Back to Journals » Drug Design, Development and Therapy » Volume 9
A novel derivative of doxorubicin, AD198, inhibits canine transitional cell carcinoma and osteosarcoma cells in vitro
Authors Rathore K, Cekanova M
Received 20 June 2015
Accepted for publication 12 August 2015
Published 25 September 2015 Volume 2015:9 Pages 5323—5335
DOI https://doi.org/10.2147/DDDT.S90859
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Shu-Feng Zhou
Kusum Rathore, Maria Cekanova
Department of Small Animal Clinical Sciences, College of Veterinary Medicine, The University of Tennessee, Knoxville, TN, USA
Abstract: Doxorubicin (DOX) is one of the most commonly used chemotherapeutic treatments for a wide range of cancers. N-benzyladriamycin-14-valerate (AD198) is a lipophilic anthracycline that has been shown to target conventional and novel isoforms of protein kinase C (PKC) in cytoplasm of cells. Because of the adverse effects of DOX, including hair loss, nausea, vomiting, liver dysfunction, and cardiotoxicity, novel derivatives of DOX have been synthesized and validated. In this study, we evaluated the effects of DOX and its derivative, AD198, on cell viability of three canine transitional cell carcinoma (K9TCC) (K9TCC#1-Lillie, K9TCC#2-Dakota, K9TCC#4-Molly) and three canine osteosarcoma (K9OSA) (K9OSA#1-Zoe, K9OSA#2-Nashville, K9OSA#3-JJ) primary cancer cell lines. DOX and AD198 significantly inhibited cell proliferation in all tested K9TCC and K9OSA cell lines in a dose-dependent manner. AD198 inhibited cell viability of tested K9TCC and K9OSA cell lines more efficiently as compared to DOX at the same concentration using MTS (3-(4,5-dimethyl-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2h-tetrazolium) assay. AD198 had lower IC50 values as compared to DOX for all tested K9TCC and K9OSA cell lines. In addition, AD198 increased apoptosis in all tested K9TCC and K9OSA cell lines. AD198 increased the caspase activity in tested K9TCC and K9OSA cell lines, which was confirmed by caspase-3/7 assay, and cleavage of poly (ADP-ribose) polymerase (PARP) was confirmed by Western blotting analysis. In addition, AD198 cleaved PKC-δ, which subsequently activated the p38 signaling pathway, resulting in the apoptosis of tested K9TCC and K9OSA cell lines. Inhibition of the p38 signaling pathway by SB203580 rescued DOX- and AD198-induced apoptosis in tested K9TCC and K9OSA cell lines. Our in vitro results suggest that AD198 might be considered as a new treatment option for K9TCC and K9OSA cell lines cancers in vivo.
Keywords: canine osteosarcoma, apoptosis, PKC-δ, canine bladder cancer, chemotherapy
Introduction
Doxorubicin (DOX, Adriamycin) is an anthracycline antibiotic that intercalates to DNA molecules causing inhibition of the topoisomerase II enzyme1 during replication of DNA. DOX stabilizes the topoisomerase II and prevents the DNA double helix from being resealed, causing inhibition of replication2 with cytotoxic effects.3 DOX is one of the most commonly used chemotherapeutic treatments for a wide range of cancers, including leukemia, lymphoma, bladder, breast, stomach, lung, ovary, thyroid, soft tissue sarcomas, and multiple myeloma.3 DOX has been extensively used in treatments of bladder transitional cell carcinoma (TCC)4,5 and osteosarcoma (OSA).6,7 Patients treated with DOX might suffer adverse events, including hair loss, nausea, vomiting, liver dysfunction, and most importantly, cardiotoxicity.8,9
N-benzyladriamycin-14-valerate (AD198) is a structural congener of DOX. AD198 is a lipophilic anthracycline analog that is therapeutically superior to DOX in murine tumor systems10 and has novel biochemical and pharmacological properties as compared to its parental compound DOX.11,12 DOX targets the nuclei of cells, blocks DNA synthesis through topoisomerase II inhibition, and increases generation of reactive oxygen species in cytoplasm of cells in order to induce apoptosis and inhibit cell growth. DOX increases the expression of P-glycoproteins (P-gp) that are associated with DOX-induced chemoresistance13 in treated cells. In contrast to DOX, AD198 targets protein kinase C (PKC) in cytoplasm of cells.14 The PKC family consists of 15 isozymes in humans; upon activation, PKC enzymes translocate to the plasma membrane and play a regulatory role in various cellular processes, including proliferation.15 AD198 has been shown to activate the PKC-δ pathway in HeLa cells.16,17 During apoptosis, PKC-δ is proteolytically cleaved by caspase-3 and generates a constitutively activated catalytic fragment that amplifies apoptosis cascades in nucleus and mitochondria.18,19 PKC-δ has been shown to activate the p38 signaling pathway, which can also lead to apoptosis.20 Apoptosis is programed cell death where the caspase-cascade system plays an important role in the induction, transduction, and amplification of intracellular apoptotic signals.21 During apoptosis, one of the first proteins to be proteolyzed by caspase-3 is poly (ADP-ribose) polymerase (PARP), when the 116 kDa form is cleaved to two fragments (89 and 24 kDa).22 The presence of cleaved PARP is generally considered as a marker of apoptosis.23 Two downstream targets of the p38 signaling pathway, cyclic AMP response element binding protein (CREB) and activating transcription factor 2 (ATF2), are transcription factors that also play an important role in apoptosis.24,25
