Back to Journals » Clinical and Experimental Gastroenterology » Volume 17
A Novel Rat Model to Simulate the Benign Esophageal Stricture Induced by Endoscopic Submucosal Dissection
Authors Luo YG , Zhang XW, Zhao H, Li JG, Tsauo JW, Gong T, Ou AX, Cong TH, Kang WD, Li X
Received 17 August 2023
Accepted for publication 29 January 2024
Published 19 February 2024 Volume 2024:17 Pages 41—50
DOI https://doi.org/10.2147/CEG.S435690
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Andreas M. Kaiser
Yin-Gen Luo,* Xiao-Wu Zhang,* He Zhao, Jin-Gui Li, Jiay-Wei Tsauo, Tao Gong, Ai-Xin Ou, Tian-Hao Cong, Wen-Di Kang, Xiao Li
Department of Interventional Therapy, National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Xiao Li; He Zhao, Department of Interventional Therapy, National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, 100021, People’s Republic of China, Tel/Fax +86 010 8778 8502, Email [email protected]; [email protected]
Objective: This study aimed to establish a rat model that simulates benign esophageal strictures induced by endoscopic submucosal dissection (ESD).
Materials and Methods: Sixteen male Sprague-Dawley rats were randomly divided into mucosal resection (n = 8) and sham-operated groups (n = 8). The rats in the mucosal resection group underwent a 5-mm three-fourths mucosal resection by way of a 3-mm incision in the distal esophagus under direct visualization via laparotomy. Rats in the sham-operated group underwent a 3-mm incision of the muscularis propria layer in the distal esophagus via laparotomy without mucosal resection. Dysphagia score, weight gain, mucosal constriction rate, and histology were evaluated 2 weeks after surgery.
Results: Technical success was achieved in all the animals. One rat in the mucosal resection group died of infection, and no other complications were observed. Weight gain (P < 0.001) and luminal diameter derived from the esophagograms (P < 0.001) were significantly lower in the mucosal resection group than those in the sham-operated group. Dysphagia score (P < 0.001) and mucosal constriction rate (P < 0.001) were significantly higher in the mucosal resection group than those in the sham-operated group. The inflammation grade (P = 0.002), damage to the muscularis propria (P < 0.001), number of nascent microvessels (P = 0.006), and degree of α-SMA positive deposition (P = 0.006) were significantly higher in the mucosal resection group.
Conclusion: A rat model of benign esophageal stricture induced by ESD was successfully and safely established by mucosal resection.
Keywords: esophageal stenosis, endoscopic mucosal resection, models, animal
Introduction
Endoscopic submucosal dissection (ESD) plays an important role in Barrett’s neoplasia and is considered the first-line treatment for esophageal squamous cell carcinoma.1 However, esophageal stricture (11–20%) is a common complication following esophageal ESD,2 especially when the lesion extends more than three-fourths of the luminal circumference (88–100%).3 Esophageal strictures can cause severe symptoms (eg, vomiting and dysphagia) and thus greatly reduce the quality of life of patients, which is often refractory to treatment.4 Many approaches have been used to prevent benign esophageal strictures after ESD (eg, periodic balloon dilation, steroids, stent placement, tissue shielding method, and tissue engineering), but no ideal prevention strategy has been established.5
Small rodent models are critical for the development of biology and medicine.6,7 Compared with larger animal models, the advantages of small rodent models include shorter gestation time, ease of use, genetic manipulation, and cost-effectiveness.8,9 These strengths make small rodent models the most commonly used models for physiology, pharmacology, genetics, and preclinical research. However, owing to the size of the instruments, animal studies related to ESD are mainly based on large animals, such as canines and swine.10,11 To our knowledge, a rodent model that can successfully simulate benign esophageal strictures induced by ESD is still scarce, limiting the in-depth research of the underlying mechanisms and development of novel treatments. Wu et al recently established a rat model of esophageal stricture by using an electrosurgical knife to remove the mucosal layer and submucosal layer of the esophagus.12 However, their method requires a high degree of proficiency and precision. Therefore, this study aimed to establish a simple, stable and reproducible rat model that could successfully simulate benign esophageal strictures induced by ESD.
