Back to Journals » OncoTargets and Therapy » Volume 12
A Novel Scoring System Based on Peripheral Blood Test in Predicting Grade and Prognosis of Patients with Glioma
Authors Wu Y , Song Z, Sun K, Rong S , Gao P, Wang F, Sun T
Received 31 October 2019
Accepted for publication 13 December 2019
Published 24 December 2019 Volume 2019:12 Pages 11413—11423
DOI https://doi.org/10.2147/OTT.S236598
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Federico Perche
Yiyang Wu,1,* Zimu Song,1,* Kuisheng Sun,2 Shikuo Rong,2 Peng Gao,1 Feng Wang,1,2 Tao Sun1,2
1Department of Neurosurgery, General Hospital of Ningxia Medical University, Yinchuan, People’s Republic of China; 2Ningxia Key Laboratory of Cerebrocranial Diseases, Incubation Base of National Key Laboratory, Ningxia Medical University, Yinchuan, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Tao Sun; Feng Wang
Department of Neurosurgery, General Hospital of Ningxia Medical University, 804 Shengli Road, Yinchuan 750001, Ningxia, People’s Republic of China
Tel +86 13909506699
; +86 13895670991
Email [email protected]; [email protected]
Purpose: To explore the value of F-NLR-AGR score based on preoperative fibrinogen, neutrophil to lymphocyte ratio (NLR), and albumin to globulin ratio (AGR) in predicting the prognosis in patients with glioma.
Patients and methods: 203 glioma patients were retrospectively analyzed. Receiver-operating characteristic (ROC) curve analysis was used to determine the optimal cut-off values for NLR, AGR, and fibrinogen. According to these cut-off values, patients with high NLR (>1.90), low AGR (<1.54), and elevated fibrinogen (>2.61 g/L) were defined as a score of 3, if none of the patients’ three parameters met these standards they were given a score of 0, if any two or one parameter met these standards they were scored as 2 or 1, respectively. The correlation between F-NLR-AGR score and glioma grade was also evaluated.
Results: The three-year overall survival (OS) rate and the mean overall survival in patients with F-NLR-AGR=3 were lower than those of patients with F-NLR-AGR = 2, 1 or 0 [17.6% vs 35.2%, 66.9% or 83.7% (26.0 vs 39.0, 64.0 or 81.0 months), P<0.001]. Multivariate analysis revealed that age (HR=2.071; 95% CI=1.195–3.588; P=0.009), WHO grade (P<0.001), and F-NLR-AGR score (P<0.001) were independent prognostic factors for OS. Spearman’s rank correlation analysis revealed that F-NLR-AGR score was positively correlated with glioma grade (r=0.278, P<0.01).
Conclusion: Preoperative F-NLR-AGR score was correlated with glioma grading, high F-NLR-AGR score was an independent predictor of poor prognosis in glioma. Therefore, the scoring system may be applied in clinical practice to identify high-risk patients.
Keywords: glioma, fibrinogen, neutrophil to lymphocyte ratio, NLR, albumin to globulin ratio, AGR, prognosis
Introduction
Glioma is the most common malignant primary brain tumor.1 Despite great breakthroughs in diagnosis and treatment, the clinical efficacy and prognosis of glioma patients have not improved significantly. Glioblastoma, in particular, has a 5-year survival rate of only about 5%, making it one of the most difficult tumors to treat nowadays.2 According to 2016 World Health Organization (WHO) standards, glioma is classified into I-IV grades,3 different grades of glioma have great differences in treatment.4 New strategies for tumor therapy are also increasingly focusing on the use of appropriate prognostic factors for appropriate risk classification and design of subsequent treatment for tumor patients. Although researchers have found a large amount of molecular markers for glioma, such as: MGMT, IDH1/IDH2, and Ki-67,5–9 these are not available preoperatively. Therefore, a simple and reliable preoperative scoring system is needed to predict the grade and prognosis of glioma patients, and provide a treatment strategy accordingly.
