Back to Journals » Journal of Pain Research » Volume 15
A Novel Rat Model to Study Postsurgical Pain After Joint Replacement Surgery
Authors Aoyama N , Izumi M , Morimoto T, Wada H, Dan J, Kasai Y, Satake Y, Aso K, Ikeuchi M
Received 4 April 2022
Accepted for publication 8 September 2022
Published 14 September 2022 Volume 2022:15 Pages 2911—2918
DOI https://doi.org/10.2147/JPR.S368130
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Professor E Alfonso Romero-Sandoval
Naoki Aoyama, Masashi Izumi, Toru Morimoto, Hiroyuki Wada, Junpei Dan, Yusuke Kasai, Yoshinori Satake, Koji Aso, Masahiko Ikeuchi
Department of Orthopedic Surgery, Kochi Medical School, Kochi University, Nankoku, Japan
Correspondence: Masashi Izumi, Department of Orthopedic Surgery, Kochi Medical School, Kochi University, 185-1 Oko-cho Kohasu, Nankoku, 783-8505, Japan, Tel +81-88-880-2386, Fax +81-88-880-2388, Email [email protected]
Purpose: The mechanisms underlying chronic postsurgical pain after joint replacement (JR) are complex, and it has been suggested that chronic postsurgical pain can develop as a result of inadequate acute pain management. Few studies have addressed acute pain after JR using specific animal models. This study aimed to develop a novel JR model focused on postsurgical pain assessment and the time course of pain recovery.
Materials and Methods: Rats were allocated to the following three groups: sham (joint exposure), joint destruction (JD; resection of the femoral head), and JR (femoral head replacement using an originally developed implant). The time course of postsurgical pain behavior was measured using a dynamic weight-bearing apparatus, along with radiological assessments. The expression of calcitonin gene-related peptide-immunoreactive (CGRP-IR) neurons in the dorsal root ganglion (DRG) was evaluated by immunohistochemistry on days 28 and 42.
Results: The ratio of weight-bearing distribution in the JR group gradually recovered from day 14 and reached the same level as that in the sham group on day 42, which was significantly greater than that in the JD group after day 7 (p< 0.05). Radiologically, no significant issues were found, except for transient central migration of the implant in the JR group. The percentage of CGRP-IR DRG neurons in the JR group was significantly lower than that in the JD group on day 28 (mean, 37.4 vs 58.1%, p< 0.05) and day 42 (mean, 32.3 vs 50.0%, p< 0.05).
Conclusion: Our novel JR model presented acute postsurgical pain behavior that was successfully recovered to the baseline level at day 42 after surgery. Difference of the pain manifestation between the JR and JD groups could be supported by the expression of CGRP-IR in DRG neurons. This model is the first step toward understanding detailed mechanisms of post-JR pain.
Keywords: joint replacement, postsurgical pain, dynamic weight bearing, CGRP
Introduction
Joint replacement (JR) is a widely performed surgery in patients with advanced joint diseases. The number of patients with JR is expected to increase exponentially with the aging population. By 2030, the incidence of total hip arthroplasty (THA) and total knee arthroplasty is predicted to increase by approximately 200% and 700%, respectively.1 While JR is one of the most successful procedures in the field of orthopedic surgery,2 it does have several drawbacks, such as postsurgical pain,3,4 bacterial infection,5,6 implant loosening,7–10 and periprosthetic fractures.11 Specifically, postsurgical pain in the acute phase after JR is extremely severe and often poorly managed.12,13 Recently, accumulating evidence revealed that if pain management is inadequate, acute postsurgical pain can progress to chronic postsurgical pain (CPSP).13,14 To develop a more effective treatment strategy, it is essential to clarify the basic mechanisms underlying postsurgical pain in the acute phase. There have been some reports of animal models exhibiting JR,8–10,16–18 but the authors have made no mention of postsurgical pain. Among them, very few studies16–18 reported on the JR model using rodents, which have been most extensively used for pain research. Therefore, we decided to develop a novel animal model of JR using rats. Initially, we attempted to develop a knee replacement model because postoperative pain is reported to be the most severe after knee replacement, among JR surgeries;20,21 however, several attempts failed because of the complex structures of the knee joint. We then changed the target joint from the knee to the hip, which is a simpler ball-and-socket joint. This study aimed to describe how we developed a hip replacement model and explain the postsurgical course, including pain recovery.