Materials and Methods
Study Design
The protocol for this animal study was approved by the Institutional Animal Care and Use Committee (approval number NCC2022A109). All animals were maintained according to the Guide for the Care and Use of Laboratory Animals. Sixteen male Sprague-Dawley rats (300–350 g) (Beijing HFK Bio-Technology Co., Ltd, Beijing, China) were randomly assigned to either a mucosal resection group (n = 8) or a sham-operated group (n = 8). All animals were individually raised in cages in a temperature-controlled room (24 ± 2 °C) with a 12 h light/dark cycle for 1 week before the operation. Mucosal resection and sham operation were performed according to the protocol described below. Body weight measurement and esophagography were performed 2 weeks after the operation. Immediately after esophagography, all animals were euthanized using inhalable carbon dioxide, followed by macroscopic and histological analyses.
Mucosal Resection and Sham Operation
The animals were fasted for 12 h before the operation. The rats were anesthetized by intramuscular injection of atropine sulfate (0.05 mg/kg) (Tianjin Jinyao Pharmaceutical Co., Ltd, Tianjin, China), xylazine (10 mg/kg) (XiYa Reagent, Shandong, China), and a mixture of tiletamine and zolazepam (50 mg/kg) (Virbac Corporation, Texas, USA). The distal esophagus was separated via a median abdominal incision, and a 3-mm incision was made in the muscularis propria of the distal esophagus 10 mm from the esophagogastric junction. Next, the mucosa underlying the muscularis propria was separated and pulled out using micro-forceps, and a 3/4 circumferential mucosal resection (length: 5 mm) was performed. After mucosal resection, the 3-mm incision in the muscularis propria was closed with continuous 7–0 polyglycolic acid absorbable sutures. Finally, the midline abdominal incision was closed in two layers using continuous 4–0 silk sutures (Figure 1). In contrast, rats in the sham-operated group underwent the same operation as that in the mucosal resection group, except for mucosal resection.
Follow-Up Examinations
The rats were housed individually in cages and fasted on the first day after the operation. All rats were given a liquid diet for 3 days, and a solid diet was given from the fourth day after the operation. When rats had trouble ingesting solid foods, they were given a semi-solid diet or even a liquid diet. Based on daily monitoring, the modified dysphagia score was evaluated in a totally blinded manner: 4 = complete dysphagia, 3 = able to eat liquid food only, 2 = able to eat semi-solid food only, 1 = able to eat solid food, and 0 = able to eat a normal diet.13 Based on body weight measurements taken before and two weeks after the operation, weight gain was calculated. And the luminal diameter of the operated esophagus was measured through the follow-up esophagograms by using RadiAnt DICOM Viewer (version 2021.1, Medixant Company, Poland).
The esophagus was excised, longitudinally dissected, and macroscopically evaluated after euthanasia. The following formula was used to calculate the rate of lateral mucosal constriction, which represents the degree of stricture at the lesion site:14 
The excised esophageal samples were fixed in 10% buffered formalin for 24 h and longitudinally cut to prepare them for paraffin embedding. The paraffin-embedded blocks were sectioned at a thickness of 4 µm and stained with hematoxylin and eosin (HE), Masson’s trichrome (MT), and α-SMA antibodies (ab21027; Abcam, Cambridge, UK). The inflammation grade and number of nascent microvessels in the lesion submucosal layer were determined using HE staining images.14,15 Damage to the muscularis propria was evaluated using MT staining images.14 The degree of α-SMA positive deposition was measured by the proportion of positive cells in each visual field using immunohistochemical staining.16 The results of the histological analysis are shown in Table 1. A BX51 microscope (Olympus, Tokyo, Japan) was used for histological analysis. Measurements were performed using Image-Pro Plus software (version 6.0; Media Cybernetics, Silver Spring, MD, USA). Three researchers who were blinded to group classification conducted all analyses.
 |
Table 1 The Scoring System of the Histologic Analysis Used in This Study |
Statistical Analysis
All data are expressed as the median (interquartile range) or mean ± standard deviation (SD). Differences between two groups were compared using Student’s t-test, Welch’s t-test, or Mann–Whitney U-test, when appropriate. Statistical significance was set at P < 0.05. GraphPad Prism 8.0.2 (GraphPad Software Inc., San Jose, CA, USA) was used for all the statistical analyses.
Results
Establishment of Rat Model
Technical success was achieved in all 16 rats. Unfortunately, one rat in the mucosal resection group died due to infection on postoperative day one, which was excluded from the analysis. The remaining rats survived without any complications until the end of the study (Figure 2).
