Back to Journals » Drug Design, Development and Therapy » Volume 10
A novel pH–enzyme-dependent mesalamine colon-specific delivery system
Authors Jin L, Ding Y, Zhang Y, Xu X, Cao Q
Received 26 February 2016
Accepted for publication 24 March 2016
Published 20 June 2016 Volume 2016:10 Pages 2021—2028
DOI https://doi.org/10.2147/DDDT.S107283
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Wei Duan
Lei Jin, Yi-cun Ding, Yu Zhang, Xiao-qing Xu, Qin Cao
Department of Gastroenterology, Putuo Hospital, Shanghai University of Traditional Chinese Medicine, Shanghai, People’s Republic of China
Abstract: The aim of the present study was to design a new pH–enzyme double-dependent mesalamine colon-specific delivery system. The drug release behaviors in vitro and pharmacokinetics and biodistribution in vivo were further evaluated. The mean particle diameters of mesalamine-coated microparticles were 312.2 µm. In vitro, a small amount of mesalamine was released in HCl at a pH of 1.2 and PBS medium at a pH of 7.4 for 5 hours, and 71% of the entrapped mesalamine was further released during the subsequent 20 hours of incubation. A greater area under the plasma concentration–time curve (AUC)0–t was obtained for the coated microparticles (1.9-fold) compared to the suspensions group, which indicated that the encapsulated mesalamine had mostly been absorbed in rats over the period of 12 hours. The AUC0–t of the coated microparticles in colon was 2.63-fold higher compared to the suspensions (P<0.05). Hence, mesalamine-coated microparticles are considered to maintain the drug concentration within target ranges for a long period of time.
Keywords: pH–enzyme, mesalamine, colon specific, pharmacokinetics, biodistribution
Introduction
Ulcerative colitis is a chronic nonspecific inflammatory bowel disease of the colon and rectum. The lesions are found in the sigmoid colon and rectum and can also be extended to the descending colon and even to the whole colon. The disease occurs at any age, but it mostly occurs at the age of 20–30 years. Mesalamine is the first-line drug used in the treatment of mild-to-moderate active ulcerative colitis and in the prevention of recurrence.1 This drug has been used for nearly 50 years, the exact mechanism of action of mesalamine, local efficacy, and the adverse reaction is known.2 Previous studies (in vitro) have shown that there is a direct relationship between the concentration of mesalamine and its therapeutic effect.3,4
This led the researchers to hope to deliver appropriate drug dosages to the inflamed tissue directly and reduce the related adverse events. In the past few years, different strategies have been reported in order to achieve specific colonic delivery of drugs. Most of the previous articles about colonic targeting have focused on the development of a colonic delivery system based on time and pH as well as systems that utilize bacteria, which colonizes the colon or enzymes produced by these bacteria that affect the drug release.5–8
However, these colon-specific delivery systems have poor specificity due to large variations in gastric emptying time and in pH of the gastrointestinal tract.9–11
The pH–enzyme double-dependent colon-specific delivery system combines the advantages of the previously explained methods; this system produces sustained target release after oral administration, with low toxicity potential for other organs.12,13 Chitosan ([1,4]-2-amino-2-deoxy-β-D-glucan) has been widely used in biomedical applications such as wound healing, drug delivery systems, coatings, and tissue engineering due to its advantages such as good biocompatibility, low toxicity, and biodegradability.14,15 So it has been considered suitable for use in sustained release delivery system as long-acting biodegradable carriers. In addition, acrylic polymers, such as Eudragit® (Evonik Industries, Essen, Germany), a copolymer of methyl acrylic acid and its esters, play a significant role in pH-dependent site-specific delivery of drugs.16 Eudragit S100 dissolves at pH 7.0 and releases the drug into the colon.
The aim of the present study was to design a new pH–enzyme double-dependent colon-specific delivery system. Mesalamine was selected as a model drug and chitosan/Eudragit S100 were selected as accessories. The release behaviors in vitro and pharmacokinetics in vivo of the drug were further evaluated.