AD198 retains the anthraquinone/daunosamine sugar complex as DOX, yet the 14-O-valerate substitution along with the proximal ring adds the lipophilicity of AD198, which causes a rapid membrane penetration of AD198 to cells in contrast to DOX. This new lipophilic anthracycline AD198 circumvents multidrug resistance conferred by overexpression of multidrug transport proteins, such as P-gp.11 AD198 is a non-cardiotoxic drug with cardioprotective effects through activation of the PKC-ε pathway in cardiomyocytes, in contrast to DOX as shown in vivo in the rat model.14 In fact, combination of low-dose AD198 along with DOX treatment has been shown to reduce cardiotoxicity of DOX in a rat model.10
Dogs diagnosed with spontaneous tumors offer unique models of human cancers to assist evaluation of new therapies for cancer treatments.26–30 Canine TCC (K9TCC) closely resembles human invasive urinary bladder cancer.31 Urinary bladder cancer is not very common in the dog, comprising only <2% of all reported canine malignancies;31,32 however, almost 97% of all diagnosed bladder tumors are malignant. Dogs with OSA represent a unique model for human OSA due to similar histopathology, clinical presentation, and molecular targets, along with similar metastatic sites.33,34 Canine OSA (K9OSA) accounts for approximately 85% of primary bone cancers in the dog. It is a more common cancer in giant-breed dogs, and it occurs primarily in the appendicular skeleton.35
DOX has been used for treatment of K9TCC36 and K9OSA37 cancers. Similarly as in people, DOX has shown cardiotoxic effects in dogs,38 therefore there is a need for new therapeutic options for treatment of both cancers with reduced toxicity and adverse events. AD198 has shown potent antitumor activity using human and murine cell lines in vitro11,17,39–41 in TRAF3-deficient mouse B-lymphoma and human multiple myeloma cells transplanted into NOD SCID mice in vivo;42 however, AD198 has never been tested on K9TCC and K9OSA cell lines primary cancer cell lines in vitro.
In this study, we evaluated the effects of AD198 in three K9TCC (K9TCC#2-Dakota, K9TCC#1-Lillie, and K9TCC#4-Molly)30,43 and three K9OSA (K9OSA#1-Zoe, K9OSA#2-Nashville, K9OSA#3-JJ) primary cancer cell lines. We compared effects of AD198 and DOX and molecular mechanisms of their actions causing cell growth inhibition and apoptosis through the activation of PKC-δ and p38 signaling pathways in K9TCC and K9OSA cells in vitro.
Methods
Antibodies and other reagents
Antibodies for BAD, PKC-δ, ATF2 (C-19), and actin were purchased from Santa Cruz Biotechnology Inc. (Dallas, TX, USA); antibodies for p-CREB and CREB were purchased from EMD Millipore (Billerica, MA, USA), and antibodies for PARP, p-p38, p38, and p-ATF2 was obtained from Cell Signaling (Boston, MA, USA). All other chemicals and reagents were purchased from Thermo Fisher Scientific (Waltham, MA, USA), unless otherwise specified. DOX was purchased from Sigma-Aldrich Co. (St Louis, MO, USA), p38 inhibitor SB203580 was purchased from Cell Signaling, and AD198 was a generous gift from Dr Leonard Lothstein from The University of Tennessee, Health Science Center in Memphis. Chemical structures of DOX and AD198 are shown in Figure 1A.
K9TCC and K9OSA primary cancer cell lines
Primary K9TCC (K9TCC#1-Lillie, K9TCC#2-Dakota, and K9TCC#4-Molly)42,43 and K9OSA (K9OSA#1-Zoe, K9OSA#2-Nashville, K9OSA#3-JJ) cell lines were isolated from tumor non-utilized portions of biopsy specimens obtained from client-owned dogs diagnosed with bladder TCC or OSA; respectively, according to the approved procedure by the University of Tennessee (Knoxville, TN, USA) Institutional Animal Care and Use Committee (IACUC). The primary K9TCC cell lines are described in detail in our previously published studies.30,43 Briefly, the tumor biopsy tissues were washed and trypsinized, and separated cells were cultured in RPMI-1640 medium (K9TCC) or Dulbecco’s Modified Eagle’s Medium (DMEM) (K9OSA) with l-glutamine supplemented with 10% fetal bovine serum, 100 IU penicillin, and 100 μg/mL streptomycin in an atmosphere of 5% CO2 at 37°C for 24 hours. Colonies of cancer cells identified under microscope were transferred into new culture dishes and expanded. K9TCC and K9OSA cell lines were progressed through at least three to five passages before using them in the experiments. All tested primary cell lines were maintained in culture for more than ten passages with no detectable changes in cell morphology (data not shown) with no detectable changes in behavior.