Materials and Methods
Animals
To assess post-JR pain, 15 adults (17–19-week-old) male Sprague-Dawley rats were used, in accordance with the guidelines of the International Association for the Study of Pain. The rats were maintained at the Institute of Laboratory Animals, Kochi Medical University. The rats were housed in plastic cages at 23 ℃ with a 14-h light/10-h dark cycle. Rats were allocated to the following three groups: sham-operated (sham: joint exposure, n=5), joint destruction (JD: resection of the femoral head, n=5), and JR (femoral head replacement using an originally developed implant, n=5). All experiments were approved by the Animal Care and Use Committee of Kochi University (N-00037).
Implant Design
The hip implant was originally developed to create an animal model of hip replacement. Femurs were collected from a 20-week-old male rat to acquire data on the appropriate femoral head size, femoral neck angle, and femoral neck length. 3D printing was used to create a mold of the femoral head and neck. Regarding implant stem design, several changes were required to ensure initial stability and easy insertion. First, we developed a prototype implant (Generation 1) consisting of a femoral head with a 4.0 mm diameter and a 7-mm long cylindrical stem with screw threads (Figure 1A). The implant was inserted into the femoral neck after rasping the medullary canal. Although the femoral head was successfully replaced with Generation 1, it was technically difficult to insert a cylindrical stem into the narrow cavity of the femoral neck. Therefore, the distal part of the stem was tapered (Generation 2: Figure 1B), which fit the femoral neck and allowed for easier implant insertion. However, a few problems were encountered during the postoperative course, including implant loosening and hip dislocation. We believed that the cause of implant loosening was inadequate stability of the stem inside the femur and that the cause of hip dislocation was insufficient repair of soft tissue around the hip joint. Therefore, we changed the stem design to one with a femoral neck angle and inserted it into the medullary canal of the femoral shaft to prevent implant loosening (Generation 3, Figure 1C). The stem in Generation 3 was made of pure titanium. The features of the implant were as follows: (1) 4.0-mm head diameter, (2) 150° neck angle, and 3) 7-mm stem length. In addition, a Surgical SimplexⓇ P Bone Cement (HowmedicaⓇ, Inc., Rutherford, NJ, USA) was inserted into the medullary canal before the stem insertion to increase fixation stability. Finally, the soft tissues around the hip joint, including the capsules, muscles, and tendons, were repaired meticulously to prevent hip dislocation.
 |
Figure 1 Implant designs. (A) Generation 1, (B) Generation 2, and (C) Generation 3. |
Surgery
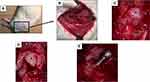
All surgeries were performed under inhalation anesthesia with 2.0% isoflurane and an oxygen flow rate of 1.0 L/min. The animals were placed in the right lateral decubitus position (Figure 2A), and a lateral approach was adopted to expose the hip joint. The skin incision was made from the greater trochanter to the middle femur. The tensor fasciae latae and gluteus medius muscles were excised (Figure 2B). The gluteus minimus was detached from the greater trochanter. The joint capsule was incised along the femoral neck, and the femoral head was exposed (Figure 2C and D). Following surgical dislocation of the hip joint, the proximal femur was cut at the level of the femoral neck, and the medullary canal of the proximal femur was reamed using an 18-gauge needle and a 1.5-mm screwdriver. After reaming the medullary canal, the implant was inserted into the cavity of the proximal femur using Surgical Simplex® P Bone Cement (Howmedica, Inc.). After hardening the cement, the hip joint was repositioned (Figure 2E), and the wound was closed with 4–0 nylon. After each step, the wounds were thoroughly washed with saline. The sham group underwent skin incision, muscle separation, and joint capsule resection to expose the hip joint. The JD group underwent resection of the proximal femur without implant insertion, which induced severe incongruency of the hip joint and subsequent JD.