Previously, the brain was considered an immune-privileged organ due to the presence of the blood-brain barrier (BBB).10 Nowadays, with the continuous progress of research, researchers gradually found that tumor related inflammation plays an important role in the progression and survival of tumor patients.11 Cancer-related inflammation, as the 7th hallmark of cancer, was proven to promote the tumor cell proliferation, invasion, and accelerate the metastasis.12 Like other malignancies, the occurrence and development of gliomas have also been demonstrated to be closely related to the inflammatory state and immune response.13–16 To date, studies have suggested that high neutrophil to lymphocyte ratio (NLR) was correlated with an unfavorable prognosis in many malignancies, including gliomas.17 In terms of systemic inflammatory response, the coagulation cascade also plays a key role in tumor progression and metastasis.18 Fibrinogen, a key factor in the coagulation cascade, has been reported as a key regulator of cancer progression.19 Recently, studies have shown that hyperfibrinogenemia is associated with poor prognosis of gliomas.20 In addition, albumin and globulin, as the main components of serum protein, are closely related to nutritional status and immune system activity. Albumin-to-globulin ratio (AGR) is considered to be an effective combination of two strong prognostic factors, it had been reported that lower AGR predicted poorer survival outcomes of many malignant tumors, including glioblastoma.21 Additionally, NLR, fibrinogen, and AGR have important prognostic significance in glioma patients and represent different pathophysiological mechanisms closely related to the prognosis of glioma patients. Because they are relatively independent and complementary, in order to provide a more accurate and comprehensive prognostic assessment tool for glioma patients, a novel prognostic scoring system based on plasma fibrinogen, NLR and AGR was proposed.
The aim of the present study was to assess correlation of F-NLR-AGR scoring system with glioma grading and overall survival (OS) in patients with glioma, so as to further clarify the clinical significance of F-NLR-AGR scoring system.
Materials and Methods
Patients
We conducted a retrospective study of glioma patients who underwent surgery in the Department of Neurosurgery, General Hospital of Ningxia Medical University between January 2010 and August 2016. The main inclusion criteria were as follows: 1) patients underwent surgical treatment and had a pathological diagnosis of gliomas; 2) tumor located in the supratentorial hemisphere; 3) patients with intact data of peripheral blood examination within 1 week before operation, including: blood routine, blood biochemistry and coagulation. 4) Complete clinical data. The main exclusion criteria were as follows: 1) patients had received preoperative treatment (including corticosteroid therapy); 2) patients with hematological disorders, active infectious diseases, or autoimmune diseases; 3) patients had other primary tumor; 4) tumor was recurrent glioma; 5) patients with a history of venous thrombosis or blood transfusion within the past 3 months; 6) patients with a history of infection within the past 1 month; 7) patient died during the perioperative period. According to inclusion and exclusion criteria, 203 patients were enrolled in the study. The last date of follow-up was 31th July 2019. OS was defined as the duration from the date of surgery to death. The Ethics Committee of Ningxia Medical University approved this study and requirement for signed informed consent from patients was waived because of the retrospective nature of the analysis. All patient data were treated with confidentiality, in accordance with the Declaration of Helsinki.
Data Collection
Based on the electronic medical record system, the patients’ clinical parameters were collected, including: gender, age, Karnofsky performance status (KPS), tumor location, tumor size, tumor grade (I/II/III/IV by WHO classification), extent of resection, postoperative adjuvant chemoradiotherapy, postoperative intracranial infection, preoperative fibrinogen, white blood cell (WBC) count, preoperative neutrophil count, preoperative lymphocyte count, preoperative albumin level, preoperative globulin level.
Calculation of the F-NLR-AGR Score
Preoperative laboratory examination data, including neutrophil count, lymphocyte count, and the levels of fibrinogen, serum albumin, and globulin were collected to evaluate the F-NLR-AGR score. The calculation formulas of NLR and AGR were as follows: NLR = neutrophil count/lymphocyte count, AGR = albumin/globulin; Receiver operating characteristic (ROC) curve analysis was performed to identify the optimal cut-off for the preoperative NLR, AGR and fibrinogen, so as to predict prognosis and establish the F-NLR-AGR scoring system. The cut-off values were 1.90 for NLR, 1.54 for AGR, and 2.61 g/L for fibrinogen (sensitivity, specificity and AUC: 68.0%, 63.2% and 0.667 for NLR; 54.7%, 71.1% and 0.600 for AGR; 80.4%, 67.9% and 0.770 for fibrinogen, respectively); Figure 1A–C. According to these cut-off values, the F-NLR-AGR score was calculated as follows: patients with high NLR (>1.90), low AGR (<1.54), and elevated fibrinogen (>2.61 g/L) were defined as a score of 3 (three abnormalities), if any two of three parameters met previously mentioned standards they were defined as a score of 2 (two abnormalities), if only one parameter met previously mentioned standards they were defined as a score of 1 (one abnormality), and if all three parameters failed to meet the standards they were defined as a score of 0 (no abnormality).