Cell morphology of K9TCC and K9OSA cell lines
All tested K9TCC and K9OSA cell lines were grown in RPMI-1640 medium and DMEM, respectively. The morphology was examined under a phase-contrast microscope with 20× objective magnification (Vistavision; VWR Radnor, PA, USA) and images were captured using a Moticam camera (VWR) with Motic 5.0 software.
Cell proliferation assay (MTS)
Cell proliferation (viability) was determined using the CellTiter96-Aqueous One Solution (MTS [3-(4,5-dimethyl-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2h-tetrazolium]) cell proliferation assay (Promega Corporation, Fitchburg, WI, USA). Cells (3×103 cells/well) were seeded in 96-well culture plates in four replicates and cultured for 24 hours, followed by the treatments with 0.1, 0.5, and 1 μM DOX and AD198 for 48 hours. After DOX and AD198 treatment, the cells were incubated with MTS reagent following the manufacturer’s protocol. The absorbance was measured at 490 nm using a plate reader (Bio-Tek Instruments, Inc., Winooski, VT, USA). The values for treatments were normalized to the control (DMSO-treated) group. IC50 values were calculated based on cell proliferation data using the online IC50 Tool Kit (http://www.ic50.tk/).
Doubling time of K9OSA cells
K9OSA cells were plated in triplicate in six-well plates. Cells were trypsinized and counted using a hemocytometer 24, 48, and 72 hours after plating. The doubling time for the cells was calculated using the following formula:
dt = t × ln2/ln(Ct/Co) | (1) |
where dt is the doubling time, t is the time between cell counts Ct and Co, Co is the initial count, Ct is the count after time t, and ln is natural log. The doubling time of cells was expressed in hours.
Apoptosis
Apoptosis in K9TCC and K9OSA cell lines was detected using the TACS® Annexin V-FITC assay (Trevigen, Gaithersburg, MD, USA) according to the manufacturer’s protocol. K9TCC and K9OSA cell lines (1.5×106 cells) were grown in 10 cm petri dishes in complete media (CM) for 24 hours. After plating the cells, the cells were treated with DOX (1 μM) and AD198 (1 μM) for 24 hours in serum-free media. After trypsinizing K9OSA and K9TCC cells, cells were washed with phosphate-buffered saline and incubated with annexin-V and propidium iodide solution at room temperature for 15 minutes. After washing with binding buffer, flow cytometry analysis was performed at the excitation and emission wavelengths of 488 and 550 nm for FITC conjugated annexin V and 488 and 645 nm for propidium iodide measurements, respectively. Both subpopulations of annexin-V–FITC-labeled cells and propidium iodide-labeled cells were validated to determine the percentage of cells undergoing apoptotic cell death using multi-cycle software (Phoenix, San Diego, CA, USA). The values for treatments were normalized to control (DMSO-treated) group.
Caspase-3/7 assay
K9TCC and K9OSA cell lines (1.5×106 cells) were grown in 10 cm petri dishes in complete media for 24 hours. After plating the cells, cells were treated with DOX (1 μM) and AD198 (1 μM) for 24 hours in serum-free media. After treatment, cells were lysed in ice-cold RIPA buffer as mentioned in the “Western blot analysis” section. Activities of caspases 3 and 7 were detected using the Caspase-Glo 3/7 Assay (Promega Corporation). Cell lysates containing 30 μg of proteins were incubated with a proluminescent substrate specific for caspase-3/7 at a 1:1 ratio in a 96-well plate at room temperature for 1 hour. The luminescence values for each treatment were measured with a luminescence plate reader (Bio-Tek Instruments, Inc.) and normalized to control groups.
Western blot analysis
K9TCC and K9OSA cell lines (1.5×106 cells) were grown in 10 cm petri dishes in CM for 24 hours, followed by treatment with DOX (1 μM) and AD198 (1 μM) for an additional 24 hours in serum-free media. After treatment, the cells were lysed in ice-cold RIPA buffer supplemented with protease and phosphatase inhibitors and kept at −80°C until cell lysates were used for Western blot (WB) analysis as described previously in detail.30 Briefly, after blocking, the membranes were incubated with primary antibodies (PARP, PKC-δ, p-p38, p38, p-ATF2, ATF2, p-CREB, CREB, and actin) at 4°C overnight and then incubated with horseradish peroxidase-conjugated secondary antibodies. Immunoreactive bands were visualized with an enhanced chemiluminescence system (Pierce Biotechnology, Rockford, IL, USA). The densitometry of protein expressions were normalized to actin from three independent WB experiments using ImageJ software (National Institutes of Health, Bethesda, MD, USA).
Statistical analysis
Statistical analyses were conducted using the paired Student’s t-test to establish significant difference between various treatment groups. Results were considered statistically significant at P<0.05, P<0.01, and P<0.001.