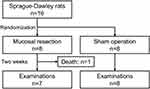 |
Figure 2 Flow chart of the study. |
Dysphagia Score and Weight Gain
In the mucosal resection group, five rats were switched to a liquid diet, and the other two were switched to a semi-solid diet. In the sham-operated group, two rats showed decreased normal diet intake, and no rats switched to a liquid or semi-solid diet. The median modified dysphagia scores were 3 (1) and 0 (1) in the mucosal resection and sham-operated groups (P < 0.001), respectively. In addition, the mean weight gain 2 weeks after the operation was lower in the mucosal resection group than that in the sham-operated group (−27.3 ± 10.7% vs 17.0 ± 5.0%, P < 0.001).
Esophagography and Gross Examination
Follow-up esophagography revealed obvious esophageal constriction in the mucosal resection group (Figure 3A). Moreover, the mean luminal diameter of the operated esophagus derived from the follow-up esophagograms was significantly lower in the mucosal resection group than that in the sham-operated group (1.2 ± 0.3 mm vs 3.8 ± 0.6 mm, P < 0.001) (Figure 3B). Macroscopic analysis indicated that all rats in the mucosal resection group had esophageal stricture, but there was no sign of esophageal stricture in the sham-operated group (Figure 3C, D). In addition, the mean rate of mucosal constriction was higher in the mucosal resection group than that in the sham-operated group (48.8 ± 9.2% vs 4.8 ± 1.4%, P < 0.001).
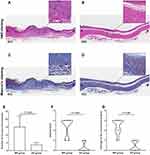
Histological Findings
Regenerative squamous epithelium, inflammation in the muscle layers, and transmural fibrotic tissue formation in the muscularis propria were evident in the mucosal resection group, and these findings were not observed in the sham-operated group (Figure 4A–D). The mean number of nascent microvessels in the submucosal layer per unit area was higher in the mucosal resection group than that in the sham-operated group (15.1 ± 7.2 vs 3.8 ± 1.4, P = 0.006) (Figure 4E). The median inflammation grades were 3 (2) and 0 (0.75) in the mucosal resection and sham-operated groups (P = 0.002), respectively (Figure 4F). The median scores of damage to the muscularis propria were 3 (1) and 0 (1) in the mucosal resection and sham-operated groups (P < 0.001), respectively (Figure 4G). In the mucosal resection group, α-SMA-positive myofibroblast-forming layers with regular horizontal arrangements were observed in the submucosal layers (Figure 5A, B). The mean degree of α-SMA positive deposition per unit area was higher in the mucosal resection group than that in the sham-operated group (1.8 ± 0.5 vs 0.5 ± 0.2, P < 0.001) (Figure 5C).
Discussion
Several methods have been used to induce benign esophageal strictures in rat models, including corrosive injury17 and stent-related methods.18 These rat models have been widely used to study prophylactic methods for corrosive esophageal burn-induced strictures and stent-induced esophageal tissue hyperplasia. However, ESD has been increasingly associated with the etiology of benign esophageal stricture formation. A rodent model that can successfully simulate benign esophageal stricture induced by ESD is still limited. Up to now, only the rat model of benign esophageal stricture established by Wu et al12 may successfully simulate benign esophageal stricture induced by ESD. Differently, we perform mucosal dissection under direct visualization through an incision in the muscularis propria of the esophagus, rather than a choledochoscope-assisted esophageal mucosal burn through a perforation of the gastric wall. Therefore, in this study, an easier rat model of benign esophageal stricture induced by mucosal resection was established with a mucosal constriction rate of 48.8%. The technical success rate was 100% (8/8) and the modeling success rate was 87.5% (7/8).
A rat model of benign esophageal stricture has traditionally been induced by corrosive injury, especially with NaOH. Corrosive esophageal burns cause full-thickness necrosis involving the mucosa, submucosa, and muscular layer. Furthermore, the mortality and complication rates of the corrosive esophageal burn model are likely to be higher in severe cases. For rats requiring gastrostomy feeding and gastric puncture postoperatively, Gehanno and Guedon17 used 50% NaOH to construct a corrosive esophageal burn model, but 20 (30.8%) of 65 rats died during the first 3 days of the experiment. Okata et al19 also reported mortality rates of 37.5% (3/8), 20% (2/10), 72.7% (8/11), and 66.7% (4/6) when the NaOH concentration was 20, 30, 40, and 50%, respectively, while the incidence of esophageal perforation was up to 66.7% (4/6) when the NaOH concentration was 50%. In the present study, one rat (12.5%, 1/8) died within 24 h postoperation due to infection in the mucosal resection group, and the remaining rats had no definite complications until the end of the study, confirming the safety of this mucosal resection rat model.
