Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 15
A Non-Interventional Study of Tiotropium/Olodaterol versus Any Triple Combination Therapy for Chronic Obstructive Pulmonary Disease: The EVELUT® Study Protocol
Authors Buhl R, Dreher M, Korn S, Taube C, Stock C , Zehendner CM, Kondla A , Vogelmeier CF
Received 20 May 2020
Accepted for publication 23 September 2020
Published 22 October 2020 Volume 2020:15 Pages 2601—2608
DOI https://doi.org/10.2147/COPD.S262746
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Richard Russell
Roland Buhl,1 Michael Dreher,2 Stephanie Korn,1 Christian Taube,3 Christian Stock,4 Christoph M Zehendner,5 Anke Kondla,5 Claus F Vogelmeier6
1Pulmonary Department, Johannes Gutenberg University Mainz, Mainz, Germany; 2Clinic of Cardiology, Pneumology, Angiology and Internal Medicine Intensive Care, University Hospital RWTH Aachen, Aachen, Germany; 3Clinic for Pneumonology, University Medicine Essen – Ruhrlandklinik, Essen, Germany; 4Biostatistics + Data Sciences Corp, Boehringer Ingelheim Pharma GmbH & Co. KG, Ingelheim am Rhein, Germany; 5HP Country Medical Affairs, Boehringer Ingelheim Pharma GmbH & Co. KG, Ingelheim am Rhein, Germany; 6Department of Pneumology, University Hospital of Giessen and Marburg, Member of the German Center for Lung Research (DZL), Marburg, Germany
Correspondence: Roland Buhl
Pulmonary Department, Johannes Gutenberg University Mainz, Langenbeckstraße 1, Mainz D-55131, Germany
Tel +49 6131 17-7271
Email [email protected]
Background: The Global Initiative for Chronic Obstructive Lung Disease 2020 report recommends that patients with chronic obstructive pulmonary disease (COPD) suffering from persistent dyspnea, despite long-acting β2-agonist (LABA)/inhaled corticosteroid (ICS) maintenance therapy, are switched to either a long-acting muscarinic antagonist (LAMA)/LABA combination regimen or LAMA/LABA/ICS triple therapy. However, to date, no studies have investigated the direct switch from LABA/ICS to LAMA/LABA therapy—instead of switching to triple therapy—in a prospective, real-world, non-interventional setting.
Methods: EVELUT® (NCT03954132) is an ongoing, prospective, open-label, multicenter, non-interventional study comparing the once-daily fixed-dose combination of tiotropium and olodaterol (tio/olo) versus any triple therapy (LAMA/LABA/ICS) in patients with COPD who are symptomatic despite LABA/ICS maintenance therapy. Patients with acute or frequent COPD exacerbations are excluded from the study. Participants will receive LABA/ICS maintenance treatment until Visit 1, followed by switching of treatment to tio/olo or LAMA/LABA/ICS. The primary endpoints are changes in modified Medical Research Council (mMRC) and COPD Assessment Test (CAT®) scores after approximately 12 weeks of treatment. Secondary endpoints are change in the patients’ general condition according to the Physician’s Global Evaluation score, the proportion of responders with a change in mMRC score of ≥ 1 and in CAT® score of ≥ 2, and patient satisfaction with the inhaler and therapy. The study is expected to enroll approximately 900 patients.
Conclusion: EVELUT results are expected to add to the current real-world evidence informing therapeutic decisions for COPD in everyday clinical practice.
Trial Registration: The European Union electronic Register of Post-authorisation Studies (EU PAS Register): EUPAS29784; the Federal Institute for Drugs and Medical Devices (BfArM): NIS Study No 7305; Clinicaltrials.gov: NCT03954132.
