the age of 6 years, the child began to suffer from chest tightness, shortness of breath, and dyspnea when she was active, which gradually aggravated, and then unable to tolerate moderate daily activities. Shortly before coming to Shijiazhuang Great Wall Cardiovascular Hospital, she had been examined at Chongqing Children’s Hospital, diagnosed with dilated cardiomyopathy and the corresponding treatment. The patient has a healthy 4-year-old younger brother and their parents are also healthy. There is no similar patient in the family.
On physical examination, the patient was not in acute distress. Her intelligence was normal. Her weight was 21kg (25–30 centile), and her height was 125cm (25–30 centile). Her body temperature was 36.4 °C, pulse 76 beats/min, respiratory rate 20 breaths/min, and blood pressure 88/65mmHg. She had sparse, dark-brown, and woolly hair (Figure 1A and B). She also suffers from agenesis of primary teeth with multiple oligodontia (7 teeth) and odontoloxia (Figure 1C). Bilateral jugular veins were dilated slightly. Thick breath sounds could be heard in bilateral lungs, without obvious dry or wet rales. The apical beat was diffuse, cardiac boundaries were enlarged to both sides, and apical pulsation was located at the 7th intercostal of the left axillary-front line. The heart rate was 76 beats/min with premature beats, and a grade 2/6 systolic blowing-like murmurs could be detected at the mitral valve auscultation area. The abdomen was flat and soft, with no tenderness. The liver was palpable 3 cm below the costal margin, and the spleen was not touched. Striate palmoplantar keratoderma was observed on both palms, fingers, soles, halluces, and heels, with a rough and chapped surface (Figure 1D and F). Leukonychia also could be found on toenails (Figure 1E). There was mild edema in both legs and joints movements barrier-free.
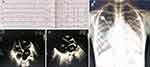
Laboratory tests showed that routine blood tests, blood glucose, liver function, renal function, electrolytes, myocardial enzymes, coagulation function, erythrocyte sedimentation rate, thyroid function, immunoglobulin (IgA, IgG, IgM), complement (C3, C4), procalcitonin, 25-hydroxyvitamin D3, trace elements were all normal. Screening indicators of hepatitis B virus, hepatitis C virus, adenovirus, Coxsackie, Epstein-Barr, syphilis, and human immunodeficiency virus were all negative and/or within the normal range. The B-type natriuretic peptide was 18,883.5 pg/mL (reference value: 0–450pg/mL). The 24-hour dynamic electrocardiogram indicated sinus rhythm, increased P wave amplitude, average heart rate of 74 beats/min, lowest heart rate of 55 beats/min, fastest heart rate of 139 beats/min, and 3561 ventricular premature beats (Figure 2A). Chest X-ray showed double-lung texture increased, together with heart shadow enlarged (Figure 2B). Cardiac magnetic resonance imaging (MRI) revealed that the heart was enlarged, especially the left heart, and the ventricular systolic and diastolic functions decreased. Color Doppler echocardiography demonstrated left ventricular end-diastolic diameter was 55 mm, left atrium 28*36*56 mm, right ventricular end-diastolic diameter 29 mm, right atrial diameter 33mm, left ventricular ejection fraction 25%, global heart enlargement, partial myocardial non-compaction of the left ventricle, diffuse decrease in ventricular septum and left ventricular wall motion amplitude, mild aortic regurgitation, moderate mitral regurgitation, mild tricuspid regurgitation, pulmonary hypertension (mild), mild pulmonary artery regurgitation, the main pulmonary artery and its branches widened. The diastolic and contractile functions of the left and right ventricles all decreased (Figure 2C and D). Abdominal color ultrasound suggested mild hepatic congestion.
Genetic testing was performed by high-throughput whole-exome sequencing (MyGenostics Inc., Beijing, China). The proband disclosed a homozygous missense variant c.4597C > T (p.Q1533X) in exon 6 of the DSP gene, ie the nucleotide at position 4597 in the coding region changed from cytosine to thymine, resulting in a senseless variant of the amino acid (Glutamine) at position 1533 (Figure 3). In reference to relevant document database, ClinVar database (https://www.ncbi.nlm.nih.gov/clinvar/), Gnomad database (http://www.gnomad-sg.org/), and the Human Gene Mutation Database (https://www.hgmd.cf.ac.uk/ac/index.php), up to now, there is no report on the variation of this gene locus. The proband’s parents were verified by Sanger sequencing (MyGenostics Inc., Beijing, China): they both carried the heterozygous variant c.4597C > T (p.Q1533X) in DSP gene and were both healthy (Figure 4).