Statistical Analysis
Receiver-operating characteristic (ROC) curve analysis was used to determine the optimal cut-off values for NLR, AGR and fibrinogen. Chi-squared test was performed to evaluate the relationship between the four F-NLR-AGR groups and other clinical variables. Categorical variables and continuous variables were presented as number and mean ± SD, respectively. Survival analysis was performed using the Kaplan-Meier survival curve. Cox proportional hazard regression model was used for univariate and multivariate analysis of clinical variables to determine the independent prognostic factors. Spearman’s rank correlation was used to analyze the correlation between NLR, AGR and fibrinogen, and the correlation between F-NLR-AGR and WHO grade. P values < 0.05 were considered statistically significant and all reported P values were two sided. All statistical analyses were performed with SPSS software version 22.0 (IBM Corporation, Armonk, NY, USA).
Results
Clinical Characteristics
A total of 203 patients (56.2% male and 43.8% female) with glioma were included in this study, ranging from 6–75 years old and mean age was 44±14.61 years. Of the enrolled patients, the distribution of tumors was as follows: frontal lobe (86, 42.4%), temporal lobe (48, 23.6%), parietal (34, 16.7%), occipital (12, 5.9%), insular (12, 5.9%) and others (11, 5.4%). The KPS were ≥70 in 141 (69.5%) patients and <70 in 62 (30.5%) patients. There were 75.4% of patients with a gross total resection, and there were 109 (53.7%) cases who received adjuvant therapy including radiotherapy or chemotherapy after surgery. The mean pre-treatment white blood cell (WBC), neutrophil, lymphocyte counts were 6.48±2.15×109/L, 3.94±1.84×109/L and 1.96±0.69×109/L, respectively. The mean pre-treatment fibrinogen, NLR and AGR were 2.77±0.86 mg/dL, 2.28±1.72 and 1.52±0.30, respectively. Approximately 19.2% of patients had F-NLR-AGR 0, 34.0% had F-NLR-AGR 1, 33.0% had F-NLR-AGR 2, 13.8% had F-NLR-AGR 3 (Table 1).
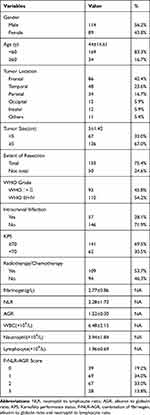 |
Table 1 Characteristics of Patients with Glioma |
Correlations Between Clinical Parameters and F-NLR-AGR
We first used Spearman’s rank correlation analysis to determine the correlation between fibrinogen, NLR and AGR. The results showed that fibrinogen was positively correlated with NLR (Figure 2A) and negatively correlated with AGR (Figure 2B), while there was no correlation between NLR and AGR. Moreover, we explored the relationship between WHO grade and the three parameters of fibrinogen, NLR and AGR. Compared with low-grade (WHO grade Ⅰ+Ⅱ), the increase of fibrinogen (Figure 3A) and NLR (Figure 3B) levels was more significant in high-grade (WHO grade Ⅲ+Ⅳ), while no significant association was found between WHO grade and AGR (Figure 3C). The correlation between F-NLR-AGR and glioma grade was further evaluated, and the result showed that F-NLR-AGR was positively correlated with glioma grade (r=0.278, P<0.01). Higher preoperative F-NLR-AGR of 2 and 3 were observed for patients with a higher WHO grade (P<0.001) (Figure 3D). We also estimated the association between the F-NLR-AGR and other clinical parameters of patients with glioma, and significant differences were observed among the F-NLR-AGR 0, 1, 2, 3 groups in terms of WHO grade (P<0.001) and intracranial infection (P=0.015). However, there were no significant differences in the distribution of gender, age, tumor location, tumor size, extent of resection, KPS score and adjuvant chemoradiotherapy (Table 2).