Results
Morphology of K9TCC and K9OSA cell lines
Primary K9TCC#1-Lillie cell line was established from a tumor biopsy sample obtained from the urethra of a 16-year-old female pointer dog.30 Primary K9TCC#2-Dakota cell line was established from a tumor biopsy sample obtained from the urinary bladder of a 13-year-old female bichon frise dog.30,42 Primary K9TCC#4-Molly cell line was established from a tumor biopsy sample of the urinary bladder of a 10-year-old female Maltese dog.30 Primary K9OSA#1-Zoe cell line was established from a tumor biopsy sample obtained from a bone of a 5-year-old female Great Pyrenees dog. Primary K9OSA#2-Nashville cell line was established from a tumor biopsy sample obtained from a bone of a 9-year-old female mixed-breed dog. Primary K9OSA#3-JJ cell line was established from a tumor biopsy sample obtained from a bone of a 6-year-old female pit bull–boxer-mix dog.
To confirm cell origin of isolated cancer cells from tumors, the cytological smears (Figure 1B) of aspirated cells were stained by the Romanowsky staining protocol and confirmed by a pathologist (data not shown). The identified K9TCC had multinucleated cells and enlarged-size cells. The identified K9OSA cells had excessive vacuoles present in cytoplasm, which is one of the characteristics of OSA cells. The morphologies of tested K9TCC and K9OSA cell lines were evaluated with a phase-contrast microscope as shown in Figure 1B. Tested K9TCC cells had polygonal epithelial-cell morphology, except K9TCC#4-Molly cells, which showed a more flattened appearance with vacuoles present in the cytoplasm of the cells. Tested K9OSA had flattened and elongated fibroblast-like morphology.
We evaluated the doubling time of K9OSA primary cancer cell lines, in addition to already reported doubling times of K9TCC.43 The values with doubling times of cells are presented in Table 1. The doubling time of the K9TCC cells ranged from 31.96 to 47.4 hours and that of K9OSA ranged from 42.86 to 46.1 hours, as shown in Table 1. The doubling times of K9TCC#4-Molly and K9OSA#3-JJ were 44.7 hours and 46.1 hours, respectively. The doubling times of tested K9TCC and K9OSA cell lines suggest that slow-growing cells might correlate with less aggressive tumors from which cells have been established.
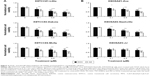
DOX and AD198 inhibited cell viability of all tested K9TCC and K9OSA cell lines
AD198 targets PKC in nonnuclear cellular compartments14 and inhibits cell proliferation. Tested K9TCC and K9OSA cell lines were treated with 0.1, 0.5, and 1 μM of DOX and AD198 for 48 hours, as shown in Figure 2. Both DOX and AD198 significantly reduced the proliferation of tested K9TCC (Figure 2A) and K9OSA (Figure 2B) cells. AD198 was significantly more effective in inhibition of cell viability of tested K9TCC and K9OSA cell lines as compared to DOX at the same concentration (P<0.001) (Figure 2). IC50 values were calculated for DOX and AD198 for K9TCC#1-Lillie, K9TCC#2-Dakota, K9TCC#4-Molly, K9OSA#1-Zoe, K9OSA#2-Nashville, K9OSA#3-JJ, as shown in Table 2. The IC50 values for AD198 were lower than those for DOX in all tested cell lines. K9TCC#4-Molly and K9OSA#3-JJ cells were among the less responsive cells to therapy as compared to other cells, as shown in Figure 2 and Table 2.
DOX and AD198 induced apoptosis in K9TCC and K9OSA cell lines
A representative cell line from each type of cancer was selected to further evaluate the molecular mechanisms of AD198 and DOX actions. We selected K9TCC#2-Dakota and K9TCC#1-Zoe cell lines to further evaluate the AD198- and DOX-induced apoptosis based on chemosensitivity tests, as shown in Figure 2 and Table 2. DOX and AD198 both significantly increased apoptosis in tested K9TCC#2-Dakota and K9OSA#1-Zoe cell lines; furthermore, AD198 was more effective in inducing apoptosis than DOX treatment in both tested cell lines, as shown in Figure 3A. DOX and AD198 increased the caspase-3/7 activities, as shown in Figure 3B. AD198 was more effective in activating caspase-3/7 enzymes as compared to DOX in K9TCC#2-Dakota and K9OSA#1-Zoe cells. In addition, increased cleavage of PARP by AD198 and DOX in both tested cell lines were confirmed (Figure 3C). Densitometry analysis of cleaved PARP protein showed an up to threefold increase in K9TCC#2-Dakota and an up to fourfold increase in K9OSA#1-Zoe when treated with AD198, as shown in Figure 3D. A statistically significant increase in PARP cleavage was observed after AD198 treatment as compared to DOX treatment in tested K9TCC and K9OSA cell lines.