Keywords: COPD, tiotropium/olodaterol, Spiolto® Respimat®, triple therapy, LAMA/LABA/ICS
Plain Language Summary
Patients with chronic obstructive pulmonary disease (COPD) are often treated with a combination of medications, including a long-acting β2-agonist (LABA) and inhaled corticosteroid (ICS) dual combination, to help improve their symptoms. However, some of the patients on LABA/ICS dual combination therapy continue to suffer from shortness of breath. In cases like these, the recommendation is for patients to switch their treatment to either a long-acting muscarinic antagonist (LAMA)/LABA dual combination therapy or LAMA/LABA/ICS triple combination therapy. Although all of these combinations have been previously studied in clinical trials, questions still remain regarding the best way to manage patients in the real-world everyday clinical setting. Most importantly, should symptomatic patients switch from LABA/ICS to LAMA/LABA, or is it better to escalate to the LAMA/LABA/ICS triple therapy? The EVELUT® study was designed to help inform these everyday clinical decisions. The study aims to include 900 patients on LABA/ICS to investigate the effect of switching to LAMA/LABA compared with LAMA/LABA/ICS on shortness of breath, the impact of COPD on patients’ lives, the patients’ general condition, and their satisfaction with the inhaler and therapy. In this paper, the study design, rationale and methods of the EVELUT study are described.
Introduction
The Global Initiative for Chronic Obstructive Lung Disease (GOLD) defines chronic obstructive pulmonary disease (COPD) as a common, preventable and treatable disease characterized by persistent respiratory symptoms and airflow limitation.1 In 2010, COPD was the fourth-leading cause of death worldwide; however, the disease burden is expected to rise, with COPD becoming the third-leading cause of death by 2020.1 In Germany, the prevalence of COPD in people over the age of 40 years is 13.3%, increasing to 40.4% in men over the age of 70 years.2 The most common respiratory symptoms of COPD include dyspnea, cough and/or sputum production. In addition, patients with COPD may experience periods of acute worsening of respiratory symptoms, known as exacerbations.1
The aims of COPD management are to reduce symptoms and the frequency and severity of exacerbations, as well as to improve health status and exercise tolerance.1,3 Long-acting β2-agonist (LABA)/inhaled corticosteroid (ICS) fixed-dose combinations are a commonly prescribed therapy for patients with COPD. However, based on the GOLD 2020 recommendations, ICS should only be considered as initial pharmacotherapy if there is a high risk of exacerbations (≥2 moderate exacerbations or ≥1 exacerbation leading to hospitalization in the previous year), a blood eosinophil count of ≥300 cells/µL, modified Medical Research Council (mMRC) score ≥2 and a COPD Assessment Test (CAT®) score ≥10.1 Patients on LABA/ICS suffering from persistent dyspnea should either be switched to a long-acting muscarinic antagonist (LAMA)/LABA combination, or escalated to a triple therapy of LAMA/LABA/ICS (Figure 1). A switch to a LAMA/LABA combination is indicated when the original indication of ICS was inappropriate (for example, when an ICS was prescribed to treat symptoms in the absence of a history of frequent exacerbations), when there has been a lack of response to ICS treatment, or following discontinuation due to ICS-related side effects, such as pneumonia.1
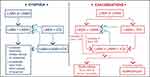 |
Figure 1 GOLD 2020 follow-up pharmacologic treatment recommendations.*Consider if eos ≥ 300 or eos ≥ 100 AND ≥ 2 moderate exacerbations/1 hospitalization. **Consider de-escalation of ICS or switch if pneumonia, inappropriate original indication or lack of response to ICS. ©2020, Global Initiative for Chronic Obstructive Lung Disease, reproduced with permission.1 Abbreviations: eos, blood eosinophil count; FEV1, forced expiratory volume in 1 second; GOLD, Global Initiative for Chronic Obstructive Lung Disease; ICS, inhaled corticosteroid; LABA, long-acting β2-agonist; LAMA, long-acting muscarinic antagonist. |