Materials and methods
Materials
Mesalamine, obtained from Xinxin Biopharma Co Ltd (Wuhan, People’s Republic of China). Chitosan (degree of deacetylation 85%) was obtained from Hongxinkang Chemical Co. (Hubei, People’s Republic of China). Eudragit S100 was kindly donated by Evonik Industries. Glutaraldehyde was purchased from Sigma-Aldrich (Perth, WA, Australia) as a 25% aqueous solution. All other analytical grade chemicals and solvents were used without further purification. Double-distilled water was used in the whole study.
Preparation of mesalamine colon-specific delivery system
The mesalamine-loaded pH–enzyme double-dependent colon-specific delivery system was prepared following a two-step protocol –preparation of chitosan microparticles and coating of microparticles. Microparticles were prepared by a recently published emulsion-chemical cross-linking technique.17 Briefly, mesalamine (20 mg) was added to 3 mL of methylene chloride and chitosan was dissolved in 1% (v/v) acetic acid solution to obtain a concentration of 2% polymer solution (w/v), respectively. Completely dissolved, the organic phase was then slowly added to 2.3% Span-60 polymer solution; the mixture was then emulsified for 30 minutes (150 W output power, each 30 seconds and hold 2 seconds). Temperature was maintained at 4°C using an ice bath during emulsification. A total of 25% glutaraldehyde solution was added to the the medium under constant magnetic stirring, followed by cross-linking for 2 hours in a dropwise manner. Then the microparticles were collected by pressure reduction filtration and lyophilized for 24 hours using freeze drier.
The coating of mesalamine microparticles was performed by fluid-bed spray technology (circulating fluidized). In short, 0.1 g of mesalamine chitosan microparticles were coated with Eudragit S100 in an aqueous phase using a fluid-bed spray coater to give a 20% weight gain. The air pressure was 0.3 MPa and the spray rate was 0.25 mL/min. The inlet and outlet temperatures were 30°C and 25°C, respectively. Eudragit S100 suspension (2.5%, swelling in ethanol) was prepared according to the recommendation by the supplier. This solution was moved to peristaltic pump nozzle for further use. The coated beads were stored in a sealed plastic bag before use.
Characterization
Scanning electron microscopy (JEOL 5200 SEM; JEOL Tokyo, Japan) was used to evaluate the morphology of mesalamine microparticles. The particle size was determined by the Nicomp 380 micron particle sizer (Particle Sizing Systems, Port Richey, FL, USA) at a fixed angle of 90° at 25°C. The samples were sealed and stored at 25°C for 6 months. Drug content, average particle size, and encapsulation efficiency were determined in triplicate.
Determination of drug content
The drug content in microparticles was determined by high-performance liquid chromatography (HPLC) method. A quantity equivalent to 100 mg of mesalamine-coated microparticles was weighed, dissolved in mobile phase, and diluted suitably.
Entrapment efficiency
Ten milligrams of microparticles were weighed accurately and dissolved in 1 mL of mobile phase. The resulting solution was analyzed for mesalamine content by HPLC method. Results were expressed as the mean ± standard error of three experiments. Encapsulation efficiency was calculated using the formula (M1 - M2)/M1×100%, where M1 and M2 are defined as the mass of the initially added mesalamine and nonencapsulated mesalamine, respectively.
In vitro release studies
The in vitro drug release studies were performed using United States Pharmacopeia type-I baskets method (100 rpm, 37°C±0.1°C). Mesalamine microparticles (100 mg) were weighed accurately and suspended in simulated gastric fluid (0.1 N HCl) (900 mL) for 2 hours. The dissolution media were then replaced with simulated intestinal fluid (pH 7.4, 0.05 M phosphate buffer) for 3 hours without added enzymes. The susceptibility of mesalamine microparticles to the enzymatic action of colonic bacteria was assessed by continuing the drug release studies in 100 mL of phosphate-buffered saline (PBS) at a pH of 6.8 containing 4% w/v rat cecal contents. The rat cecal contents were prepared according to previous reports.18 At predetermined time points of 0.5, 1, 2, 3, 5, 7, 9, 11, 15, 20, and 25 hours, a 3 mL aliquot was withdrawn and immediately replaced with an equal volume of dissolution medium at the same temperature. The aliquot withdrawn was filtered through 0.45 μm Millipore membrane filter, diluted adequately, and analyzed. The mesalamine suspensions (0.5% microcrystalline cellulose) were investigated as a contrast.