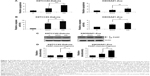
AD198 activated the PKC-δ signaling pathway in K9TCC and K9OSA cell lines
To better understand the AD198- and DOX-induced apoptosis in K9TCC and K9OSA cell lines, we further investigated the role of the PKC-δ signaling pathway. AD198 increased the cleavage of the PKC-δ in both tested K9TCC#2-Dakota and K9OSA#1-Zoe cell lines, as is shown in Figure 4A. A study has shown that activation of PKC-δ causes apoptosis through the activation of the p38 signaling pathway in human leukemia cells.20 Therefore, we tested the role of the p38 pathway in AD198-induced apoptosis. AD198 increased the phosphorylation of p38 more as compared to DOX in K9TCC#2-Dakota and K9OSA#1-Zoe cells (Figure 4A). Further, we studied the activation of downstream transcription factors of the p38 signaling pathway, such as p-CREB and p-ATF2, CREB and ATF2, both of which were activated by AD198 in tested K9TCC#2-Dakota and K9OSA#1-Zoe cells (Figure 4A). No changes in the expression of total CREB were detected by AD198 or DOX treatments in tested canine cancer cells. Unfortunately, we did not detect any total ATF2 expressions using tested ATF2 antibodies due to antibodies not reacting with canine proteins (data not shown). Densitometry analysis of cleaved PKC-δ, p-p38, p-ATF2, and p-CREB protein expressions were normalized to actin in control and DOX- and AD198-treated cells, as shown in Figure 4B. Statistically significant increases of cleaved PKC-δ, p-p38, p-ATF2, and p-CREB protein levels were observed in AD198-treated samples as compared to controls in both K9TCC#2-Dakota and K9OSA#1-Zoe cells.
To confirm the role of the p38 signaling pathway in AD198- and DOX-induced apoptosis, we co-treated cells with a p38 inhibitor, SB203580. The inhibition of the p38 signaling pathway by SB203580 rescued DOX- and AD198-induced apoptosis in tested K9TCC#2-Dakota and K9OSA#1-Zoe cell lines, as shown in Figure 5. SB203580 rescued DOX- and AD198-induced caspase-3/7 activity (Figure 5A), inhibited the production of cleaved PARP (Figure 5B and C), and inhibited the phosphorylation of p-ATF2 and p-CREB (Figure 5D and E) in tested K9TCC and K9OSA cell lines.
AD198 increased apoptosis in K9TCC and K9OSA cell lines through the activation of PKC-δ and p38 signaling pathways. DOX and AD198 did not affect the other MAP kinases, such as ERK and AKT, in tested K9TCC#2-Dakota and K9OSA#1-Zoe cells (data not shown).
Discussion
Spontaneously occurring cancers in companion animals are more similar to human malignancies as compared to the chemically or genetically induced tumor mouse models.44 As in humans, spontaneous carcinomas in dogs are influenced by age and environmental factors.45 Current treatment for OSA in humans includes chemotherapeutic drugs, including DOX, methotrexate, cisplatin, ifosfamide, and etoposide, by themselves or in a combination regimen.7 Other treatment options for OSA include PEGylated interferon-alpha,46 small-molecule kinase inhibitors that inhibit serine/threonine kinases,47 and bisphosphonates.48 The most common treatments of OSA in dogs include limb amputation and chemotherapy including DOX, with 50% surviving 1 year and 20% of dogs surviving 2 years or longer.44 Treatment options for TCC include chemotherapeutic mitomycin into the bladder49 and immunotherapy using infusions of Bacillus Calmette–Guérin,50 and chemotherapy for TCC consists of the GC regimen (gemcitabine and cisplatin) or MVAC regimen (methotrexate, vinblastine, Adriamycin/DOX, and cisplatin).51 In dogs, combination protocols using chemotherapy and the nonsteroidal anti-inflammatory drugs are the most promising treatment options for TCC. Surgery and radiation therapy are useful treatment modalities in select cases.52
In this study, we evaluated the mechanisms of DOX and its derivative, AD198, actions in three K9TCC and three K9OSA cells in vitro. AD198, as a structural congener of DOX, has shown to be non-cardiotoxic in contrast to DOX in a rodent model in vivo.10 AD198 has been shown to activate the PKC-δ pathway.16,17 In the present study, we confirmed that AD198 significantly reduced cell proliferation of K9TCC and K9OSA cell lines as compared to DOX. The IC50 values of AD198, which is an important indicator for chemosensitivity of a drug, were approximately half the IC50 values for DOX in tested K9TCC and K9OSA cell lines in vitro. AD198 was the more effective chemotherapy drug as compared to its parental DOX in tested K9TCC and K9OSA cell lines in vitro. The lower effective dose of AD198 chemotherapy used in tested K9TCC and K9OSA cell lines in vitro together with the reduced cardiotoxicity detected in the rat model in vivo10 provide evidence for AD198 as a new promising chemotherapy drug that might replace DOX treatment for patients diagnosed with TCC and OSA cancers.