The benefits of a LAMA/LABA or LAMA/LABA/ICS versus a LABA/ICS combination have already been demonstrated in randomized clinical trials of COPD.4,5 The FLAME® study (NCT01782326) reported that glycopyrronium/indacaterol (LAMA/LABA) was more efficacious than salmeterol/fluticasone (LABA/ICS) in preventing COPD exacerbations in patients with a history of exacerbations in the previous year.4 Data from the ENERGITO® study showed that once-daily tiotropium/olodaterol (tio/olo; LAMA/LABA) was superior to twice-daily salmeterol/fluticasone propionate (LABA/ICS), in terms of lung function improvements, in patients with moderate-to-severe COPD requiring maintenance therapy.5 The IMPACT study demonstrated a reduction in moderate or severe exacerbations, including those leading to hospitalization, with umeclidinium/vilanterol/fluticasone furoate (LAMA/LABA/ICS) compared with LABA/ICS dual therapy.6
LAMA/LABA has also been compared with LAMA/LABA/ICS triple therapy in observational and database studies of COPD patients.7,8 In the DACCORD non-interventional study, LAMA/LABA was associated with fewer exacerbations and improved health status compared with LAMA/LABA/ICS.7 In contrast, a real-world database study reported LAMA/LABA/ICS to be more effective in reducing exacerbation risk than LAMA/LABA.8 This likely reflects the different patient populations in the two studies in terms of prior exacerbations, as most patients in the DACCORD study did not have exacerbations in the 6 months prior to study entry, whereas the database study included frequently exacerbating patients (≥2 exacerbations in the preceding year).7,8
Dyspnea, particularly exertional dyspnea, is a cardinal symptom of COPD, often leading to COPD diagnosis and therefore to the initiation or change of maintenance treatment.9,10 Although clinical studies have demonstrated that LAMA/LABA treatment with tio/olo significantly improved dyspnea in patients with COPD, real-world data demonstrating the effects of a switch to tio/olo versus triple therapy are lacking in patients experiencing dyspnea and other symptoms despite current LABA/ICS maintenance therapy.3,11,12
This manuscript describes the rationale, study design and methodology of the EVELUT study. The non-interventional EVELUT study is designed to investigate the most appropriate strategy for the real-world treatment of patients with COPD who remain symptomatic on LABA/ICS maintenance therapy. In Germany, almost two-thirds of patients with COPD receive ICS; however, only a small subpopulation of these patients exacerbates, indicating that ICS may currently be routinely prescribed even in the absence of frequent exacerbations.13,14 EVELUT will investigate the comparative effectiveness of tio/olo LAMA/LABA therapy versus any triple therapy in dyspneic patients switching from LABA/ICS as maintenance therapy, as per their treating physician’s recommendation. The results of the EVELUT study are expected to generate further evidence to help address a key question physicians face daily in clinical practice, namely should a symptomatic patient on LABA/ICS maintenance therapy be switched to LAMA/LABA or LAMA/LABA/ICS?
Methods
Study Design
EVELUT (NCT03954132) is an ongoing, prospective, open-label, multicenter, non-interventional study of tio/olo administered via the Respimat® re-usable inhaler (Boehringer Ingelheim, Germany), versus any triple therapy (LAMA/LABA/ICS), in patients with COPD who are symptomatic despite LABA/ICS maintenance therapy and for whom their physician has already decided to switch therapy to either tio/olo or LAMA/LABA/ICS. The study aims to recruit 900 patients from around 150 sites in Germany and is assessing comparative effectiveness and safety in a real-world setting. Patients will continue to receive their LABA/ICS maintenance treatment until Visit 1 of the study, followed by switching of treatment to tio/olo or any LAMA/LABA/ICS at the discretion of the attending physician, and according to routine clinical practice (Figure 2). Patients will take tio/olo (5 µg of tiotropium plus 5 µg of olodaterol once daily at the same time of day; two puffs) or the recommended dosage of the prescribed LAMA/LABA/ICS (once or twice daily at the same time of day). Patients are enrolled consecutively and will be followed for an observational period of approximately 12 weeks, which is the average time between two medical consultations.