Postoperative Assessments
Behavioral Assessments
Postsurgical pain was assessed with the dynamic weight-bearing (DWB) 2.0 (Bioseb Development, Vitrolles, France), consisting of a biometric floor instrumented cage (22×22×30 cm), a high-precision force sensor, and a high-definition camera that allows us to assess postsurgical pain in freely moving animals. Pain behavior was monitored and recorded for 5 min at each time point. We assessed the ratio of weight-bearing distribution calculated as left/right hind paw, as previously reported.18 Stimulus-evoked responses, such as the von Frey test, have been widely used in pain research to assess pain-related behavior. However, we selected DWB in this study because asymmetric weight distribution partially reflects spontaneous pain during free walking in unilateral paw pain models,25–27 which appears to be more relevant and clinically important for assessing our novel JR model.
Radiographic Assessments
Anteroposterior and lateral radiographs of the hip joint were taken under anesthesia at weekly intervals until the rats were euthanized on day 42. Particular attention was paid to implant displacement and peri-implant bone remodeling.
Immunohistochemistry Assessment
On days 28 and 42 after surgery, the animals were euthanized with carbon dioxide gas, and the left L3 and L4 dorsal root ganglia (DRGs) were collected referring to the previous reports about sensory innervation of the hip joint.15,19 The DRGs were placed in 10% formalin and 30% sucrose solution overnight, embedded in OCT compound (Sakura Finetek USA, Inc., Torrance, CA, USA), and kept frozen at −70 ℃ until sectioning. Frozen sections with a thickness of 14 µm were cut using a cryostat. Approximately 10–15 sections were obtained for each DRG. A double-immunofluorescence method was used to assess DRGs. The primary antibody used in this study was rabbit anti-α-CGRP (1:1000 Peninsula Laboratories, San Carlos, CA, USA) diluted in phosphate-buffered saline (PBS) with 10% goat serum and 0.05% Triton X-100. The secondary antibody used in this study was goat anti-rabbit IgG-FITC (1:500), which was diluted in PBS with 10% goat serum, 0.05% Triton X-100, and 4’,6-diamidino-2-phenylindole. On the first day, the sections were blocked in 3% normal goat serum for 1 h, immersed two times in PBS for 5 min, and then incubated with the primary antibody overnight at room temperature. On the next day, the sections were incubated with the secondary antibody for 1 h after being immersed two times in PBS for 5 min. Finally, the sections were mounted with VECTASHIELD (Vector, Burlingame, CA, USA). To avoid double counting, the numbers of CGRP-immunoreactive (IR) neurons and all DRG neurons were counted in every fifth section using a fluorescent microscope (Nikon Corp., Tokyo, Japan). We calculated the percentage of CGRP-IR DRG neurons (CGRP-IR neurons/all DRG neurons*100) to minimize the effect of individual differences between the rats on days 28 and 42. We compared the percentage of CGRP-IR DRG neurons in the three groups.
Statistical Analysis
IBM SPSSⓇ Statistics for Windows version 26 (IBM Corp., Armonk, NY, USA) was used for the statistical analysis. All data are presented as mean ± SEM. For between-group comparisons of pain behaviors and neuron counts, a one-way analysis of variance with an unpaired t-test was used. The Mann–Whitney U-test was used to compare the percentage of CGRP-IR DRG neurons between days 28 and 42 after surgery in each group. The results were considered significant if the p-value was <0.05.
Results
Behavioral Assessment
Figure 3 shows the time course of postsurgical pain. The ratio of weight-bearing distribution in the JR group gradually recovered from day 14 and reached the same level as that in the sham group on day 42, which was significantly greater than that in the JD group after day 7 (p < 0.05).
Radiological Assessments
In the sham group, no apparent changes were found in the hip joint after 42 days. The JR group showed no joint dislocation or implant loosening throughout the study period. Although there were no major issues in the final follow-up, subtle changes were detected in the series of radiographs. Up to day 21, a slight implant movement towards the acetabulum was found, ie, central migration (Figure 4A–D). Subsequent to the movement of the implant, central bulging of the acetabulum was confirmed on days 28–35. By day 42, bone remodeling associated with implant movement was completed (Figure 4E–G). These findings were observed in all the cases.