AD198 increased apoptosis in the K9TCC#2-Dakota and K9OSA#1-Zoe cells, which was confirmed by caspase-3/7 activities and is in agreement with results from previously published studies.11,17,42 AD198 activated the caspase-3/7 that in turn activated PKC-δ protein in K9OSA and K9TCC cells. Edwards et al42 discovered that AD198 can also act through a PKC-δ-independent mechanism in human TRAF-3 negative multiple myeloma by suppressing c-Myc expression. The different mechanisms of action of AD198 could suggest that cell origins of tumors might play an important role. It is also to be noted that DOX increased apoptosis and PKC-δ activation in K9OSA cells, but not in K9TCC cells. This finding could be due to DOX-induced caspases leading to activation of the PKC-δ pathway, which has been previously shown in Jurkat lymphoma cells.53 Studies have shown that stress-activated pathways, such as the p38 MAPK pathway, are also involved in the regulation of tumor cell apoptosis.54 Activation of the p38 MAPK pathway has been shown to induce apoptosis in human bladder cancer55,56 and OSA cells.57,58 In this study, we showed that AD198 increased phosphorylation of p38 and its downstream effectors p-CREB and p-ATF2 in K9TCC and K9OSA cell lines, leading to further apoptosis. Inhibition of the p38 signaling pathway by SB203580 rescued DOX- and AD198-induced apoptosis in tested K9TCC and K9OSA cell lines, confirming the importance of p-ATF2 and p-CREB as downstream proteins of the p38 signaling pathway. Our results involving p38/ATF2 and p38/CREB signaling pathways in cell apoptosis are in agreement with previously published studies.24,25
Conclusion
Our results suggest that AD198 was more effective in the inhibition of K9TCC and K9OSA cell lines as compared to parental compound DOX. AD198 led to apoptosis through activation of PKC-δ and p38 signaling pathways in K9TCC and K9OSA cell lines in vitro. Our in vitro results suggest that AD198 is a promising new chemotherapy drug that might be considered for clinical trials to evaluate the efficacy of AD198 as an alternative treatment option for dogs diagnosed with TCC and OSA in vivo.
Acknowledgments
We thank Dr Leonard Lothstein for providing the AD198 compound for this study. We thank Dr Alfred Legendre with assisting to identify dogs diagnosed with OSA and with obtaining OSA tumor tissue samples. We thank Dr Joseph Bartges and Ms Amanda Callens for their assistance with obtaining bladder cancer tissue samples during cystoscopy procedures of dogs. We thank the National Institutes of Health (R15-CA182850-01A1, Principal Investigator: Cekanova), the University of Tennessee Center of Excellence in Livestock Diseases and Human Health grant (R181721333; Principal Investigator: Cekanova), and Department of Small Animal Clinical Sciences (E180120; Principal Investigator: Cekanova) for supporting this research.
Disclosure
The authors report no conflicts of interest in this work.
References
Pommier Y, Leo E, Zhang H, Marchand C. DNA topoisomerases and their poisoning by anticancer and antibacterial drugs. Chem Biol. 2010;17(5):421–433. | ||
Jamieson D, Boddy AV. Pharmacogenetics of genes across the doxorubicin pathway. Expert Opin Drug Metab Toxicol. 2011;7(10):1201–1210. | ||
Tacar O, Sriamornsak P, Dass CR. Doxorubicin: an update on anticancer molecular action, toxicity and novel drug delivery systems. J Pharm Pharmacol. 2013;65(2):157–170. | ||
Yagoda A. Chemotherapy of urothelial tract tumors. Cancer. 1987;60(3 Suppl):574–585. | ||
Rozzi A, Santini D, Salerno M, et al. Pegylated liposomal doxorubicin as third-line chemotherapy in patients with metastatic transitional cell carcinoma of urothelial tract: results of a phase II study. Med Oncol. 2013;30(1):407. | ||
Ettinger LJ, Douglass HO Jr, Mindell ER, et al. Adjuvant adriamycin and cisplatin in newly diagnosed, nonmetastatic osteosarcoma of the extremity. J Clin Oncol. 1986;4(3):353–362. | ||
Luetke A, Meyers PA, Lewis I, Juergens H. Osteosarcoma treatment – where do we stand? A state of the art review. Cancer Treat Rev. 2014;40(4):523–532. | ||
Hershman DL, McBride RB, Eisenberger A, Tsai WY, Grann VR, Jacobson JS. Doxorubicin, cardiac risk factors, and cardiac toxicity in elderly patients with diffuse B-cell non-Hodgkin’s lymphoma. J Clin Oncol. 2008;26(19):3159–3165. | ||
Volkova M, Russell R 3rd. Anthracycline cardiotoxicity: prevalence, pathogenesis and treatment. Curr Cardiol Rev. 2011;7(4):214–220. | ||
Cai C, Lothstein L, Morrison RR, Hofmann PA. Protection from doxorubicin-induced cardiomyopathy using the modified anthracycline N-benzyladriamycin-14-valerate (AD 198). J Pharmacol Exp Ther. 2010;335(1):223–230. | ||