In addition, several clinical studies have shown that strictures often occur 3 weeks after corrosive esophageal injuries20,21 but 2–4 weeks after ESD.22,23 For this reason, many experimental studies related to the rat model of corrosive esophageal burns choose 3 weeks or longer postoperatively as the time of sacrifice.15,24,25 In the current rat model, after mucosal resection, postoperative stricture formation after 2 weeks was responsible for feeding difficulties (Figure 3), and the epithelium was regenerated (Figure 4A), consistent with the research of Wu et al.12 Compared with corrosive esophageal burn rat models, our mucosal resection rat model seems to have the potential to shorten the study duration for evaluating new preventive strategies for esophageal stricture formation.
The mechanisms of esophageal stricture formation induced by alkali (eg, NaOH) and ESD differ. Alkalis cause liquefactive necrosis, leading to severe esophageal damage. The liquefactive necrosis process often lasts 3–4 days and is related to mucosal inflammation and vascular thrombosis, causing extensive or focal ulceration and sloughing. In general, the natural process is fibrosis and collagen deposition, followed by stricture formation in the second or third week.26 In contrast, ESD causes a large area of esophageal mucosal defects, which means the loss of a barrier against saliva, food, gastric acid, and microbes. Esophageal stricture development is a healing process followed by scarring of the mucosal tissue defect, with severe inflammation, atrophy, and fibrosis in the muscularis propria. In the current study, severe inflammation, angiogenesis, atrophy, and fibrosis were found in the mucosal resection group, which is consistent with the results of previous investigations.12,14 Another important pathological feature in the healing process of mucosal defects after ESD is the appearance of myofibroblasts with horizontal extension and a parallel arrangement of spindle cells.27 As expected, we also found a spindle-shaped α-SMA-positive myofibroblast cambium in a regular horizontal arrangement (Figure 5A). Therefore, it is plausible that the proposed mucosal resection rat model is appropriate for further studies of post-ESD esophageal strictures related to mechanisms, treatment strategies, etc.
To date, numerous anti-inflammatory and anti-fibrotic agents for preventing esophageal stricture formation in corrosive esophageal burn rat models have been studied.28–30 However, it is unclear whether these agents can also be applied in preventing and treating post-ESD esophageal strictures since the underlying mechanisms of stricture formation after corrosive injuries and ESD differ. Interestingly, corticosteroids have been reported to be effective in preventing post-ESD stricture formation;2,31,32 however, they are unlikely to generate obvious benefits for preventing esophageal stricture after corrosive injuries.33,34 Therefore, a rat model of mucosal resection is essential for evaluating new medications or other prevention and treatment strategies for post-ESD stricture formation.
This study has several limitations. First, mucosal resection was performed in a rat model under direct visualization via laparotomy rather than intraluminally, as in ESD. However, the size of the rodent model and the available endoluminal devices limit the possibility of performing ESD in rodent models. Second, the healing process of mucosal defects was not recorded or explored in depth in this study. Further studies on the mechanism of post-ESD esophageal stricture formation in rodent models are warranted.
Conclusion
In summary, the proposed rat model of benign esophageal strictures induced by mucosal resection is simple, stable, and reproducible, which can also help to shorten study duration time and improve cost effectiveness. This model may serve as a useful tool to further investigate the pathogenesis of and develop novel prevention and treatment strategies for benign esophageal strictures after ESD.
Abbreviations
ESD, Endoscopic submucosal dissection; DICOM, Digital Imaging and Communications in Medicine; HE, hematoxylin and eosin; MT, Masson’s trichrome; SD, standard deviation; NAOH, sodium hydroxide.
Data Sharing Statement
All data are available upon request from the corresponding authors on reasonable request.
Ethics Approval and Informed Consent
This animal study was approved by the Animal Care and Use Committee of National Cancer Center/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College (Approval No. NCC2022A109).
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
All authors report no conflicts of interest in this work.