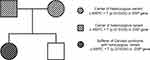 |
Figure 4 Pedigree chart of the family. |
According to clinical manifestations and DSP gene test results, the patient was diagnosed as (1) Carvajal syndrome; (2) dilated cardiomyopathy, heart failure (NYHA grade III), and arrhythmia as ventricular premature contraction. Then, the patient received comprehensive treatments: for heart failure and ventricular premature, using the diuretic, cardiotonic, inhibition of myocardial remodeling, inhibition of sympathetic excitation, traditional Chinese medicine decoction, and the symptoms were significantly improved; for palmoplantar keratoderma, oral vitamin A and E capsules were given once every other day, and 0.1% retinoic acid ointment and urea ointment were applied externally twice a day. One month later, the hand and foot palmoplantar keratoderma was better than before. The electrocardiogram remained premature ventricular contraction. Three months later, there was a significant improvement in palmoplantar keratoderma, and premature ventricular contraction was found to be reduced, but there was no significant change in color Doppler echocardiography. At present, the cardiology department and dermatology department are following up.
Discussion
This study, for the first time, identified a novel DSP variant of c.4597C > T (p.Q1533X) in exon 6, leading to the Carvajal syndrome in a sporadic case of patient, who manifested as dilated cardiomyopathy, woolly hair, palmoplantar keratosis, and teeth loss. Both the parents carried this variant in a heterozygous state, and they were both healthy, which indicated that this novel variant causes Carvajal syndrome in an autosomal recessive manner.
At present, Carvajal syndrome is believed to be mainly secondary to DSP gene variant.5 Desmosomes are tough and strong intercellular junctions, particularly abundant in the myocardium and epidermis, and DSP is the most abundant protein of desmosome.6 Therefore, once the DSP gene variant occurs, the desmosome undergoes structural changes, resulting in phenotypic changes in the heart, skin and other related organs and tissues. We summarized the characteristics of patients with Carvajal syndrome in published literature in Table 1. Most patients with Carvajal syndrome are autosomal recessive inheritance, and the variant hotspots are mainly located in exons 237–12 or 244,13–16 of DSP gene, while a few of them were autosomal dominant inheritance, and the variant regions were mainly in other exons of DSP gene.6,17–20 Furthermore, the earliest and most reports related to Carvajal syndrome were homozygous variants in DSP gene c.7901delG.4 To date, apart from a few special cases of Carvajal syndrome without DPS variant,5 many other DSP gene variants have been discovered successively4,6–32 (Table 1). While, in this case, a novel homozygous missense variant c.4597C > T (p.Q1533X) in exon 6 of the DSP gene was found for the first time.
 |
Table 1 Characteristics of Patients with Carvajal Syndrome in Published Literatures |
The phenotype of DSP variant is related to its inheritance mode. In the past, it was believed that Carvajal syndrome caused by DSP variant was a recessive inheritance, and in fact most cases were reported to be so.4,7,10,11,13–17,24,26,27,32 Nevertheless, with the gradual increasing and deepening of studies, some reports have also found spontaneous variant9,10,16,20,21,28 and dominant inheritance.18,19,23,25,30 Whether inherited or acquired, the main clinical manifestations of Carvajal syndrome are woolly hair, palmoplantar keratoderma, and cardiomyopathy involving more frequently the left ventricle.33 However, some cases showed biventricular7,14,17,18,21 or right ventricular4,12,13,21,23 involvement, and even no cardiac abnormalities.16,19,20,24,32 In this case, the main manifestations of the proband were global dilated cardiomyopathy, partial left ventricular myocardial no-compaction, wool hair, palmoplantar keratoderma, and leukonychia. Other reports suggest that other features of Carvajal syndrome include paratrichosis (alopecia11,12,27 or hypotrichosis)4,7,14,20,21,24,28,29,32, dental anomalies (oligodontia5,18–22,25 or enamel abnormalities)14, fingernail/toenail abnormalities (onychodystrophy,5,11,14,20,21 leukonychia,19,21,22,28 onychorrhexis,18 or onychogryphosis)24, recurrent syncopes,13,18,25 mucocutaneous blisters,11,14,24 and other rare manifestations papules (such as bilateral external rotation of the fifth toe,24 recurrent pharyngeal infections, recurrent diarrhea, left-sided hypoacusis,25 and hand wart-like lesions).26 The clinical phenotypes of Carvajal syndrome are varied, which is closely related to the variants of different DSP genes and their influence on the changes of N-terminal and C-terminal domain.4,14,17