Lothstein L, Savranskaya L, Barrett CM, Israel M, Sweatman TW. N-benzyladriamycin-14-valerate (AD 198) activates protein kinase C-delta holoenzyme to trigger mitochondrial depolarization and cytochrome c release independently of permeability transition pore opening and Ca2+ influx. Anticancer Drugs. 2006;17(5):495–502. | ||
Traganos F, Israel M, Silber R, Seshadri R, Kirschenbaum S, Potmesil M. Effects of new N-alkyl analogues of adriamycin on in vitro survival and cell cycle progression of L1210 cells. Cancer Res. 1985;45(12 Pt 1):6273–6279. | ||
Bao L, Haque A, Jackson K, et al. Increased expression of P-glycoprotein is associated with doxorubicin chemoresistance in the metastatic 4T1 breast cancer model. Am J Pathol. 2011;178(2):838–852. | ||
Hofmann PA, Israel M, Koseki Y, et al. N-Benzyladriamycin-14-valerate (AD 198): a non-cardiotoxic anthracycline that is cardioprotective through PKC-epsilon activation. J Pharmacol Exp Ther. 2007;323(2):658–664. | ||
Mellor H, Parker PJ. The extended protein kinase C superfamily. Biochem J. 1998;332(Pt 2):281–292. | ||
Roaten JB, Kazanietz MG, Caloca MJ, et al. Interaction of the novel anthracycline antitumor agent N-benzyladriamycin-14-valerate with the C1-regulatory domain of protein kinase C: structural requirements, isoform specificity, and correlation with drug cytotoxicity. Mol Cancer Ther. 2002;1(7):483–492. | ||
He Y, Liu J, Durrant D, et al. N-benzyladriamycin-14-valerate (AD198) induces apoptosis through protein kinase C-delta-induced phosphorylation of phospholipid scramblase 3. Cancer Res. 2005;65(21):10016–10023. | ||
Kato K, Yamanouchi D, Esbona K, et al. Caspase-mediated protein kinase C-delta cleavage is necessary for apoptosis of vascular smooth muscle cells. Am J Physiol Heart Circul Physiol. 2009;297(6):H2253–H2261. | ||
Zhao M, Xia L, Chen GQ. Protein kinase cδ in apoptosis: a brief overview. Arch Immunol Ther Exp (Warsz). 2012;60(5):361–372. | ||
Lee YJ, Lee DH, Cho CK, et al. HSP25 inhibits radiation-induced apoptosis through reduction of PKCdelta-mediated ROS production. Oncogene. 2005;24(23):3715–3725. | ||
Fan TJ, Han LH, Cong RS, Liang J. Caspase family proteases and apoptosis. Acta Biochim Biophys Sin (Shanghai). 2005;37(11): 719–727. | ||
Soldani C, Lazzè MC, Bottone MG, et al. Poly(ADP-ribose) polymerase cleavage during apoptosis: when and where? Exp Cell Res. 2001;269(2):193–201. | ||
Duriez P, Shah GM. Cleavage of poly(ADP-ribose) polymerase: a sensitive parameter to study cell death. Biochem Cell Biol. 1997;75(4):337–349. | ||
Lu C, Shi Y, Wang Z, et al. Serum starvation induces H2AX phosphorylation to regulate apoptosis via p38 MAPK pathway. FEBS Lett. 2008;582(18):2703–2708. | ||
Liu WH, Chou WM, Chang LS. p38 MAPK/PP2Acα/TTP pathway on the connection of TNF-α and caspases activation on hydroquinone-induced apoptosis. Carcinogenesis. 2013;34(4):818–827. | ||
Dhawan D, Ramos-Vara JA, Stewart JC, Zheng R, Knapp DW. Canine invasive transitional cell carcinoma cell lines: in vitro tools to complement a relevant animal model of invasive urinary bladder cancer. Urol Oncol. 2009;27(3):284–292. | ||
Khanna C, Lindblad-Toh K, Vail D, et al. The dog as a cancer model. Nat Biotechnol. 2006;24(9):1065–1066. | ||
MacEwen EG. Spontaneous tumors in dogs and cats: models for the study of cancer biology and treatment. Cancer Metastasis Rev. 1990;9(2):125–136. | ||
Rathore K, Alexander M, Cekanova M. Piroxicam inhibits Masitinib-induced cyclooxygenase 2 expression in oral squamous cell carcinoma cells in vitro. Transl Res. 2014;164(2):158–168. | ||
Cekanova M, Uddin MJ, Bartges JW, et al. Molecular imaging of cyclooxygenase-2 in canine transitional cell carcinomas in vitro and in vivo. Cancer Prev Res (Phila). 2013;6(5):466–476. | ||
Mutsaers AJ, Widmer WR, Knapp DW. Canine transitional cell carcinoma. J Vet Intern Med. 2003;17(2):136–144. | ||
Norris AM, Laing EJ, Valli VE, et al. Canine bladder and urethral tumors: a retrospective study of 115 cases (1980–1985). J Vet Intern Med. 1992;6(3):145–153. | ||
Morello E, Martano M, Buracco P. Biology, diagnosis and treatment of canine appendicular osteosarcoma: similarities and differences with human osteosarcoma. Vet J. 2011;189(3):268–277. | ||
LaRue SM, Withrow SJ, Powers BE, et al. Limb-sparing treatment for osteosarcoma in dogs. J Am Vet Med Assoc. 1989;195(12):1734–1744. | ||
Mayer MN, Grier CK. Palliative radiation therapy for canine osteosarcoma. Can Vet J. 2006;47(7):707–709. | ||