References
1. Pimentel-Nunes P, Libanio D, Bastiaansen BAJ, et al. Endoscopic submucosal dissection for superficial gastrointestinal lesions: European society of gastrointestinal endoscopy (esge) guideline - update. Endoscopy. 2022;54(6):591–622. doi:10.1055/a-1811-7025
2. Abe S, Iyer PG, Oda I, Kanai N, Saito Y. Approaches for stricture prevention after esophageal endoscopic resection. Gastrointest Endosc. 2017;86(5):779–791. doi:10.1016/j.gie.2017.06.025
3. Oliveira JF, Moura EG, Bernardo WM, et al. Prevention of esophageal stricture after endoscopic submucosal dissection: a systematic review and meta-analysis. Surg Endosc. 2016;30(7):2779–2791. doi:10.1007/s00464-015-4551-9
4. Siersema PD. Treatment options for esophageal strictures. Nat Clin Pract Gastro Hepatol. 2008;5(3):142–152. doi:10.1038/ncpgasthep1053
5. Hikichi T, Nakamura J, Takasumi M, et al. Prevention of stricture after endoscopic submucosal dissection for superficial esophageal cancer: a review of the literature. J Clin Med. 2020;10(1):10. doi:10.3390/jcm10010010
6. Halfmann PJ, Iida S, Iwatsuki-Horimoto K, et al. Sars-cov-2 omicron virus causes attenuated disease in mice and hamsters. Nature. 2022;603(7902):687–692. doi:10.1038/s41586-022-04441-6
7. Oikawa M, Kobayashi H, Sanbo M, et al. Functional primordial germ cell-like cells from pluripotent stem cells in rats. Science. 2022;376(6589):176–179. doi:10.1126/science.abl4412
8. Hetze S, Sure U, Schedlowski M, Hadamitzky M, Barthel L. Rodent models to analyze the glioma microenvironment. ASN Neuro. 2021;13:17590914211005074. doi:10.1177/17590914211005074
9. Gotz J, Bodea LG, Goedert M. Rodent models for Alzheimer disease. Nat Rev Neurosci. 2018;19(10):583–598. doi:10.1038/s41583-018-0054-8
10. Ji R, Yang JL, Yang XX, et al. Simplified robot-assisted endoscopic submucosal dissection for esophageal and gastric lesions: a randomized controlled porcine study (with videos). Gastrointest Endosc. 2022;96(1):140–147. doi:10.1016/j.gie.2022.01.004
11. Zhao LM, Gong M, Wang R, et al. Accelerating esd-induced gastric ulcer healing using a ph-responsive polyurethane/small intestinal submucosa hydrogel delivered by endoscopic catheter. Regen Biomater. 2021;8(1):rbaa056. doi:10.1093/rb/rbaa056
12. Wu R, Fu M, Tao HM, et al. Benign esophageal stricture model construction and mechanism exploration. Sci Rep. 2023;13(1):11769. doi:10.1038/s41598-023-38575-y
13. Mellow MH, Pinkas H. Endoscopic laser therapy for malignancies affecting the esophagus and gastroesophageal junction. Analysis of technical and functional efficacy. Arch Intern Med. 1985;145(8):1443–1446. doi:10.1001/archinte.1985.00360080117017
14. Honda M, Hori Y, Nakada A, et al. Use of adipose tissue-derived stromal cells for prevention of esophageal stricture after circumferential EMR in a canine model. Gastrointest Endosc. 2011;73(4):777–784. doi:10.1016/j.gie.2010.11.008
15. Anayurt M, Karaman A, Balci O, Ozguner IF, Karaman I. The effects of hesperidin on stricture formation in corrosive esophageal burns: an experimental study. Esophagus. 2022;19(1):189–196. doi:10.1007/s10388-021-00861-x
16. Zhao H, Fu Y, Tsauo J, et al. Silver nanoparticle-coated self-expandable metallic stent suppresses tissue hyperplasia in a rat esophageal model. Surg Endosc. 2022;36(1):66–74. doi:10.1007/s00464-020-08238-4
17. Gehanno P, Guedon C. Inhibition of experimental esophageal lye strictures by penicillamine. Arch Otolaryngol. 1981;107(3):145–147. doi:10.1001/archotol.1981.00790390011004
18. Kim EY, Shin JH, Jung YY, Shin DH, Song HY. A rat esophageal model to investigate stent-induced tissue hyperplasia. J Vasc Interv Radiol. 2010;21(8):1287–1291. doi:10.1016/j.jvir.2010.04.023