Immunohistochemistry Assessments
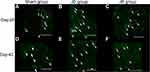
Representative fluorescent photographs showed CGRP-IR DRG neurons (Figure 5A–F). The percentage of CGRP-IR DRG neurons in the JD group was significantly higher than that in the sham group (*p=0.027 on day 28 and p=0.010 on day 42) and JR group (*p=0.036 on day 28 and p=0.004 on day 42), (Table 1). However, the expression of CGRP was not significantly different between day 28 and day 42 in each group (Table 1).
 |
Table 1 Percentage of CGRP-IR DRG Neurons in the Sham, JD, and JR Groups |
Discussion
The mechanisms underlying CPSP after JR are complex and have not yet been elucidated.3 Recent systematic reviews13,24,28 provided possible risk factors for CPSP development such as preoperative pain intensity, pain sensitization, psychosocial problems, surgical procedures, acute postoperative care; however, these outcomes were derived from clinical studies that could not address the detailed basic pain mechanisms. To resolve this issue, an animal model mimicking JR that can be used for postsurgical pain assessment is required.
In this study, we developed a simple and reproducible animal model to evaluate the course of pain after JR using rat hip joints. The ratio of weight-bearing distribution in the JR group recovered within 42 days, and the percentage of CGRP-IR DRG neurons in the JR group was significantly lower than that in the JD group. These results suggest that our model demonstrates a recovery process of postsurgical pain behavior, which is similar to clinical observations.
Although postsurgical pain mechanisms have been documented using various animal models,29–31 pain following joint surgery has not been well studied. Two recent reports have used a rat model to drill a hole in the articular surface of the knee joint.22,23 Buvanendran et al reported the features of postsurgical pain-related behavior and its recovery following systemic and intrathecal administration of morphine, ketorolac, and celecoxib.22 Majuta et al reported that spontaneous postsurgical pain behavior was attenuated using a monoclonal antibody against the nerve growth factor.23 They evaluated pain behaviors after joint surgery and the effects of pharmacological treatment; however, their models were completely different from a model of JR surgery.
Regarding JR models in rats, Powers et al and Paish et al recently developed a THA model, but they mainly reported on implant design and surgical techniques without postsurgical pain assessment. In this study, we documented pain after hip replacement surgery in rats, which may pave the way for future research into CPSP after JR.
Pain recovery after JR was initiated from day 14, which was slower than expected. A plausible mechanism for this delay is the joint incongruency between the artificial femoral head and the original acetabulum. Up to day 21, wear of the acetabulum due to the artificial femoral head was observed, which possibly induced an inflammatory reaction and decreased the ratio of weight-bearing distribution to the operated paw. On day 28, bone remodeling in the acetabulum was completed, after which stability of the hip joint improved and pain recovery was probably accelerated. Essentially, the percentage of CGRP-IR DRG neurons was comparable between the JR and sham groups on day 28, which supports this interpretation from a neurological aspect.
This study had some limitations. First, it took time to recover the weight-bearing distribution ratio after the JR. As mentioned, joint incongruency between the artificial femoral head and the original acetabulum was possibly associated with this issue, and some improvements may be needed, such as a more congruent and smoother surface of the artificial femoral head and/or acetabular implants for THA. Second, the assessments of postsurgical pain were limited. The usefulness of this model could have been emphasized by additional evaluations such as stimulus-evoked pain behavior, histology, and proinflammatory cytokine measurement. Third, the observation period was relatively short. Although we observed a complete recovery of weight-bearing distribution within the observation period, a longer follow-up period is required to evaluate the sustainability of the implant. Lastly, there was no size variation in the artificial femoral head. Paish et al created implants to fit the body size of rats, whereas we created only one size. To obtain better results, additional implants tailored to the size of each rat may be necessary.
Despite hurdles to overcome, our novel JR model has the potential to address some basic mechanisms of CPSP, which will be clinically important for developing new treatment strategies.
Conclusion
Our novel JR model presented acute postsurgical pain behavior that successfully recovered to the baseline level on day 42. The difference in pain manifestation between the JR and JD groups is neurologically supported by the expression of CGRP-IR in DRG neurons. This model is the first step in understanding the detailed mechanisms of pain after JR.