Pharmacokinetic studies
Sprague Dawley rats (male and female, 12 weeks old, 200±30 g), supplied by the Experimental Animal Center of Shanghai Jiao Tong University (Shanghai, People’s Republic of China), were used for pharmacokinetic study. All animal-handling procedures were performed according to the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health and followed the guidelines of the Animal Welfare Act. All animal experiments were approved by the Experimental Animal Ethical Committee of Shanghai University of Traditional Chinese Medicine. Before administration, rats of similar weight were selected for in vivo studies, kept in well-spaced ventilated cages, and maintained on a normal diet (water ad libitum). The mesalamine-coated microparticles or mesalamine suspensions (1% carboxymethyl cellulose solution) were administered to rats by intragastric administration (10 mg/kg). Blood samples (0.5 mL) were collected in heparinized tubes from the caudal vein at 0.5, 1, 2, 4, 6, 8, 10, 12, 16, and 24 hours after intragastric administration and stored at -20°C as soon as possible until analysis.
Pharmacokinetic parameters were calculated against the plasma concentration–time data. The maximum peak concentration (Cmax) and time (Tmax) were directly calculated from the plasma concentration vs time curve. The elimination rate constant (Kel) was determined from the terminal stage of the log plasma concentration vs time curve by least square regression analysis. From this, Kel is calculated as Kel = slope ×2.303. The elimination half-life is calculated as t1/2=0.693/Kel. The area under the plasma concentration–time curve from 0–t (AUC0~t) and from 0–∞ (AUC0~∞) and mean residence time were calculated using trapezoidal rule.
Biodistribution studies
Sixty mice were divided randomly into two groups, each containing 30 mice. The mesalamine-coated microparticles or mesalamine suspensions were orally administrated to the mice (10 mg/kg). At various time intervals after administration (1, 4, 8, 12, and 24 hours), six mice in each group were picked up randomly. The stomach, small intestine, and colon were immediately removed, and approximately 100 mg of tissue slices were excised, weighed, and stored at -20°C until analysis.
Histopathological studies
After 24 hours of administration of the formulation, the mice were sacrificed by excess anesthesia, and the stomach, small intestine, colon were dissected and washed with cold saline. The organs were pressed between filter pads and weighed. Stomach, small intestine, and colon tissues were fixed in 10% neutral formalin using standard techniques and stained with hematoxylin and eosin for histopathological examination. All tissue samples were examined and graded under light microscope with 500× magnification.
LC-MS/MS condition
The LC-MS/MS experiments were conducted with an API4000 tandem mass spectrometer (Thermo Fisher Scientific, Waltham, MA, USA) equipped with a binary and a quaternary pump (Agilent series 1200; Agilent Technologies, Santa Clara, CA, USA). Analyses were performed on a C18 column with a mobile phase composed of 17.5 mmol/L acetic acid (pH 3.3): acetonitrile =85:15 (v/v) at 0.2 mL/min flow rate. Selective detection was performed by tandem mass spectrometry with electrospray source, operating in negative ionization mode and in multiple reaction monitoring acquisition (m/z 152 – 108 for mesalamine).19,20
Plasma sample (100 μL) was transferred to a 5 mL plastic tube with 10 μL of internal standard solution (1 mg/mL, 4-ASA). After 30 seconds of vortex shaking (5432 vortex mixer; Eppendorf, Hamburg, Germany), 300 μL of HClO4 was added for deproteination to occur, and the mixture was vortexed for another 2 minutes by centrifuging at 3,000 rpm for 10 minutes, and 5 μL of the aliquot solution was injected into the LC-MS/MS system for analysis.
Tissue samples (100 mg) were homogenized in a normal saline solution (0.2 mL). A volume of 0.1 mL of the homogenized sample was transferred to a 5 mL plastic test tube. The other preparation process was the same as the plasma samples.
Statistical analysis
Data are presented as mean ± standard deviation. Unpaired Student’s t-test was used for statistical analysis. A probability (P) less than 0.05 was considered statistically significant.