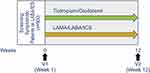 |
Figure 2 EVELUT® study overview. Abbreviations: ICS, inhaled corticosteroid; LABA, long-acting β2-agonist; LAMA, long-acting muscarinic antagonist; V, visit. |
Study Population
Eligible for inclusion in the study are patients ≥40 years of age who have a diagnosis of COPD (as determined by the treating physician) and who are symptomatic (mMRC score ≥1 and CAT score ≥10). Patients must be on LABA/ICS maintenance therapy prior to study entry, switching to either tio/olo (delivered via the reusable inhaler) or any triple combination (LAMA/LABA/ICS) at Visit 1. Patients must also be willing and able to follow the procedures outlined in the protocol and must provide written informed consent prior to study participation. Key exclusion criteria are contraindications to either treatment regimen as per the respective summary of product characteristics, patients not on LABA/ICS maintenance therapy at study start, acute exacerbation of COPD (within 4 weeks prior to Visit 1), and frequently exacerbating patients (ie, ≥2 moderate exacerbations or ≥1 exacerbation leading to hospitalization within the last 12 months). Exacerbations are defined as follows: mild (additional use of short-acting bronchodilators and treated by the patient without consulting a physician); moderate (medical prescription of a systemic corticosteroid and/or antibiotic); and severe (exacerbation leading to hospitalization). Patients with allergic rhinitis or lung cancer within the last 5 years, acute respiratory failure (pH<7.35 and/or respiratory rate >30/min) within 3 months prior to Visit 1, a current diagnosis/history of asthma, asthma–COPD overlap, pregnant or lactating females, and those who are participating in a parallel interventional clinical trial, are also excluded from the study.
Study Endpoints
The co-primary endpoints of the study are the changes in mMRC and CAT scores between the baseline measurement (Visit 1) and the follow-up measurement after approximately 12 weeks of treatment (Visit 2). Secondary endpoints are the change in the patients’ general condition according to the Physician’s Global Evaluation score and the proportion of responders with a change in mMRC score of ≥1 and in CAT score of ≥2, all assessed over the same time period. A further secondary endpoint is patient satisfaction with the inhaler and therapy according to a 7-point ordinal scale at Visit 2.
Baseline and Other Follow-Up Data
The following data will be collected and assessed at Visit 1 and/or Visit 2 to allow for further characterization of the patients:
- specialty of attending physician (general practitioner, pulmonologist, internal specialist)
- patient demographics (age, gender, height, weight)
- history of COPD
- rationale for changing COPD maintenance therapy
- reported number and severity of exacerbations in the last 12 months
- number of exacerbations leading to hospitalization in the last 12 months
- number and severity of exacerbations during the study and any prescription of oral corticosteroids and/or antibiotics
- device training (yes/no), and the reason for lack of training (if applicable)
- GOLD patient groups (A, B) based on GOLD guidelines 2020
- GOLD spirometric classifications (1, 2, 3, 4) of airflow limitations based on post-bronchodilator forced expiratory volume in 1 second (FEV1) (and date of examination, if available)
- eosinophils in peripheral blood and date of examination, where available
- smoking history, current smoking status (current smokers, former smokers and never smokers) and pack-years
- concomitant diseases and comorbidities (eg, cardiovascular diseases, diabetes, musculoskeletal impairment, renal diseases, liver diseases, osteoporosis, gastroesophageal reflux)
- current (within the last 6 months of Visit 1) COPD-related or other relevant concomitant medications (eg, beta-blockers, beta-agonists, corticosteroids, or proton pump inhibitors)
- safety: serious and non-serious adverse drug reactions, fatal adverse events and pregnancies during the study
- the patient’s willingness to continue or discontinue treatment with either tio/olo or the triple therapy at the end of the study, and the rationale for treatment discontinuation (if applicable).
Statistical Considerations
All patients receiving ≥1 dose of tio/olo or LAMA/LABA/ICS triple combination will be included in the analyses. The study is to be considered exploratory in design.
Main Statistical Analyses
Estimation of relative treatment effects in the real-world setting is subject to potential confounding and requires adjusted analyses. In order to assess the sensitivity of the estimated treatment effects on the analytical approach, different types of analyses will be performed for the two primary endpoints. The main analysis of the primary endpoints will be based on propensity score matching, and sensitivity analyses will be based on propensity score weighting and multivariable regression modeling. Comparative analyses of secondary endpoints will be assessed based on propensity score matching only.
