Back to Journals » Neuropsychiatric Disease and Treatment » Volume 18
A Nomogram for Individualized Prediction of Stress-Related Gastrointestinal Bleeding in Critically Ill Patients with Primary Intracerebral Hemorrhage
Authors Liu S, Wang Y, Gao B, Peng J
Received 5 October 2021
Accepted for publication 18 January 2022
Published 9 February 2022 Volume 2022:18 Pages 221—229
DOI https://doi.org/10.2147/NDT.S342861
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Jun Chen
Shucheng Liu,1 Yilin Wang,2 Bin Gao,2 Jun Peng2
1Department of Urology, The First Affiliated Hospital, Hengyang Medical School, University of South China, Hengyang City, Hunan Province, People’s Republic of China; 2Department of Neurology, The First Affiliated Hospital, Hengyang Medical School, University of South China, Hengyang City, Hunan Province, People’s Republic of China
Correspondence: Jun Peng
Department of Neurology, The First Affiliated Hospital, Hengyang Medical School, University of South China, No. 69 Chuanshan Road, Hengyang City, Hunan Province, People’s Republic of China
, Tel +86 19958919270
, Fax +86 734-8578774
, Email [email protected]
Purpose: To establish and validate a nomogram model for predicting stress-related gastrointestinal bleeding in critically ill patients with primary intracerebral hemorrhage.
Patients and Methods: From January 2018 to March 2021, we conducted a hospital-based study by screening eligible patients with acute intracerebral hemorrhage. Univariate and multivariate logistic regression analyses were performed to determine the predictors for stress-related gastrointestinal bleeding in patients with primary intracerebral hemorrhage. The nomogram was constructed on the basis of multivariate logistic model and was internally validated by bootstrap resampling. The discriminative performance of the nomogram was evaluated using the calibration and concordance index (C-index), which was equal to the area under the curve of receiver-operating characteristics. Hosmer-Lemeshow test was performed to check the model’s goodness of fit. A decision curve analysis was used to assess its clinical utility.
Results: A total of 410 patients were enrolled in this study. Stress-related gastrointestinal bleeding occurred in 115 patients (28.0%). Multivariate analysis demonstrated that gastric pH at admission [odds ratio (OR): 0.52, 95% confidence interval (CI): 0.41– 0.66, P < 0.001], ICH volume (OR: 1.03, 95% CI: 1.02– 1.05, P < 0.001) and sepsis (OR: 2.56, 95% CI: 1.54– 4.25, P < 0.001) were independent predictors for stress-related gastrointestinal bleeding in critically ill patients with ICH. The nomogram including gastric pH at admission, ICH volume and sepsis presented good discrimination with C-index of 0.770 (95% CI: 0.716 to 0.822), which was confirmed to be 0.764 through bootstrapping validation. The calibration plot showed good agreement between the predicted and observed outcomes. The Hosmer–Lemeshow test showed a goodness-of-fit (Chi-Square = 8.085, DF = 8, P = 0.425). Decision curve analysis demonstrated that the nomogram was clinically beneficial.
Conclusion: The proposed nomogram based on gastric pH at admission, ICH volume and sepsis can accurately predict the risk of stress-related gastrointestinal bleeding in critically ill patients with primary intracerebral hemorrhage.
Keywords: nomogram, stress-related gastrointestinal bleeding, intracerebral hemorrhage, prediction, decision curve analysis
Introduction
Upper gastrointestinal bleeding is a common and serious complication in critically ill patients in the intensive care unit (ICU), which is called stress-related gastrointestinal bleeding (SGIB) or stress ulcer. Usually, patients with intracerebral hemorrhage (ICH) are at high risk for SGIB, which is significantly associated with unfavorable outcomes.1–3 Thus, patients with ICH who are susceptible to SGIB are routinely recommended to accept acid-suppressive therapy as stress ulcer prophylaxis (SUP) due to the contribution of gastric acid to stress ulcer.4–7 However, not all ICH patients benefit from acid suppressants on account of their adverse reactions.8 Hence, it is necessary and important to identify those patients at high risk for SGIB. To date, there have been a few studies concerning risk factors associated with SGIB in ICH patients.1,9,10 However, the performances of the previous models have not been tested, which are limited to clinical use.