Furthermore, what counts is some related diseases with similar clinical phenotypes need to be differentiated, especially the Naxos disease. With the main clinical features of arrhythmogenic right ventricular cardiomyopathy (ARVC), woolly hair, and palmoplantar keratoderma, Naxos disease was first described by Protonotarios et al in four families from Naxos island of Greece in 1986.34 Because both show similar heart, hair and skin lesions, earlier studies considered Carvajal syndrome and Naxos disease as one syndrome, or Carvajal syndrome as a variant of Naxos disease.8,23,35,36 However, with the increasing number of case reports and the deepening of investigations, researchers began to realize that Carvajal syndrome and Naxos disease are two different diseases. First, left cardiac involvement was more common in Carvajal syndrome,5,9–11,21,22,24–26,28,29,31 while right-ventricle involvement was more common in Naxos disease.23,36–38 Second, the plantopalmar keratoderma in Carvajal syndrome was often striate,4,18,21,29,32 which in Naxos disease was often diffuse.33,35–38 Third, Carvajal syndrome was often accompanied by dental abnormalities5,14,18–22,25 or nail abnormalities,5,11,14,18–22,24,28 while Naxos disease was less seen. However, with the increasing number of cases reported, the phenotypic differences between them gradually become less obvious. Then, genetic testing can be used to differentiate them——Carvajal syndrome is mainly caused by DSP gene variant,4,7–32,34 while Naxos disease is mainly caused by plakoglobin gene variant.34,37,38
Moreover, woolly hair is generally the first sign that patients with Carvajal syndrome may be detected at birth. However, woolly hair is not unique to Carvajal syndrome; it is also found in Naxos disease, mentioned above, and in other rare diseases such as Menkes disease (MD),39,40 skin fragility/woolly hair syndrome (SFWHS),41,42 and tricho-dento-osseus syndrome (TDOS).43,44 MD occurs due to X-linked genetic variant in the ATP7A gene. Woolly, sparse, lusterless, and tangled hair usually together with progressive neurodegeneration and connective tissue disturbances become the main features of MD.39,40 Similar to Carvajal syndrome, SFWHS is also caused by variants of DSP gene and presents with woolly hair and palmokeratosis. However, SFWHS is often accompanied by increased skin fragility and recurrent blisters at birth.41,42 TDOS is a rare autosomal-dominant ectodermal dysplasia, with woolly hair at birth, caused by variants in the DLX3 gene. However, as patients with TDOS age, their hair becomes thicker and straighter. Besides, almost all TDOS patients exhibit dental abnormalities.43,44 It is worth noting that neither SFWHS nor TDOS will present with cardiac disease, which can be as the basis for distinguishing them from Carvajal syndrome.41–44
Our study has several limitations. First, apart from the proband and her parents, we have been unable to trace the genotypes of other family members. Second, due to the limited information available, we could not further explore the function of the mutated exon. Third, more cases need to be collected to obtain the epidemiological data.
Conclusion
In this study, we presented a case of Carvajal syndrome performed as global dilated cardiomyopathy sparse woolly hair, striate palmoplantar keratoderma on both palms and soles, leukonychia, and dental dysplasia. A novel heterozygous variant c.4597C > T (p.Q1533X), never reported before, was found in exon 6 in DSP gene in our patient); there is moderate evidence for this variant to be considered pathogenic (MutationTaster, http://www.mutationtaster.org).45 The parents were both heterozygous for the identified nonsense variant without the phenotype of Carvajal syndrome, implying that the novel variant in DSP caused the disease in an autosomal recessive manner. As more cases are reported, more clinical phenotypes of Carvajal syndrome are emerging. Thus, future studies need to pay more attention to the correlation between genotype and phenotype.