The propensity score will be estimated as the probability of receiving triple therapy using a range of pre-specified baseline variables (including age, sex, mMRC score, CAT score, exacerbation history, smoking history, FEV1, eosinophil levels and physician specialty), with missing data being accounted for by multiple imputation. One-to-one matching will be performed using greedy nearest-neighbor matching on the logit of the propensity score with a caliper of 0.2 standard deviations of the logit of the propensity score. Balance across propensity score-matched samples will be compared by standardized differences and graphical methods. If imbalances are negligible (defined as standardized difference of <0.1 for all matching variables), unequal fixed- and variable-ratio matching—eg 1:2 (triple:tio/olo) and 1:3 (triple:tio/olo)—will be considered and favored if balance can be maintained. The primary outcome models will use the matched samples and will be linear regression models adjusted for the respective baseline covariates and for clusters of matched patients (using generalized estimating equations). Sensitivity analyses will be done by inverse probability of treatment weighting based on the propensity score and multivariable regression modeling, with adjustment for all covariates also included in the propensity score model. For secondary endpoints, the analytical approach will be similar to the primary endpoints, using generalized linear models in case of categorical endpoints. 95% confidence intervals for estimated relative differences between treatment groups will be computed, but no formal statistical testing will be applied and no sensitivity analyses will be conducted. All endpoints will be analyzed in a descriptive and non-comparative way in both the matched and the treated set.
No adjustment for multiplicity will be performed; thus, the results are to be interpreted as exploratory.
Sample Size Calculation and Population
The study sample size is based on the assumption that tio/olo is at least non-inferior to any triple therapy using two-sample t-tests (one-sided alpha-level 2.5% with 90% power). The respective minimal clinically important differences (MCIDs) are treated as non-inferiority margins. The MCID for the mMRC score is 1 point and a standard deviation of 1 point is assumed.15 The MCID for the CAT score is 2 points and a standard deviation of 7 points is assumed.15 Overall, 44 patients (22 per group) are required in order to assess non-inferiority between tio/olo and the triple combination therapy in terms of the mMRC questionnaire; 518 patients (259 per group) are required in order to assess non-inferiority in terms of the CAT questionnaire. However, it is expected that approximately 40% of patient data will not be available for evaluation at the end of the study (10% drop-out based on experiences with German non-interventional studies, and an additional 30% due to the need to discard patients that cannot be matched). Therefore, a total number of 864 patients (432 patients per group) are required to evaluate non-inferiority with respect to the CAT score, and 74 patients (37 patients per group) to evaluate non-inferiority with regards to the mMRC score.
Further Analyses
Baseline characteristics and further endpoints will be compared using descriptive statistics (ie, without formal statistical testing). Subgroup analyses for primary endpoints will be performed according to the GOLD spirometric classification (1, 2, 3, or 4) and GOLD patient groups (based on the GOLD ABCD assessment scheme). Descriptive analyses concerning the handling of the reusable inhaler and other user experiences will be performed. Additional group-wise analyses will be performed for the following:
- number of exacerbations (overall and stratified by levels of eosinophils)
- number of hospitalizations due to COPD exacerbations
- adherence to medication regimen
- levels of eosinophils (if available)
- improvement in dyspnea (as measured via mMRC questionnaire) and responder rates (overall and stratified by levels of eosinophils)
- improvement in lung function (overall and stratified by levels of eosinophils).
In descriptive analyses, the fraction of missing observations will be reported.
Ethical Considerations
Boehringer Ingelheim International GmbH is located within the geographical area covered by the Rhineland-Palatinate State Medical Association, Mainz, Germany (a corporation under public law and Competent Authority according to national and international regulations). As such, the legal documentation required for initiation of this study was reviewed and approved by the ethics committee of the Rhineland-Palatinate State Medical Association (Ethics approval number: 2019–14258-andere Forschung erstvotierend). Written informed consent is obtained from each patient prior to study start, according to the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use Good Clinical Practice guidelines, and according to German regulatory and legal requirements. The study is conducted in accordance with the Declaration of Helsinki.
Study Update
The EVELUT study started in June 2019, and patient recruitment is ongoing. An update on study timelines can be found at https://clinicaltrials.gov/ct2/show/NCT03954132.
Study Organization
To improve and secure data quality, automatic data checks upon data entry are done within the electronic case report form (eCRF). Source data verification is planned at every recruiting site of the study. EVELUT is fully sponsored by Boehringer Ingelheim, Germany.