Nomogram, a graphical statistical instrument, has been generally performed in medical decision-making or other specialties by calculating the continuous probability of a particular outcome for an individual patient.11,12 In the present study, we aimed at identifying predictors for SGIB in critically ill ICH patients and constructing a nomogram based on a multivariable regression model. Furthermore, the performance and clinical benefits of the nomogram were assessed.
Materials and Methods
Study Design
This was a retrospective and observational study. Patients with ICH who were admitted to the neurological and neurosurgical intensive care unit of First Affiliated Hospital of University of South China from January 2018 to March 2021 participated in the investigation. This study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Institutional Ethical Committee of The First Affiliated Hospital, Hengyang Medical School, University of South China. We obtained written informed consent from the patients or their legal surrogates in accordance with national regulations.
Participants
Patients aged ≥18 years with spontaneous ICH within 24 hours of onset were screened. Primary ICH was defined as intraparenchymal bleeding, which was proven by computed tomography (CT), excluding those secondary causes: arteriovenous malformation or aneurysmal hemorrhage, brain tumor, brain trauma, hemorrhagic transformation of a cerebral infarction. Obviously, the patients with primary intraventricular hemorrhage (IVH) were excluded. In order to eliminate the possible confounder effect of disorders, patients with a history of pre-existing gastritis, gastric/duodenum ulcer, gastrointestinal hemorrhage, liver and kidney disease, bleeding diathesis and medication of antiplatelet, anticoagulant, nonsteroidal anti-inflammatory drugs (NSAIDS) or steroids were excluded.
Clinical Data
Characteristics of the patients at baseline included age, sex, systolic blood pressure (SBP) and diastolic blood pressure (DBP) on admission, gastric pH at admission, Glasgow Coma Scale (GCS) at admission, hematoma location, ICH volume, intraventricular extension, midline shift, surgical treatments for ICH (such as external ventricular drain placement and hematoma evacuation), mechanical ventilation (MV) and sepsis. The hematoma size was calculated as A × B × C/2.13 The pH of the gastric aspirates was measured with a precision acidity meter (PHS-2C, KUANSONS, Shanghai) and recorded within 2 hours after admission. GCS was used to assess the severity of consciousness impairment. The diagnostic criteria for Systemic Inflammatory Response Syndrome (SIRS) was considered if at least two of the following features were present: (1) temperature >38°C or <36°C, (2) heart rate >90/min, (3) respiratory rate >20/min or PaCO2<32mmHg (4.3 kPa), (4) leucocyte counts >12,000/mm3 or <4000/mm3 or more than 10% immature bands. Sepsis is defined as SIRS caused by a dysregulated host response to infection.14
Definition of Gastrointestinal Bleeding
Gastrointestinal bleeding, which occurred during hospitalization, was defined as any episode of fresh blood or coffee ground materials in nasogastric aspirate, hematemesis, melena or bloody stool.15 Of note, the patients were routinely administered with intravenously PPIs (pantoprazole or omeprazole) with 40 mg q12h for SUP within 24 hours after admission.