Acknowledgments
We would like to thank Editage for English language editing.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis, and interpretation, or in all these areas; took part in drafting, revising, or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was supported by JSPS KAKENHI Grant Number 17K09034.
Disclosure
Dr Yoshinori Satake reports grants from Eli Lilly Japan K. K., outside the submitted work. The authors report no other conflicts of interest in this work.
References
1. Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projection of primary and revision Hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89(4):80–85. doi:10.2106/00004623-200704000-00012
2. Neuprez A, Neuprez AH, Kaux JF, et al. Total joint replacement improves pain, functional quality of life, and health utilities in patients with last-stage knee and Hip osteoarthritis for up to 5 years. Clin Rheumatol. 2020;39(3):861–871. doi:10.1007/s10067-019-04811-y
3. Wylde V, Hewlett S, Learmonth ID, Dieppe P. Persistent pain after joint replacement: prevalence, sensory qualities, and postoperative determinants. Pain. 2011;152(3):566–572. doi:10.1016/j.pain.2010.11.023
4. Grosu I, Lavand’homme P, Thienpont E. Pain after knee arthroplasty: an unresolved issue. Knee Surg Sports Traumatol Arthrosc. 2014;22(8):1744–1758. doi:10.1007/s00167-013-2750-2
5. Abrman K, Musil D, Stehlik J. Treatment of acute periprosthetic infections with DAIR (debridement, antibiotics and implant retention) - success rate and risk factors of failure. Acta Chir Orthop Traumatol Cech. 2019;86(3):181–187.
6. Gehrke T, Alijanipour PJ, Parvizi J. The management of an infected total knee arthroplasty. Bone Joint J. 2015;97(10 Suppl A):20–29. doi:10.1302/0301-620X.97B10.36475
7. El-Warrak AO, Olmstead M, Schneider R, et al. An experimental animal model of aseptic loosening of Hip prostheses in sheep to study early biochemical changes at the interface membrane. BMC Musculoskelet Disord. 2004;5:7. doi:10.1186/1471-2474-5-7
8. Volstad NJ, Schaefer SL, Snyder LA, Meinen JB, Sample SJ. Metallosis with pseudotumour formation: long-term complication following cementless total Hip replacement in a dog. Vet Comp Orthop Traumatol. 2016;29(4):283–289. doi:10.3415/VCOT-16-02-0028
9. DiVincenzo MJ, Frydman GH, Kowaleski MP, et al. Metallosis in a dog as a long-term complication following total Hip arthroplasty. Vet Pathol. 2017;54(5):828–831. doi:10.1177/0300985817716261
10. Deyoung DJ, Schiller RA. Radiographic criteria for evaluation of uncemented total Hip replacement in dogs. Vet Surg. 1992;21(2):88–98. doi:10.1111/j.1532-950X.1992.tb00021.x
11. King SW, Lamb JN, Cage ES, Pandit H. Periprosthetic femoral fractures following total Hip and total knee arthroplasty. Maturitas. 2018;117:1–5. doi:10.1016/j.maturitas.2018.08.010
12. Pinto PR, McIntyre T, Ferrero R, Almeida A, Araújo-Soares V. Predictors of acute postsurgical pain and anxiety following primary total Hip and knee arthroplasty. J Pain. 2013;14(5):502–515. doi:10.1016/j.jpain.2012.12.020
13. Glare P, Aubrey KR, Myles PS. Transition from acute to chronic pain after surgery. Lancet. 2019;393(10180):1537–1546. doi:10.1016/S0140-6736(19)30352-6
14. Beswick AD, Wylde V, Gooberman-Hill R, Blom A, Dieppe P. What proportion of patients report long-term pain after total Hip or knee replacement for osteoarthritis? A systematic review of prospective studies in unselected patients. BMJ Open. 2012;2(1):e000435. doi:10.1136/bmjopen-2011-000435
15. Nakajima T, Ohtori S, Nakamura J. Inflammatory pain-related traits of sensory DRG neurons innervating the Hip joints. J Orthop Sci. 2017;22(2):325–329. doi:10.1016/j.jos.2016.12.001
16. Powers DL, Claassen B, Black J. The rat as an animal model for total Hip replacement arthroplasty. J Invest Surg. 1995;8(5):349–362. doi:10.3109/08941939509015381
17. Paish ADM, Nikolov HN, Welch ID, et al. Image‐based design and 3D‐metal printing of a rat Hip implant for use in a clinically representative model of joint replacement. J Orthop Res. 2020;38(7):1627–1636. doi:10.1002/jor.24706
18. Lecocq M, Linares JM, Chaves-Jacob J, et al. Total knee arthroplasty with a Ti6Al4V/PEEK prosthesis on an osteoarthritis rat model: behavioral and neurophysiological analysis. Sci Rep. 2020;10;5277:1639–1641.