Abbreviations
ARVC, arrhythmogenic right ventricular cardiomyopathy; DSP, desmoplakin; MD, Menkes disease; MRI, magnetic resonance imaging; SFWHS, skin fragility/woolly hair syndrome; TDOS, tricho-dento-osseus syndrome.
Ethics Approval and Informed Consent
All procedures performed in this study involving human participants were approved by the Ethics Committee of the Shijiazhuang Great Wall Cardiovascular Hospital. Publication of the case details was also approved by the Shijiazhuang Great Wall Cardiovascular Hospital.
Consent for Publication
Written informed consent was obtained from the patient’s father for publication of this Case report and any accompanying images.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was supported by Key R & D project of Hebei Province, China (grant number 182777229) and Medical Research Project of Hebei Province, China (grant number 20190449). The funding bodies played no role in design of the study, collection, analysis, and interpretation of the data and in writing the manuscript.
Disclosure
All authors report no conflicts of interest in this work.
References
1. Stöllberger C, Vujic I, Wollmann E, et al. Carvajal syndrome with oligodontia, hypoacusis, recurrent infections, and noncompaction. Int J Cardiol. 2016;203:825–827. doi:10.1016/j.ijcard.2015.11.050
2. Rao BH, Reddy IS, Chandra KS. Familial occurrence of a rare combination of dilated cardiomyopathy with palmoplantar keratoderma and curly hair. Indian Heart J. 1996;48(2):161–162.
3. Carvajal-Huerta L. Epidermolytic palmoplantar keratoderma with woolly hair and dilated cardiomyopathy. J Am Acad Dermatol. 1998;39(3):418–421. doi:10.1016/S0190-9622(98)70317-2
4. Norgett EE, Hatsell SJ, Carvajal-Huerta L, et al. Recessive mutation in desmoplakin disrupts desmoplakin-intermediate filament interactions and causes dilated cardiomyopathy, woolly hair and keratoderma. Hum Mol Genet. 2000;9(18):2761–2766. doi:10.1093/hmg/9.18.2761
5. Nehme N, El Malti R, Roux-Buisson N, et al. Evidence for genetic heterogeneity in Carvajal syndrome. Cell Tissue Res. 2012;348(2):261–264. doi:10.1007/s00441-012-1351-6
6. Norgett EE, Lucke TW, Bowers B, et al. Early death from cardiomyopathy in a family with autosomal dominant striate palmoplantar keratoderma and woolly hair associated with a novel insertion mutation in desmoplakin. J Invest Dermatol. 2006;126(7):1651–1654. doi:10.1038/sj.jid.5700291
7. Uzumcu A, Norgett EE, Dindar A, et al. Loss of desmoplakin isoform I causes early onset cardiomyopathy and heart failure in a Naxos-like syndrome. J Med Genet. 2006;43(2):e5. doi:10.1136/jmg.2005.032904
8. Prompona M, Kozlik-Feldmann R, Mueller-Hoecker J, et al. Images in cardiovascular medicine. Magnetic resonance imaging characteristics in Carvajal syndrome (variant of Naxos disease). Circulation. 2007;116(20):e524–30. doi:10.1161/CIRCULATIONAHA.107.704742
9. Krishnamurthy S, Adhisivam B, Hamilton RM, et al. Arrhythmogenic dilated cardiomyopathy due to a novel mutation in the desmoplakin gene. Indian J Pediatr. 2011;78(7):866–869. doi:10.1007/s12098-010-0319-3
10. Williams T, Machann W, Kühler L, et al. Novel desmoplakin mutation: juvenile biventricular cardiomyopathy with left ventricular non-compaction and acantholytic palmoplantar keratoderma. Clin Res Cardiol. 2011;100(12):1087–1093. doi:10.1007/s00392-011-0345-9
11. Antonov NK, Kingsbery MY, Rohena LO, et al. Early-onset heart failure, alopecia, and cutaneous abnormalities associated with a novel compound heterozygous mutation in desmoplakin. Pediatr Dermatol. 2015;32(1):102–108. doi:10.1111/pde.12484
12. Erolu E, Akalın F, Saylan Çevik B, et al. Arrhythmogenic right ventricular dysplasia, cutaneous manifestations and desmoplakin mutation: carvajal syndrome. Pediatr Int. 2018;60(10):987–989. doi:10.1111/ped.13683
13. Alcalai R, Metzger S, Rosenheck S, et al. A recessive mutation in desmoplakin causes arrhythmogenic right ventricular dysplasia, skin disorder, and woolly hair. J Am Coll Cardiol. 2003;42(2):319–337. doi:10.1016/S0735-1097(03)00628-4
14. Mahoney MG, Sadowski S, Brennan D, et al. Compound heterozygous desmoplakin mutations result in a phenotype with a combination of myocardial, skin, hair, and enamel abnormalities. J Invest Dermatol. 2010;130(4):968–978. doi:10.1038/jid.2009.357
15. Yermakovich D, Sivitskaya L, Vaikhanskaya T, et al. Novel desmoplakin mutations in familial Carvajal syndrome. Acta Myol. 2018;37(4):263–266.