19. Okata Y, Hisamatsu C, Hasegawa T, Nishijima E, Okita Y. Development of a model of benign esophageal stricture in rats: the optimal concentration of sodium hydroxide for stricture formation. Pediatr Surg Int. 2011;27(1):73–80. doi:10.1007/s00383-010-2711-5
20. Karaman I, Koc O, Karaman A, et al. Evaluation of 968 children with corrosive substance ingestion. Indian J Crit Care Med. 2015;19(12):714–718. doi:10.4103/0972-5229.171377
21. Arnold M, Numanoglu A. Caustic ingestion in children-a review. Semin Pediatr Surg. 2017;26(2):95–104. doi:10.1053/j.sempedsurg.2017.02.002
22. Mizuta H, Nishimori I, Kuratani Y, Higashidani Y, Kohsaki T, Onishi S. Predictive factors for esophageal stenosis after endoscopic submucosal dissection for superficial esophageal cancer. Dis Esophagus. 2009;22(7):626–631. doi:10.1111/j.1442-2050.2009.00954.x
23. Ono S, Fujishiro M, Niimi K, et al. Predictors of postoperative stricture after esophageal endoscopic submucosal dissection for superficial squamous cell neoplasms. Endoscopy. 2009;41(08):661–665. doi:10.1055/s-0029-1214867
24. Turkyilmaz Z, Sonmez K, Demirtola A, et al. Mitomycin c prevents strictures in caustic esophageal burns in rats. J Surg Res. 2005;123(2):182–187. doi:10.1016/j.jss.2004.08.009
25. Guven A, Demirbag S, Uysal B, et al. Effect of 3-amino benzamide, a poly(adenosine diphosphate-ribose) polymerase inhibitor, in experimental caustic esophageal burn. J Pediatr Surg. 2008;43(8):1474–1479. doi:10.1016/j.jpedsurg.2007.10.001
26. Contini S. Caustic injury of the upper gastrointestinal tract: a comprehensive review. World J Gastro. 2013;19(25):3918–3930. doi:10.3748/wjg.v19.i25.3918
27. Nonaka K, Miyazawa M, Ban S, et al. Different healing process of esophageal large mucosal defects by endoscopic mucosal dissection between with and without steroid injection in an animal model. BMC Gastroenterol. 2013;13(1):72. doi:10.1186/1471-230X-13-72
28. Turkyilmaz Z, Sonmez K, Karabulut R, et al. Mitomycin c decreases the rate of stricture formation in caustic esophageal burns in rats. Surgery. 2009;145(2):219–225. doi:10.1016/j.surg.2008.10.007
29. Aciksari K, Yanar HT, Hepgul G, et al. The effect of beta-aminopropionitrile and prednisolone on the prevention of fibrosis in alkali esophageal burns: an experimental study. Gastroenterol Res Pract. 2013;2013:574260. doi:10.1155/2013/574260
30. Orozco-Perez J, Aguirre-Jauregui O, Salazar-Montes AM, Sobrevilla-Navarro AA, Lucano-Landeros MS, Armendariz-Borunda J. Pirfenidone prevents rat esophageal stricture formation. J Surg Res. 2015;194(2):558–564. doi:10.1016/j.jss.2014.11.009
31. Kochhar R, Makharia GK. Usefulness of intralesional triamcinolone in treatment of benign esophageal strictures. Gastrointest Endosc. 2002;56(6):829–834. doi:10.1016/S0016-5107(02)70355-6
32. Yamaguchi N, Isomoto H, Nakayama T, et al. Usefulness of oral prednisolone in the treatment of esophageal stricture after endoscopic submucosal dissection for superficial esophageal squamous cell carcinoma. Gastrointest Endosc. 2011;73(6):1115–1121. doi:10.1016/j.gie.2011.02.005
33. Pelclova D, Navratil T. Do corticosteroids prevent oesophageal stricture after corrosive ingestion? Toxicol Rev. 2005;24(2):1287–1289. doi:10.2165/00139709-200524020-00006
34. Fulton JA, Hoffman RS. Steroids in second degree caustic burns of the esophagus: a systematic pooled analysis of fifty years of human data: 1956–2006. Clin Toxicol. 2007;45(4):402–408. doi:10.1080/15563650701285420
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.