Results
Characteristics of mesalamine microparticles
In this study, mesalamine microparticles were prepared successfully by emulsion–chemical cross-linking method; these microparticles were spherical in shape (Figure 1). The mean particle diameters of mesalamine microparticles before and after coating were 220.4 and 312.2 μm, respectively, which were the ideal range for colon target treatment according to the previous report.21 The zeta potential of mesalamine-coated microparticles was -17.6 mV and the entrapment efficiency was more than 91.5%.
  | Figure 1 Scanning electron microscopy of mesalamine-coated microparticles. |
In vitro release studies
Release profiles of mesalamine-coated microparticles and mesalamine suspensions are illustrated in Figure 2. It was noticed that the release rate of mesalamine-coated microparticles was significantly lower than that of mesalamine suspensions. Approximately 100% of drug in suspensions was released within 5 hours, suggesting a burst release effect in suspensions and indicating that nearly all the drug was released before they reached colon. However, mesalamine-coated microparticles showed a completely different release curve. The initial release phase did not show a burst release and the drug released from the coated microparticles was low. A small amount of drug was detected in HCl at a pH of 1.2 and PBS medium at a pH 7.4 of for 5 hours, which indicates that the release of drug was suppressed by the “burst effect” to some extent. Only 3.2%±0.4% of drug was released after 5 hours. Then 71% of the entrapped mesalamine was further released during the subsequent 20 hours of incubation. The mesalamine release profile was prominently prolonged by the encapsulation of coated microparticles.
Pharmacokinetic studies
Plasma concentration–time profiles of mesalamine after oral administration of mesalamine-coated microparticles and mesalamine suspensions are shown in Figure 3. Pharmacokinetic parameters are shown in Table 1. As shown in Figure 3, drug plasma concentration reached a higher level (more than 12 μg/mL) in the initial 2 hours after oral administration of mesalamine suspensions. The half-life of the suspensions (2.8 hours) was shorter than that of the coated microparticles (16.2 hours), suggesting that the suspensions were taken up by other tissues more rapidly than coated microparticles. A greater AUC0–t was obtained for the coated microparticles (1.9-fold) compared to the suspensions, which indicated that the encapsulated mesalamine had mostly been absorbed over the period of 12 hours. The clearance of coated microparticles was 5.1 L/h, smaller than that (21.3 L/h) of the suspensions.
  | Figure 3 Plasma concentration–time profiles of mesalamine in rats after oral administration of mesalamine-coated microparticles and mesalamine suspensions (n=6). |
Biodistribution and histopathological studies
Both mesalamine-coated microparticles and mesalamine suspensions were taken up by the stomach, small intestine, and colon of mice. Figure 4 reflects the tissue distribution results in samples taken 1, 4, 8, 12, and 24 hours after intragastric administration of single mesalamine preparations to mice. The total amount of drug accumulated in each organ within 24 hours (AUC0–t) was calculated, and the results are shown in Table 2. The results showed that the maximum concentration of mesalamine (9.6 μg/M) was observed in small intestine after 8 hours of intragastric administration of suspensions, and a small amount reached the colon (Figure 4). Only a 3.1 μg/mL concentration of drug was measured in colon after 24 hours.
The coated microparticles of mesalamine were found to be intact in the upper gastrointestinal tract. A negligible amount of the drug was found in the stomach; small amounts in the small intestine and a maximum percentage of drugs were observed in the colon following 8 hours of administration. Drug concentrations in the stomach, small intestine, and colon were significantly different between the coating microparticles and the suspensions. The AUC0–t of the coated microparticles was 2.63-fold higher compared to suspensions in colon (P<0.05).
Discussion
Based on these results, we concluded that the characteristics of microparticles were affected by the ratio of the amount of drug/chitosan and the amount of cross-linking agent. Due to the decrease in the drug/chitosan ratio from 1:1 to 1:10, entrapment rate decreased; this might be due to higher amount of polymer which produces small-sized droplets which in turn increases the surface area with rapid diffusion of drug microparticles, resulting in loss of drug with a concomitant decrease in encapsulation efficiency. The encapsulation efficiency of the microparticles decreased with increased amount of the cross-linking agent. The microparticles are more rigid and the free volume space inside the matrix gets reduced, resulting in a lower encapsulation efficiency.