16. Wu W, Zheng L, Pan C, et al. Novel desmoplakin mutations in a child with Carvajal syndrome. Chin J Dermatol. 2020;53(4):271–274. Chinese.
17. Tanaka A, Lai-Cheong JE, Café ME, et al. Novel truncating mutations in PKP1 and DSP cause similar skin phenotypes in two Brazilian families. Br J Dermatol. 2009;160(3):692–697. doi:10.1111/j.1365-2133.2008.08900.x
18. Chalabreysse L, Senni F, Bruyère P, et al. A new hypo/oligodontia syndrome: carvajal/Naxos syndrome secondary to desmoplakin-dominant mutations. J Dent Res. 2011;90(1):58–64. doi:10.1177/0022034510383984
19. Bitar F, Najjar T, Hayashi R, et al. A novel heterozygous mutation in desmoplakin gene in a Lebanese patient with Carvajal syndrome and tooth agenesis. J Eur Acad Dermatol Venereol. 2016;30(12):e217–9. doi:10.1111/jdv.13549
20. Zhang B, Liu L, Yu J, et al. A case of Carvajal syndrome caused by a spontaneous mutation in the desmoplakin gene. Chin J Dermatol. 2019;52(11):812–816. Chinese.
21. Keller DI, Stepowski D, Balmer C, et al. De novo heterozygous desmoplakin mutations leading to Naxos-Carvajal disease. Swiss Med Wkly. 2012;142:w13670. doi:10.4414/smw.2012.13670
22. Boulé S, Fressart V, Laux D, et al. Expanding the phenotype associated with a desmoplakin dominant mutation: carvajal/Naxos syndrome associated with leukonychia and oligodontia. Int J Cardiol. 2012;161(1):50–52. doi:10.1016/j.ijcard.2012.06.068
23. Tomberli B, Fornaro A, Bardi S, et al. A novel desmoplakin dominant mutation responsible for Carvajal/Naxos syndrome identified by exome sequencing. Eur Heart J. 2013;34(1):2959. doi:10.1093/eurheartj/eht309.P2959
24. Molho-Pessach V, Sheffer S, Siam R, et al. Two novel homozygous desmoplakin mutations in carvajal syndrome. Pediatr Dermatol. 2015;32(5):641–646. doi:10.1111/pde.12541
25. Finsterer J, Stöllberger C, Wollmann E, et al. Autosomal dominant Carvajal plus syndrome due to the novel desmoplakin mutation c.1678A > T (p.Ile560Phe). Mol Genet Metab Rep. 2016;8:1–3. doi:10.1016/j.ymgmr.2016.05.005
26. Ramoğlu MG, Uçar T, Ceylaner S, et al. A novel mutation in the desmoplakin gene in two female siblings with a rare form of dilated cardiomyopathy: carvajal syndrome. Anatol J Cardiol. 2017;18(6):435–436. doi:10.14744/AnatolJCardiol.2017.7867
27. Paller AS, Czarnowicki T, Renert-Yuval Y, et al. The spectrum of manifestations in desmoplakin gene (DSP) spectrin repeat 6 domain mutations: immunophenotyping and response to ustekinumab. J Am Acad Dermatol. 2018;78(3):498–505.e2. doi:10.1016/j.jaad.2017.10.026
28. Akdogan N, Incel-Uysal P, Cavdarli B, et al. A case of Carvajal syndrome associated with cervical neuroblastoma in an 8-year-old girl. Int J Dermatol. 2019;58(5):611–613. doi:10.1111/ijd.14154
29. Guerra L, Magliozzi M, Baban A, et al. Palmoplantar keratoderma and woolly hair revealing asymptomatic arrhythmogenic cardiomyopathy. Acta Derm Venereol. 2019;99(9):831–832. doi:10.2340/00015555-3216
30. De Gregorio MG, Girolami F, Tomberli B, et al. Comprehensive risk management in arrhythmogenic cardiomyopathy associated with autosomal dominant carvajal syndrome: protecting families. JACC Case Rep. 2020;2(6):925–929. doi:10.1016/j.jaccas.2020.03.033