 |
Table 2 Association Between F-NLR-AGR and the Clinicopathological Characteristics of Patients with Glioma (n=203) |
Survival Analysis of F-NLR-AGR
Kaplan–Meier curves for OS based on preoperative fibrinogen, NLR and AGR demonstrated that patients with high fibrinogen (≥2.61 g/L), high NLR (≥1.90) and low AGR (≤1.54) had significantly shorter OS than those with low fibrinogen (<2.61 g/L), low NLR (<1.90) and high AGR (>1.54) [(P<0.001 for all), Figure 4A-C]. Kaplan-Meier analysis was also used to determine the survival differences among the four groups divided by F-NLR-AGR score, the three-year OS rate and the mean overall survival in patients with F-NLR-AGR 3 were lower than those of patients with F-NLR-AGR = 2, 1 or 0 [17.6% vs 35.2%, 66.9% or 83.7% (26.0 vs 39.0, 64.0 or 81.0 months), P< 0.001, (Figure 4D)].
Univariate and Multivariate Analyses for OS
The age (HR=2.308; 95% CI=1.441–3.698; P=0.001), tumor size (HR=1.693; 95% CI=1.060–2.704; P=0.027), extent of resection (HR=1.729; 95% CI=1.136–2.633; P=0.011), WHO grade (HR=5.863; 95% CI=3.567–9.636; P<0.001), KPS (HR=1.604; 95% CI=1.066–2.413; P=0.023) and F-NLR-AGR score (P<0.001) were significantly associated with OS in the univariate analysis. Futhermore, multivariate analysis revealed that age (HR = 2.071; 95% CI=1.195–3.588; P =0.009), WHO grade (HR = 5.863; 95% CI=3.567–9.636; P<0.001), and F-NLR-AGR score (P<0.001) were independent prognostic factors for OS (Table 3).
 |
Table 3 The Univariate and Multivariate Cox Analyses of OS |
Discussion
Although great progress has been made in the diagnosis and treatment of glioma, there is still a lack of comprehensive preoperative assessment tools to appropriately classify the risk of glioma patients, thereby guiding further individualized treatment. In this study, we analyzed the preoperative fibrinogen, NLR and AGR of 203 glioma patients, and combined these three prognostic parameters to establish a novel F-NLR-AGR scoring system. As far as we know, this is the first study to determine the clinical significance of the F-NLR-AGR scoring system in patients with malignancies.
There is increasing evidence that has revealed that activation of coagulation cascade plays an important pathophysiological role in tumor progression. Fibrinogen, a key factor in the coagulation cascade, has been reported as a crucial regulator of tumor progression and systemic inflammatory response in various malignant tumors. Hyperfibrinogenemia has also been reported to be associated with the high invasiveness of malignant tumors, including gliomas.20 Although many studies have found the link between hyperfibrinogenemia and malignancy, the mechanism remains unclear. Possible reasons are as follows: fibrinogen builds a provisional framework in the tumor extracellular matrix, which promotes tumor angiogenesis and increases the potential of tumor cell adhesion, migration and invasion.22–24 Furthermore, the tumor cell-associated platelet-fibrin deposition forms a physical barrier on the periphery of tumor cells, preventing NK cells from making contact with target tumor cells, thereby reducing the clearance rate of tumor cells.25 Fibrinogen, as an acute-phase protein, is usually released in the presence of malignant tumors or systemic inflammation. It has been reported that interleukin-6 produced by cancer cells can stimulate the host to release fibrinogen,26 cancer cells can also synthesize fibrinogen by themselves. The resulting fibrinogen in turn promotes tumor proliferation and angiogenesis by interacting with VEGF and fibroblast growth factor-2.27,28 In the present study, we also further found that glioma patients with high preoperative fibrinogen had shorter OS than those with low preoperative fibrinogen. Therefore, preoperative plasma fibrinogen is an indispensable parameter for predicting the prognosis of gliomas.