In addition, use of the same amount of glutaraldehyde increased the degree of cross-linking and thus the cross-linking time was increased (data not shown). The cross-linking degree increased significantly from the second to fourth hour of cross-linking time (P<0.05) but not from the fourth to eighth hour. It has been hypothesized that this might be due to the fact that the outer layers of microparticles were cross-linked, thereby limiting cross-linking of inner layers. The colon-specific delivery system is not only to protect the drug from being released in the gastrointestinal physiological environment but also to release the drug in the colon after enzymatic degradation of colonic bacteria. Therefore, the in vitro drug release studies were carried out by using PBS at a pH of 6.8, containing 4% rat cecal contents. The results of this study show that mesalamine was protected from the acidic conditions of the gastric juice by a membrane coating. Above pH 6.8, the coating film began to gradually dissolve, and chitosan polymers were exposed.22
Conclusion
Based on the in vitro drug release results, we concluded that chitosan-coated particles are efficient in delivering mesalamine to its target tissue, the colon.
In order to investigate the kinetic modeling of drug release from microparticles, the dissolution profiles were fitted to zero order (Q = k0·t), first order (ln [100 - Q] = ln Q0 - k1·t), Higuchi (Q = kH·t1/2), and Ritger–Peppas models. The model that fitted best for mesalamine released from the microparticles was the Ritger–Peppas model with a correlation coefficient r=0.9981. Then the drug was released from microparticles following diffusion and erosion mechanism.23
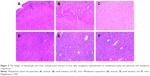
In vivo, mesalamine-coated microparticles are thought to have the potential to maintain the concentration of mesalamine in the target range for a long time, reduce the influence caused by fluctuations in the concentrations of adverse, ensure the efficiency of treatment and reduce the frequency of administration, and improve patient compliance. In case of targeted drug-delivery system such as coated microparticles, a large portion of the drug and excipient is accumulated in specific tissues and, therefore, evaluating the compatibility between the tissues and formulations becomes a necessity to ensure the safety of the formulations. The cell structure of the tissue did not show any significant difference (degenerative changes) compared to the coated microparticles preparations and placebo group. Based on this observation, it was concluded that no histological changes were observed in the organs after the administration of mesalamine-coated microparticles (Figure 5).
Acknowledgment
This research was supported by the Department of Gastroenterology, Key Discipline of Shanghai Putuo District Central Hospital (2013XK150I).
Disclosure
The authors report no conflicts of interest in this work.
References
Stange EF, Travis SP, Vermeire S, et al. European evidence-based Consensus on the diagnosis and management of ulcerative colitis: definitions and diagnosis. J Crohns Colitis. 2008;2(1):1–23. | ||
Brogden RN, Sorkin EM, Mesalazine. A review of its pharmacodynamic and pharmacokinetic properties, and therapeutic potential in chronic inflammatory bowel disease. Drugs. 1989;38(4):500–523. | ||
Stevens C, Lipman M, Fabry S, et al. 5-Aminosalicylic acid abrogates T-cell proliferation by blocking interleukin-2 production in peripheral blood mononuclear cells. J Pharmacol Exp Ther. 1995;272(1):399–406. | ||
Mahida YR, Lamming CE, Gallagher A, Hawthorne AB, Hawkey CJ. 5-Aminosalicylic acid is a potent inhibitor of interleukin 1 beta production in organ culture of colonic biopsy specimens from patients with inflammatory bowel disease. Gut. 1991;32(1):50–54. | ||