31. Akintoye E, Ashwath ML. Cardiac magnetic resonance imaging findings in primary arrhythmogenic left ventricular cardiomyopathy with cardiocutaneous phenotype-Carvajal syndrome. Heart Rhythm Case Rep. 2021;7(5):312–315. doi:10.1016/j.hrcr.2021.01.024
32. Pigors M, Schwieger-Briel A, Cosgarea R, et al. Desmoplakin mutations with palmoplantar keratoderma, woolly hair and cardiomyopathy. Acta Derm Venereol. 2015;95(3):337–340. doi:10.2340/00015555-1974
33. Polivka L, Bodemer C, Hadj-Rabia S. Combination of palmoplantar keratoderma and hair shaft anomalies, the warning signal of severe arrhythmogenic cardiomyopathy: a systematic review on genetic desmosomal diseases. J Med Genet. 2016;53(5):289–295. doi:10.1136/jmedgenet-2015-103403
34. Protonotarios N, Tsatsopoulou A, Patsourakos P, et al. Cardiac abnormalities in familial palmoplantar keratosis. Br Heart J. 1986;56(4):321–326. doi:10.1136/hrt.56.4.321
35. Protonotarios N, Tsatsopoulou A. Naxos disease and Carvajal syndrome: cardiocutaneous disorders that highlight the pathogenesis and broaden the spectrum of arrhythmogenic right ventricular cardiomyopathy. Cardiovasc Pathol. 2004;13(4):185–194. doi:10.1016/j.carpath.2004.03.609
36. Salam AA, Remadevi KS, Kurup RP. Naxos disease and Carvajal variant. Indian Pediatr. 2013;50(6):596–598.
37. Protonotarios N, Tsatsopoulou A. Naxos disease: cardiocutaneous syndrome due to cell adhesion defect. Orphanet J Rare Dis. 2006;1:4. doi:10.1186/1750-1172-1-4
38. Baykan A, Olgar Ş, Argun M, et al. Different clinical presentations of Naxos disease and Carvajal syndrome: case series from a single tertiary center and review of the literature. Anatol J Cardiol. 2015;15(5):404–408. doi:10.5152/akd.2014.5413
39. Tümer Z, Møller LB. Menkes disease. Eur J Hum Genet. 2010;18(5):511–518. doi:10.1038/ejhg.2009.187
40. Ramani PK, Parayil Sankaran B. Menkes Kinky Hair Disease. In: StatPearls. Treasure Island (FL): StatPearls Publishing; 2023.
41. Whittock NV, Wan H, Morley SM, et al. Compound heterozygosity for non-sense and mis-sense mutations in desmoplakin underlies skin fragility/woolly hair syndrome. J Invest Dermatol. 2002;118(2):232–238. doi:10.1046/j.0022-202x.2001.01664.x
42. Peter DCV, Thomas M, Wilson NJ, et al. Skin fragility, woolly hair syndrome with a desmoplakin mutation - A case from India. Int J Dermatol. 2018;57(9):e73–5. doi:10.1111/ijd.14096
43. Li Y, Han D, Zhang H, et al. Morphological analyses and a novel de novo DLX3 mutation associated with tricho-dento-osseous syndrome in a Chinese family. Eur J Oral Sci. 2015;123(4):228–234. doi:10.1111/eos.12197
44. Perandones-González H, Rusiñol-Batlle L, Bosquez D, et al. Woolly hair in tricho-dento-osseous syndrome. Pediatr Dermatol. 2023. doi:10.1111/pde.15309
45. Richards S, Aziz N, Bale S, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American college of medical genetics and genomics and the association for molecular pathology. Genet Med. 2015;17(5):405–424. doi:10.1038/gim.2015.30
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.