Recently, there has also been a large number of evidence suggesting that inflammation is closely related to the occurrence and development of malignant tumors, the concept of cancer-related inflammation has also been proposed.29 Some inflammatory parameters have been associated with adverse clinical outcomes of gliomas and have the ability to predict prognosis of gliomas.30 Among them, the most representative is NLR, which is calculated by dividing the neutrophil count by the lymphocyte count.31 Meanwhile, the neutrophil-lymphocyte ratio (NLR) has been proposed as an indicator of cancer-related inflammation.32 Lymphocytes, as important components of anticancer immunity, have key functions in suppressing tumor migration and proliferation.33 Neutrophils, in contrast, have been shown to play a positive role in tumor angiogenesis and metastasis.34 The recruitment of neutrophils and lymphocytes plays a critical role in the pathogenesis of gliomas.14 Like other solid tumors, increased peripheral neutrophil to lymphocyte ratio (NLR) is associated with poor prognosis in gliomas.17 Our study also demonstrated that glioma patients with high NLR have shorter OS than patients with low NLR. Therefore, NLR can represent cancer-related systemic inflammation response to evaluate the prognosis of gliomas.
AGR, as an effective combination of two strong prognostic factors, can reflect nutrition status and systemic inflammation in cancer patients.35 Albumin is the largest component of serum protein and plays a vital role in the human body. Low albumin is not only a symbol of malnutrition, but also closely associated with inadequate antitumor immunity.36,37 It directly and indirectly leads to decreased capacity for treatment tolerance, increased mortality, and worse quality of life in the postoperative period.38 Globulin is a comprehensive protein which contains certain immunoglobulins and other acute-phase proteins, such as CRP, complements, et al.39,40 These proteins are produced in a state of inflammation; an increased level of globulin is thought to reflect the presence of continuous systemic inflammation.41 Previous studies have shown that chronic inflammation stimulates the occurrence, proliferation, survival, metastasis and recurrence of cancer;42 low AGR can be used as an immunonutritional marker to identify glioblastoma patients with poor prognosis.43 Based on all of this, low AGR may relatively indicate malnutrition, inadequate antitumor immunity, existance of cancer-related inflammation or both, all of these are unfavorable for cancer prognosis. In the present study, we also further observed that glioma patients with high preoperative AGR had longer OS than those with low preoperative AGR. Therefore, AGR can represent immunonutritional status to evaluate the prognosis of glioma patients.
In recent years, many studies have established a series of scoring systems to evaluate the prognosis of malignant tumors based on preoperative hematological indicators. For example, combining fibrinogen and NLR to produce F-NLR score,44 combining fibrinogen and albumin to produce FA score,20 dividing albumin by NLR to produce ANS score,45 and so on. However, these scoring systems are still not comprehensive enough. Therefore, on the basis of these existing scoring systems, we have developed a novel scoring system based on fibrinogen, neutrophil-lymphocyte ratio (NLR) and albumin-globulin ratio (AGR), which is the F-NLR-AGR scoring system. In order to verify the significance of the F-NLR-AGR scoring system, a retrospective study of 203 glioma patients showed that F-NLR-AGR score was an independent predictor of prognosis, the three-year OS rate and the mean overall survival gradually decreased with the increase of patients’ F-NLR-AGR score. It should be noted that although the whole scoring system had P value of <0.001, score 1 was not statistically significant (Table 3). This may be due to the fact that we used score 0 as the reference variable and the prognostic difference between score 0 and score 1 was not significant enough. In addition, the correlation between F-NLR-AGR score and glioma grade was further evaluated, and the result showed that F-NLR-AGR score was positively correlated with glioma grade. In the subgroup of F-NLR-AGR 3 and 2, the number of patients with high-grade (WHO grade Ⅲ+Ⅳ) gliomas was significantly more than those with low-grade (WHO grade Ⅰ+Ⅱ) gliomas. In the future, the F-NLR-AGR score can be validated as predictive factor of the pathological grade of glioma with results of more studies.