Discussion
This non-interventional study is comparing symptomatic patients with COPD switching from LABA/ICS maintenance therapy to either tio/olo or any triple combination therapy, in terms of reducing dyspnea (as per mMRC score) and symptom burden (as per CAT score) in a real-world setting. Both questionnaires, which are recommended by national guidelines and the GOLD strategy report1,16 are widely used, easy to complete, relate well to other measures of health status and respond well to interventions.17,18 To date, prospective clinical data evaluating the direct switch from LABA/ICS in symptomatic patients to LAMA/LABA in comparison with a switch to LAMA/LABA/ICS do not exist.7 EVELUT aims to bridge the gap between the data generated by randomized controlled trials (RCTs) and real-world clinical effects in this group of patients.
Although RCTs are the gold standard that assure the highest internal validity for assessing therapeutic benefit of a new treatment regimen versus placebo or the standard of care, they sometimes lack generalizability. A limitation of traditional RCTs evaluating efficacy is that participants may not reflect the heterogeneity of patients encountered in routine clinical settings due to rather restrictive inclusion and exclusion criteria; trial populations therefore may not be a good representation of a typical patient population.19 Real-world comparative effectiveness studies, which are usually observational (non-interventional), are an attractive, increasingly used alternative. They reflect the realities of routine clinical practice in broadly representative populations and assess endpoints that are directly relevant to clinical and policy decisions. However, non-interventional studies assessing the effectiveness of pharmacotherapies are prone to a number of biases (eg, confounding by indication and immortal time bias). They, therefore, need to be designed, conducted, analyzed and interpreted with caution. Thus, the observational nature of EVELUT presents some limitations. It must be acknowledged that due to the non-interventional nature of the study, treatments and analyses are performed according to the discretion of the treating physician, including time points that may not strictly adhere to those recommended by the study protocol.
The EVELUT study was designed to ensure both high internal and external validity in the real-world setting. The study is prospective and follows an active-comparator and new-user design. Eligibility criteria are non-restrictive, which enables the enrollment of a patient population that is more representative of clinical daily practice. To minimize site-level selection bias, the goal is to include centers with access to all available and approved treatment options for the targeted patients with COPD in Germany. To address patient-level selection bias, consecutive enrollment will be employed. Information bias will be reduced by the use of standard eCRFs and questionnaires, and by ensuring that physicians are trained on the study protocol. Further, the analyses take a large number of potential confounders into account and thereby minimize potential residual confounding. Additionally, EVELUT considers different analytical approaches in sensitivity analyses to assess the robustness of results.
Patients taking part in the LAMA/LABA arm of EVELUT will inhale tio/olo via the Respimat reusable inhaler, which was developed to simplify assembly and daily use, to optimize the dose indicator, and to allow reuse.20 Inhalation satisfaction is important for adherence, and EVELUT will therefore generate data on patients’ satisfaction with the reusable device.
The results from the EVELUT study will address the following clinical questions about the management of COPD:
- Which type of patients actually benefited from LABA/ICS (ie, experienced a reduction in benefit when switched to LAMA/LABA)?
- What are the characteristics of patients who benefit from the switch to LAMA/LABA?
- Is there a clinically relevant difference between the effect of LAMA/LABA and LAMA/LABA/ICS?
- What is the safety profile of LAMA/LABA compared with LAMA/LABA/ICS?
- What are the main reasons underlying physicians’ preferences for switching their patients’ therapy to LAMA/LABA or LAMA/LABA/ICS?
Conclusions
In conclusion, the real-word study EVELUT aims to prospectively address a key situation in daily clinical practice: how to manage symptomatic patients with COPD already receiving LABA/ICS maintenance therapy, who require a switch to LAMA/LABA or triple therapy. Currently, no prospective clinical evidence is available to support a direct switch from LABA/ICS to LAMA/LABA therapy instead of triple therapy when there is no indication for an ICS. This study aims to assess whether patients who are symptomatic on LABA/ICS maintenance therapy are able to switch to LAMA/LABA rather than the triple therapy, without a reduction in benefit.