Robat C, Burton J, Thamm D, Vail D. Retrospective evaluation of doxorubicin-piroxicam combination for the treatment of transitional cell carcinoma in dogs. J Small Anim Pract. 2013;54(2):67–74. | ||
Mauldin GN, Matus RE, Withrow SJ, Patnaik AK. Canine osteosarcoma. Treatment by amputation versus amputation and adjuvant chemotherapy using doxorubicin and cisplatin. J Vet Intern Med. 1988;2(4):177–180. | ||
Herman EH, Ferrans VJ, Myers CE, Van Vleet JF. Comparison of the effectiveness of (+/−)-1,2-bis(3,5-dioxopiperazinyl-1-yl)propane (ICRF-187) and N-acetylcysteine in preventing chronic doxorubicin cardiotoxicity in beagles. Cancer Res. 1985;45(1):276–281. | ||
Ganapathi R, Grabowski D, Sweatman TW, Seshadri R, Israel M. N-benzyladriamycin-14-valerate versus progressively doxorubicin-resistant murine tumours: cellular pharmacology and characterisation of cross-resistance in vitro and in vivo. Br J Cancer. 1989;60(6):819–826. | ||
Harstrick A, Vanhoefer U, Schleucher N, et al. Activity of N-benzyladriamycin-14-valerate (AD198), a new anthracycline derivate, in multidrug resistant human ovarian and breast carcinoma cell lines. Anticancer Drugs. 1995;6(5):681–685. | ||
Lothstein L, Hosey LM, Sweatman TW, Koseki Y, Dockter M, Priebe W. N-benzyladriamycin-14-valerate (AD 198)-resistant cells exhibit highly selective cross-resistance to other anthracyclines that circumvent multidrug resistance. Oncol Res. 1993;5(6–7):229–234. | ||
Edwards SK, Moore CR, Liu Y, Grewal S, Covey LR, Xie P. N-benzyladriamycin-14-valerate (AD 198) exhibits potent anti-tumor activity on TRAF3-deficient mouse B lymphoma and human multiple myeloma. BMC Cancer. 2013;13:481. | ||
Rathore K, Cekanova M. Animal model of naturally occurring bladder cancer: characterization of four new canine transitional cell carcinoma cell lines. BMC Cancer. 2014;14:465. | ||
Cekanova M, Rathore K. Animal models and therapeutic molecular targets of cancer: utility and limitations. Drug Des Devel Ther. 2014;8:1911–1921. | ||
Lerner SP, Schoenberg MP, Sternberg CN, editors. Textbook of Bladder Cancer. Oxon, United Kingdom, CRC press; 2006. | ||
Strander H. Interferons and osteosarcoma. Cytokine Growth factor Rev. 2007;18(5–6):373–380. | ||
Spreafico A, Schenone S, Serchi T, et al. Antiproliferative and proapoptotic activities of new pyrazolo[3,4-d]pyrimidine derivative Src kinase inhibitors in human osteosarcoma cells. FASEB J. 2008;22(5):1560–1571. | ||
Brown HK, Holen I. Anti-tumour effects of bisphosphonates – what have we learned from in vivo models? Curr Cancer Drug Targets. 2009;9(7):807–823. | ||
Di Stasi SM, Giannantoni A, Stephen RL, et al. Intravesical electromotive mitomycin C versus passive transport mitomycin C for high risk superficial bladder cancer: a prospective randomized study. J Urol. 2003;170(3):777–782. | ||
Alexandroff AB, Jackson AM, O’Donnell MA, James K. BCG immunotherapy of bladder cancer: 20 years on. Lancet. 1999;353(9165):1689–1694. | ||
von der Maase H, Hansen SW, Roberts JT, et al. Gemcitabine and cisplatin versus methotrexate, vinblastine, doxorubicin, and cisplatin in advanced or metastatic bladder cancer: results of a large, randomized, multinational, multicenter, phase III study. J Clin Oncol. 2000;18(17):3068–3077. | ||
Henry CJ. Management of transitional cell carcinoma. Vet Clin North Am Small Anim Pract. 2003;33(3):597–613. | ||
Panaretakis T, Laane E, Pokrovskaja K, et al. Doxorubicin requires the sequential activation of caspase-2, protein kinase Cdelta, and c-Jun NH2-terminal kinase to induce apoptosis. Mol Biol Cell. 2005;16(8):3821–3831. | ||
Wada T, Penninger JM. Mitogen-activated protein kinases in apoptosis regulation. Oncogene. 2004;23(16):2838–2849. | ||
Zhang S, Ding D, Zhang X, Shan L, Liu Z. Maslinic acid induced apoptosis in bladder cancer cells through activating p38 MAPK signaling pathway. Mol Cell Biochem. 2014;392(1–2):281–287. | ||
Wang H, Jiang D, Liu J, et al. Compound K induces apoptosis of bladder cancer T24 cells via reactive oxygen species-mediated p38 MAPK pathway. Cancer Biother Radiopharm. 2013;28(8):607–614. | ||
Chou CT, He S, Jan CR. Paroxetine-induced apoptosis in human osteosarcoma cells: activation of p38 MAP kinase and caspase-3 pathways without involvement of [Ca2+]i elevation. Toxicol Appl Pharmacol. 2007;218(3):265–273. | ||
Hennessy B, Mills G, Coombes K, Gonzalez-Anguelo A, Carey M, inventors; Hennessy BT, Mills GB, Coombes K, Gonzalez-Anguelo A, Carey M, assignees. Proteomic patterns of cancer prognostic and predictive signatures. United States patent US 20080108091 A1. 2008 May 8. |
 © 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.