Our study had certain limitations. First, this was a retrospective analysis with a single-center design, which may have introduced selection bias. Second, this study did not further clarify which parameters were abnormal in F-NLR-AGR score 1 and 2 groups, so the difference in the prognostic effects of different pathophysiological mechanisms is unclear. Third, molecular markers such as MGMT, IDH status and ki-67 were not included in the study because the immunohistochemical analysis results of some early cases were incomplete. Fourth, with limited data availability, some confounding factors, such as liver disease, malnutrition, BMI information and anticoagulation treatment information (heparin subQ or home oral anticoagulants), may affect preoperative albumin and fibrinogen levels, but cannot be analyzed. Furthermore, sensitivity and specificity for fibrinogen, NLR, AGR are very moderate. Therefore, a future multicenter cohort prospective study with larger samples and more comprehensive data should be conducted again to further validate the scoring system.
Conclusion
In any case, WHO grade is still the gold standard for evaluating the prognosis and treatment selection of gliomas. However, WHO grade can only be obtained through postoperative pathology, which undoubtedly brings difficulties to preoperative survival prediction and further treatment decisions. Thus, as a simple, convenient, cheap, easily acquired parameter in clinical practice, the F-NLR-AGR score can be used as a novel preoperative assessment tool to predict the prognosis of glioma patients, thereby guiding further individualized treatment. In addition, it may serve as a complementary to the WHO grade to identify high-risk patients among patients with the same WHO grade.
Acknowledgments
This study was supported by the Key Research and Development Program of Ningxia Hui Autonomous Region (No. 2018BFG02007) and the National Natural Science Foundation of China (No. 81660226).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Ostrom QT, Gittleman H, Stetson L, et al. Epidemiology of gliomas. Cancer Treat Res. 2015;163(3):1–14.
2. Anton K, Baehring JM, Mayer T. Glioblastoma multiforme: overview of current treatment and future perspectives. Hematol Oncol Clin North Am. 2012;26(4):825–853. doi:10.1016/j.hoc.2012.04.006
3. Louis DN, Perry A, Reifenberger G, et al. The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol. 2016;131(6):803–820. doi:10.1007/s00401-016-1545-1
4. Nabors LB, Portnow J, Ammirati M, et al. NCCN guidelines insights: central nervous system cancers, Version 1.2017. J Natl Compr Canc Netw. 2017;15(11):1331–1345. doi:10.6004/jnccn.2017.0166
5. Brito C, Azevedo A, Esteves S, et al. Clinical insights gained by refining the 2016 WHO classification of diffuse gliomas with: EGFR amplification, TERT mutations, PTEN deletion and MGMT methylation. BMC Cancer. 2019;19(1):968. doi:10.1186/s12885-019-6177-0
6. Labussiere M, Boisselier B, Mokhtari K, et al. Combined analysis of TERT, EGFR, and IDH status defines distinct prognostic glioblastoma classes. Neurology. 2014;83(13):1200–1206. doi:10.1212/WNL.0000000000000814
7. Leu S, von Felten S, Frank S, et al. IDH/MGMT-driven molecular classification of low-grade glioma is a strong predictor for long-term survival. Neuro-Oncology. 2013;15(4):469–479. doi:10.1093/neuonc/nos317