Abbreviations
CAT, COPD Assessment Test; COPD, chronic obstructive pulmonary disease; eCRF, electronic case report form; FEV1, forced expiratory volume in 1 second; GOLD, Global Initiative for Chronic Obstructive Lung Disease; ICS, inhaled corticosteroid; LABA, long-acting β2-agonist; LAMA, long-acting muscarinic antagonist; MCID, minimal clinically important difference; mMRC, modified Medical Research Council; RCT, randomized controlled trial; tio/olo, tiotropium and olodaterol.
Data Sharing Statement
Boehringer Ingelheim is committed to responsible sharing of clinical study reports, related clinical documents, and patient-level clinical study data. Researchers are invited to submit enquiries via the Clinical Study Data Request website (https://www.clinicalstudydatarequest.com).
Acknowledgments
Medical writing assistance, in the form of the preparation and revision of the manuscript, was supported financially by Boehringer Ingelheim, and provided by MediTech Media (London, UK) under the authors’ conceptual direction and based on feedback from the authors.
Funding
This work was supported by Boehringer Ingelheim International GmbH.
Disclosure
Roland Buhl reports grants and personal fees from Boehringer Ingelheim, GlaxoSmithKline, Novartis, and Roche, and personal fees from AstraZeneca, Chiesi, Cipla, Sanofi and Teva, outside the submitted work. Michael Dreher received speaking fees from Actelion, AstraZeneca, Bayer, Berlin Chemie, Boehringer Ingelheim, Chiesi, Hamilton, Heinen und Löwenstein, Intermune, Linde, Novartis, Pfizer, Philips Respironics, ResMed, Roche, and Weinmann, and honoraria for advising from Almirall, Boehringer Ingelheim, Hamilton, Linde, Novartis, Pfizer, Philips Respironics, ResMed, and Roche; and grants from Linde, Philips Respironics, ResMed and the German Federal Ministry of Education and Research (BMBF). Stephanie Korn received personal fees from AstraZeneca, Boehringer Ingelheim, Chiesi, GlaxoSmithKline, Novartis, Roche, Sanofi and Teva, outside the submitted work. Christian Taube reports no conflict of interest. Christian Stock, Christoph M. Zehendner and Anke Kondla are employees of Boehringer Ingelheim. Claus F Vogelmeier reports personal fees from Almirall, Cipla, Berlin Chemie/Menarini, CSL Behring and Teva; grants and personal fees from AstraZeneca, Boehringer Ingelheim, Chiesi, GlaxoSmithKline, Grifols, MedUpdate, Mundipharma, Novartis, Nuvaira, OmniaMed, and Takeda; and grants from the German Federal Ministry of Education and Research (BMBF) Competence Network Asthma and COPD (ASCONET), Bayer Schering Pharma AG, MSD and Pfizer, outside the submitted work. The authors report no other conflicts of interest in this work.
References
1. Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease (2020 report). Published 2019. Available from: https://goldcopd.org/wp-content/uploads/2019/11/GOLD-2020-REPORT-ver1.0wms.pdf.
2. Kardos P, Vogelmeier C, Buhl R, Criee CP, Worth H. The prospective non-interventional DACCORD study in the national COPD registry in Germany: design and methods. BMC Pulm Med. 2015;15:2. doi:10.1186/1471-2466-15-2
3. Buhl R, Maltais F, Abrahams R, et al. Tiotropium and olodaterol fixed-dose combination versus mono-components in COPD (GOLD 2-4). Eur Respir J. 2015;45(4):969–979. doi:10.1183/09031936.00136014
4. Wedzicha JA, Banerji D, Chapman KR, et al. Indacaterol-glycopyrronium versus salmeterol-fluticasone for COPD. N Engl J Med. 2016;374(23):2222–2234. doi:10.1056/NEJMoa1516385
5. Beeh KM, Derom E, Echave-Sustaeta J, et al. The lung function profile of once-daily tiotropium and olodaterol via Respimat® is superior to that of twice-daily salmeterol and fluticasone propionate via Accuhaler® (ENERGITO® study). Int J Chron Obstruct Pulmon Dis. 2016;11:193–205. doi:10.2147/COPD.S95055
6. Lipson DA, Barnhart F, Brealey N, et al. Once-daily single-inhaler triple versus dual therapy in patients with COPD. N Engl J Med. 2018;378(18):1671–1680. doi:10.1056/NEJMoa1713901
7. Buhl R, Criee CP, Kardos P, et al. Dual bronchodilation vs triple therapy in the “real-life” COPD DACCORD study. Int J Chron Obstruct Pulmon Dis. 2018;13:2557–2568. doi:10.2147/COPD.S169958
8. Voorham J, Corradi M, Papi A, et al. Comparative effectiveness of triple therapy versus dual bronchodilation in COPD. ERJ Open Res. 2019;5(3):00106–02019. doi:10.1183/23120541.00106-2019
9. Rabe KF. Improving dyspnea in chronic obstructive pulmonary disease: optimal treatment strategies. Proc Am Thorac Soc. 2006;3(3):270–275. doi:10.1513/pats.200601-002SF
10. Beeh KM. A descriptive analysis of COPD patients in a large cohort in private practices in Germany. Am J Respir Crit Care Med. 2004;169:A603.