19. Nakajima T, Ohtori S, Inoue G, et al. The characteristics of dorsal-root-ganglia and sensory innervation of the Hip in rats. J Bon Jt Surg Br. 2008;90(2):254–257. doi:10.1302/0301-620X.90B2.19808
20. Wylde V, Rooker J, Halliday L, Blom A. Acute postoperative pain at rest after Hip and knee arthroplasty: severity, sensory qualities and impact on sleep. Orthop Traumatol Surg Res. 2011;97(2):139–144. doi:10.1016/j.otsr.2010.12.003
21. Thomas T, Robinson C, Champion D, Mckell M, Pell M. Prediction and assessment of the severity of postoperative pain and of satisfaction with management. Pain. 1998;75(2–3):177–185. doi:10.1016/S0304-3959(97)00218-2
22. Buvanendran A, Kroin JS, Kari MR, TumanTH KJ. A new surgery model in rats to evaluate functional measures of postoperative pain. Anesth Analg. 2008;107(1):300–308. doi:10.1213/ane.0b013e3181732f21
23. Majuta LA, Guedon JG, Mitchell SAT, Ossipov MH, Mantyh PW. Anti-nerve growth factor therapy increases spontaneous day/night activity in mice with orthopedic surgery-induced pain. Pain. 2017;158(4):605–617. doi:10.1097/j.pain.0000000000000799
24. Bourne RB, Chesworth BM, Davis AM, Mahomed NN, Charron KD. Patient satisfaction after total knee arthroplasty: who is satisfied and who is not? Clin Orthop Relat Res. 2010;468(1):57–63. doi:10.1007/s11999-009-1119-9
25. Lu F, Kato J, Toramaru T, Sugai M, Zhang M, Morisaki H. Objective and quantitative evaluation of spontaneous pain-like behaviors using dynamic weight-bearing system in mouse models of postsurgical pain. J Pain Res. 2022;15:1601–1612. doi:10.2147/JPR.S359220
26. Pertici V, Pin-Barre C, Felix M, Laurin J, Brisswalter J, Decherchi P. A new method to assess weight-bearing distribution after central nervous system lesions in rats. Behav Brain Res. 2014;259:78–84. doi:10.1016/j.bbr.2013.10.043
27. Quadros AU, Pinto LG, Fonseca MM, Kusuda R, Cunha FQ, Cunha TM. Dynamic weight bearing is an efficient and predictable method for evaluation of arthritic nociception and its pathophysiological mechanisms in mice. Sci Rep. 2015;29:5.
28. Lewis GN, Rice DA, McNair PJ, Kluger M. Predictors of persistent pain after total knee arthroplasty: a systematic review and meta-analysis. Br J Anaesth. 2015;114(4):551–561. doi:10.1093/bja/aeu441
29. Cata JP, Patino M, Lacagnina MJ, et al. A rat model to investigate quality of recovery after abdominal surgery. Pain Rep. 2021;6(2):e943. doi:10.1097/PR9.0000000000000943
30. Yang J, Yuan F, Ye G, et al. Skin/muscle incision and retraction induces evoked and spontaneous pain in mice. Pain Res Manag. 2019;2019:6528528. doi:10.1155/2019/6528528
31. Timothy JB. Pathophysiology of postoperative pain. Pain. 2011;152(3 Suppl):S33–S40. doi:10.1016/j.pain.2010.11.005
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.