8. Molenaar RJ, Verbaan D, Lamba S, et al. The combination of IDH1 mutations and MGMT methylation status predicts survival in glioblastoma better than either IDH1 or MGMT alone. Neuro-Oncology. 2014;16(9):1263–1273. doi:10.1093/neuonc/nou005
9. Li J, Niu X, Gan Y, et al. Clinical and pathologic features and prognostic factors for recurrent gliomas. World Neurosurg. 2019;128:e21–e30. doi:10.1016/j.wneu.2019.02.210
10. Louveau A, Harris TH, Kipnis J. Revisiting the mechanisms of CNS immune privilege. Trends Immunol. 2015;36(10):569–577. doi:10.1016/j.it.2015.08.006
11. Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674. doi:10.1016/j.cell.2011.02.013
12. Colotta F, Allavena P, Sica A, et al. Cancer-related inflammation, the seventh hallmark of cancer: links to genetic instability. Carcinogenesis. 2009;30(7):1073–1081. doi:10.1093/carcin/bgp127
13. Michelson N, Rincon-Torroella J, Quiñones-Hinojosa A, et al. Exploring the role of inflammation in the malignant transformation of low-grade gliomas. J Neuroimmunol. 2016;297:132–140. doi:10.1016/j.jneuroim.2016.05.019
14. Massara M, Persico P, Bonavita O, et al. Neutrophils in Gliomas. Front Immunol. 2017;8:1349. doi:10.3389/fimmu.2017.01349
15. Zhou W, Ke SQ, Huang Z, et al. Periostin secreted by glioblastoma stem cells recruits M2 tumour-associated macrophages and promotes malignant growth. Nat Cell Biol. 2015;17(2):170–182. doi:10.1038/ncb3090
16. Komohara Y, Ohnishi K, Kuratsu J, et al. Possible involvement of the M2 anti-inflammatory macrophage phenotype in growth of human gliomas. J Pathol. 2008;216(1):15–24. doi:10.1002/path.v216:1
17. Templeton AJ, McNamara MG, Seruga B, et al. Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: a systematic review and meta-analysis. J Natl Cancer Inst. 2014;106(6):dju124. doi:10.1093/jnci/dju124
18. Rondon AMR, Kroone C, Kapteijn MY, et al. Role of tissue factor in tumor progression and cancer-associated thrombosis. Semin Thromb Hemost. 2019;45(4):396–412. doi:10.1055/s-0039-1687895
19. Perisanidis C, Psyrri A, Cohen EE, et al. Prognostic role of pretreatment plasma fibrinogen in patients with solid tumors: a systematic review and meta-analysis. Cancer Treat Rev. 2015;41(10):960–970. doi:10.1016/j.ctrv.2015.10.002
20. He ZQ, Duan H, Ke C, et al. Evaluation of cumulative prognostic score based on pretreatment plasma fibrinogen and serum albumin levels in patients with newly diagnosed high-grade gliomas. Oncotarget. 2017;8(30):49605–49614. doi:10.18632/oncotarget.17849
21. He J, Pan H, Liang W, et al. Prognostic effect of albumin-to-globulin ratio in patients with solid tumors: a systematic review and meta-analysis. J Cancer. 2017;8(19):4002–4010. doi:10.7150/jca.21141
22. Palumbo JS, Talmage KE, Massari JV, et al. Platelets and fibrin(ogen) increase metastatic potential by impeding natural killer cell-mediated elimination of tumor cells. Blood. 2005;105(1):178–185. doi:10.1182/blood-2004-06-2272
23. Simpson-Haidaris PJ, Rybarczyk B. Tumors and fibrinogen. The Role of Fibrinogen as an Extracellular Matrix Protein. Ann N Y Acad Sci. 2001;936(1):406–425.
24. Liu X, Liu Z, Lin E, et al. A cumulative score based on preoperative fibrinogen and the neutrophil-lymphocyte ratio to predict outcomes in resectable gastric cancer. Cancer Manag Res. 2018;10:3007–3014. doi:10.2147/CMAR.S174656
25. Nieswandt B, Hafner M, Echtenacher B, et al. Lysis of tumor cells by natural killer cells in mice is impeded by platelets. Cancer Res. 1999;59(6):1295–1300.