11. Halpin DMG. The role of tiotropium+olodaterol dual bronchodilator therapy in the management of chronic obstructive pulmonary disease. Tuberc Respir Dis. 2018;81(1):13–18. doi:10.4046/trd.2017.0098
12. Ingelheim B. Spiolto® Respimat® (tiotropium/olodaterol) is Boehringer Ingelheim’s new advance in COPD maintenance therapy. Published 2018. Available from: https://www.boehringer-ingelheim.com/copd/copd/information-tovito-clinical-trial-program.
13. Graf J, Jörres RA, Lucke T, Nowak D, Vogelmeier CF, Ficker JH. Medical treatment of COPD: an analysis of guideline-adherent prescribing in a large national cohort (COSYCONET). Dtsch Arztebl Int. 2018;155(37):599–605. doi:10.3238/arztebl.2018.0599
14. Kardos P, Vogelmeier C, Worth H, et al. A two-year evaluation of the ‘real life’ impact of COPD on patients in Germany: the DACCORD observational study. Respir Med. 2017;124:57–64. doi:10.1016/j.rmed.2017.02.007
15. Tsiligianni IG, Alma HJ, de Jong C, et al. Investigating sensitivity, specificity, and area under the curve of the clinical COPD questionnaire, COPD Assessment Test, and modified Medical Research Council scale according to GOLD using St George’s Respiratory Questionnaire cutoff 25 (and 20) as reference. Int J Chron Obstruct Pulmon Dis. 2016;11:1045–1052. doi:10.2147/COPD.S99793
16. Vogelmeier C, Buhl R, Burghuber O, et al. Guideline for the diagnosis and treatment of COPD patients-issued by the German Respiratory Society and the German Atemwegsliga in cooperation with the Austrian Society of Pneumology. Pneumologie. 2018;72(4):253–308. doi:10.1055/s-0043-125031
17. Bestall JC, Paul EA, Garrod R, Garnham R, Jones PW, Wedzicha JA. Usefulness of the medical research council (MRC) dyspnoea scale as a measure of disability in patients with chronic obstructive pulmonary disease. Thorax. 1999;54(7):581–586. doi:10.1136/thx.54.7.581
18. Dodd JW, Hogg L, Nolan J, et al. The COPD assessment test (CAT): response to pulmonary rehabilitation. A multicentre, prospective study. Thorax. 2011;66(5):425–429. doi:10.1136/thx.2010.156372
19. Herland K, Akselsen J-P, Skjønsberg OH, Bjermer L. How representative are clinical study patients with asthma or COPD for a larger “real life” population of patients with obstructive lung disease? Respir Med. 2005;99(1):11–19. doi:10.1016/j.rmed.2004.03.026
20. Dhand R, Eicher J, Hansel M, Jost I, Meisenheimer M, Wachtel H. Improving usability and maintaining performance: human-factor and aerosol-performance studies evaluating the new reusable Respimat inhaler. Int J Chron Obstruct Pulmon Dis. 2019;14:509–523. doi:10.2147/COPD.S190639
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