Sanka K, Pragada RR, Veerareddy PR. A pH-triggered delayed-release chronotherapeutic drug delivery system of aceclofenac for effective management of early morning symptoms of rheumatoid arthritis. J Microencapsul. 2015;32(8):794–803. | ||
Naeem M, Choi M, Cao J, et al. Colon-targeted delivery of budesonide using dual pH- and time-dependent polymeric nanoparticles for colitis therapy. Drug Des Devel Ther. 2015;9:3789–3799. | ||
Solanki A, Thakore S. Cellulose crosslinked pH-responsive polyurethanes for drug delivery: α-hydroxy acids as drug release modifiers. Int J Biol Macromol. 2015;80:683–691. | ||
Guo F, Zhang M, Gao Y, et al. Modified nanoparticles with cell-penetrating peptide and amphipathic chitosan derivative for enhanced oral colon absorption of insulin: preparation and evaluation. Drug Deliv. 2015;1–12. | ||
Davis SS, Hardy JG, Taylor MJ, Stockwell A, Whalley DR, Wilson CG. The in-vivo evaluation of an osmotic device (Osmet) using gamma scintigraphy. J Pharm Pharmacol. 1984;36(11):740–742. | ||
Ashford M, Fell JT, Attwood D, et al. An in vivo investigation into the suitability of pH dependent polymers for colonic targeting. Int J Pharm. 1993;95:193–199. | ||
Evans DF, Pye G, Bramely R, Clark AG, Dyson TJ, Hardcastle JD. Measurement of gastrointestinal pH profiles in normal ambulant human subjects. Gut. 1988;29(8):1035–1041. | ||
Rabito MF, Reis AV, Freitas Ados R, Tambourgi EB, Cavalcanti OA. A pH/enzyme-responsive polymer film consisting of Eudragit FS 30 D and arabinoxylane as a potential material formulation for colon-specific drug delivery system. Pharm Dev Technol. 2012;17(4):429–436. | ||
Zhao XL, Li KX, Zhao XF, Pang DH, Chen DW. Study on colon-specific 5-Fu pH-enzyme Di-dependent chitosan microspheres. Chem Pharm Bull (Tokyo). 2008;56(7):963–968. | ||
Agnihotri SA, Mallikarjuna NN, Aminabhavi TM. Recent advances on chitosan-based micro- and nanoparticles in drug delivery. J Control Release. 2004;100(1):5–28. | ||
Sinha VR, Singla AK, Wadhawan S, et al. Chitosan microspheres as a potential carrier for drugs. Int J Pharm. 2004;274(1–2):1–33. | ||
Palmieri GF, Michelini S, Di Martino P, Martelli S. Polymers with pH dependent solubility: possibility of use in the formulation of gastroresistant and controlled release matrix tablets. Drug Dev Ind Pharm. 2000;26(8):837–845. | ||
Nair AB, Kaushik A, Attimarad M, Al-Dhubiab BE. Enhanced oral bioavailability of calcium using bovine serum albumin microspheres. Drug Deliv. 2012;19(6):277–285. | ||
Raghavan CV, Muthulingam C, Jenita JA, Ravi TK. An in vitro and in vivo investigation into the suitability of bacterially triggered delivery system for colon targeting. Chem Pharm Bull (Tokyo). 2002;50(7):892–895. | ||
Pastorini E, Locatelli M, Simoni P, Roda G, Roda E, Roda A. Development and validation of a HPLC-ESI-MS/MS method for the determination of 5-aminosalicylic acid and its major metabolite N-acetyl-5-aminosalicylic acid in human plasma. J Chromatogr B Analyt Technol Biomed Life Sci. 2008;872(1–2):99–106. | ||
Nobilis M, Vybíralová Z, Sládková K, Lísa M, Holcapek M, Kvetina J. High-performance liquid-chromatographic determination of 5-aminosalicylic acid and its metabolites in blood plasma. J Chromatogr A. 2006;1119(1–2):299–308. | ||
Madhavi M, Madhavi K, Jithan AV. Preparation and in vitro/in vivo characterization of curcumin microspheres intended to treat colon cancer. J Pharm Bioallied Sci. 2012;4(2):164–171. | ||
Ganguly K, Kulkarni AR, Aminabhavi TM. In vitro cytotoxicity and in vivo efficacy of 5-fluorouracil-loaded enteric-coated PEG-crosslinked chitosan microspheres in colorectal cancer therapy in rats. Drug Deliv. 2015;1–14. | ||
Noppakundilograt S, Piboon P, Graisuwan W, Nuisin R4, Kiatkamjornwong S. Encapsulated eucalyptus oil in ionically cross-linked alginate microcapsules and its controlled release. Carbohydr Polym. 2015;131:23–33. |
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.