26. Yamaguchi T, Yamamoto Y, Yokota S, et al. Involvement of interleukin-6 in the elevation of plasma fibrinogen levels in lung cancer patients. Jpn J Clin Oncol. 1998;28(12):740–744. doi:10.1093/jjco/28.12.740
27. Sahni A, Francis CW. Vascular endothelial growth factor binds to fibrinogen and fibrin and stimulates endothelial cell proliferation. Blood. 2000;96(12):3772–3778. doi:10.1182/blood.V96.12.3772
28. Sahni A, Simpson-Haidaris PJ, Sahni SK, et al. Fibrinogen synthesized by cancer cells augments the proliferative effect of fibroblast growth factor-2 (FGF-2). J Thromb Haemost. 2008;6(1):176–183. doi:10.1111/j.1538-7836.2007.02808.x
29. Mantovani A, Allavena P, Sica A, et al. Cancer-related inflammation. Nature. 2008;454(7203):436–444. doi:10.1038/nature07205
30. Zheng SH, Huang JL, Chen M, et al. Diagnostic value of preoperative inflammatory markers in patients with glioma: a multicenter cohort study. J Neurosurg. 2018;129(3):583–592. doi:10.3171/2017.3.JNS161648
31. Lei YY, Li YT, Hu QL, et al. Prognostic impact of neutrophil-to-lymphocyte ratio in gliomas: a systematic review and meta-analysis. World J Surg Oncol. 2019;17(1):152. doi:10.1186/s12957-019-1686-5
32. Lorente D, Mateo J, Templeton AJ, et al. Baseline neutrophil-lymphocyte ratio (NLR) is associated with survival and response to treatment with second-line chemotherapy for advanced prostate cancer independent of baseline steroid use. Ann Oncol. 2015;26(4):750–755. doi:10.1093/annonc/mdu587
33. Shankaran V, Ikeda H, Bruce AT, et al. IFNgamma and lymphocytes prevent primary tumour development and shape tumour immunogenicity. Nature. 2001;410(6832):1107–1111. doi:10.1038/35074122
34. Jablonska J, Leschner S, Westphal K, et al. Neutrophils responsive to endogenous IFN-beta regulate tumor angiogenesis and growth in a mouse tumor model. J Clin Invest. 2010;120(4):1151–1164. doi:10.1172/JCI37223
35. Liu J, Dai Y, Zhou F, et al. The prognostic role of preoperative serum albumin/globulin ratio in patients with bladder urothelial carcinoma undergoing radical cystectomy. Urol Oncol. 2016;34(11):
36. Ataseven B, Du Bois A, Reinthaller A, et al. Pre-operative serum albumin is associated with post-operative complication rate and overall survival in patients with epithelial ovarian cancer undergoing cytoreductive surgery. Gynecol Oncol. 2015;138(3):560–565.
37. Chiang JM, Chang CJ, Jiang SF, et al. Pre-operative serum albumin level substantially predicts post-operative morbidity and mortality among patients with colorectal cancer who undergo elective colectomy. Eur J Cancer Care (Engl). 2017;26:2. doi:10.1111/ecc.12403
38. Kanda M, Mizuno A, Tanaka C, et al. Nutritional predictors for postoperative short-term and long-term outcomes of patients with gastric cancer. Medicine. 2016;95(24):e3781. doi:10.1097/MD.0000000000003781
39. Azab B, Kedia S, Shah N, et al. The value of the pretreatment albumin/globulin ratio in predicting the long-term survival in colorectal cancer. Int J Colorectal Dis. 2013;28(12):1629–1636. doi:10.1007/s00384-013-1748-z
40. Gabay C, Kushner I. Acute-phase proteins and other systemic responses to inflammation. N Engl J Med. 1999;340(6):448–454. doi:10.1056/NEJM199902113400607
41. Li Q, Meng X, Liang L, et al. High preoperative serum globulin in rectal cancer treated with neoadjunctive chemoradiation therapy is a risk factor for poor outcome. Am J Cancer Res. 2015;5(9):2856–2864.
42. Gast MC, Van Gils CH, Wessels LF, et al. Serum protein profiling for diagnosis of breast cancer using SELDI-TOF MS. Oncol Rep. 2009;22(1):205–213. doi:10.3892/or_00000426
43. Xu WZ, Li F, Xu ZK, et al. Preoperative albumin-to-globulin ratio and prognostic nutrition index predict prognosis for glioblastoma. Onco Targets Ther. 2017;10:725–733. doi:10.2147/OTT.S127441
44. Huang W, Wang S, Zhang H, et al. Prognostic significance of combined fibrinogen concentration and neutrophil-to-lymphocyte ratio in patients with resectable non-small cell lung cancer. Cancer Biol Med. 2018;15(1):88–96. doi:10.20892/j.issn.2095-3941.2017.0124
45. Kao HK, Lofstrand J, Loh CY, et al. Nomogram based on albumin and neutrophil-to-lymphocyte ratio for predicting the prognosis of patients with oral cavity squamous cell carcinoma. Sci Rep. 2018;8(1):13081. doi:10.1038/s41598-018-31498-z
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.