Statistical Analysis
The t-test or Mann–Whitney U-test was used to analyze the continuous variables. The Chi-square test was used to assess the categorical variables. All variables were analyzed using univariate logistic regression to determine their association with SGIB in ICH patients. Variables that showed a significant (P<0.05) univariate association with SGIB or a biological plausibility on factors that might influence SGIB were included in the final model (stepwise multiple logistic regression analysis). Based on the risk factor identified in multivariate analysis, a nomogram was built. The calibration, discrimination and clinical usefulness of the nomogram were calculated to evaluate its performance. The predictive accuracy and discrimination of the nomogram was evaluated by the concordance index (C-index), which was identical to the area under the curve (AUC) of the receiver- operating characteristic curve (ROC). Bootstrapping validation (1000 bootstrap resamples) was used to calculate a relatively corrected C-index.16 Calibration curve (1000 bootstrap resamples) was conducted to verify the calibration of the prediction nomogram. In general, a C-index value >0.70 indicated that the model was good for discrimination. Hosmer-Lemeshow test was performed to check the model’s goodness of fit. Decision curve analysis (DCA) was performed to assess the clinical usefulness of the nomogram by quantifying the standardized net benefits at different threshold probabilities. Statistical analyses were conducted using SPSS (ver.23.0; SPSS Inc., IL, USA) and R software, version 4.0.2 (The R Foundation for Statistical Computing, Vienna, Austria; http://www.R-project.org/). A two-sided p <0.05 was considered statistically significant.
Results
Baseline Characteristics
This study included 410 participants. A flowchart of the study population is shown in Figure 1, and the clinical characteristics of the participants are summarized in Table 1. 231 men and 179 women were included. The median age of the patients was 69 years (IQR, 61–76 years). The median volume of hematomas was 27 mL (IQR, 17–43 mL). The median GCS at admission was 7 (IQR, 6–8). The median measured gastric pH was 2.48 (IQR, 1.74–3.56). SGIB occurred in 115 patients (28.0%). There were no significant differences in clinical characteristics including age, sex, blood pressure at admission, GCS at admission, ICH position, ratios of surgical treatment, occurrence of intraventricular extension and midline shift between SGIB and non-SGIB groups. Clinical characteristics including gastric pH at admission (P<0.001), MV (P=0.048), ICH volume (P< 0.001) and occurrence of sepsis (P< 0.001) were significantly different between the two groups, indicating potential roles of these factors in logistics model.
 |
Table 1 Clinical Characteristics of Participants with or without SGIB |
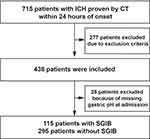 |
Figure 1 Participant flowchart. ICH indicates intracerebral hemorrhage. |
Univariate and Multivariate Analysis
Univariate analysis revealed significant associations between SGIB in ICH patients and gastric pH at admission (OR:0.50, 95% CI:0.39–0.63, P< 0.001), ICH volume (OR:1.04, 95% CI:1.02–1.05, P<0.001), sepsis (OR:2.30, 95% CI:1.46–3.62, P<0.001) and MV (OR:1.61, 95% CI:1.03–2.51, P = 0.037). All these candidate factors were included in the multivariate analysis. In the final prediction model based on multivariable logistic regression, gastric pH at admission (OR: 0.52, 95% CI: 0.41–0.66, P<0.001), ICH volume (OR: 1.03, 95% CI: 1.02–1.05, P<0.001) and sepsis (OR: 2.56, 95% CI: 1.54–4.25, P<0.001) remained independent predictors. The results were shown in Figure 2.
Construction and Validation of the Nomogram
A nomogram was established with the three factors obtained from the multivariable regression model. The nomogram was generated by assigning a weighted score to each of the independent predictors. The probability of SGIB in ICH patients ranged from 0.05 to 0.95. A higher score calculated from the sum of the assigned points for each predictor in the nomogram corresponded to a higher probability of SGIB in ICH patients. The nomogram including gastric pH at admission, ICH volume and sepsis presented good discrimination with C-index of 0.770 (95% CI: 0.716 to 0.822), which was equal to the AUC of the ROC plot. The corrected C-index was confirmed to be 0.764 through bootstrapping validation. The calibration plot showed good agreement between the predicted and observed outcomes. The Hosmer-Lemeshow test showed a goodness-of-fit (Chi-Square = 8.085, DF = 8, P = 0.425). The results are shown in Figure 3.
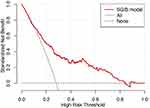
The DCA showed the net benefits obtained from the application of our nomogram with threshold probabilities of 0.097 to 0.843. This corroborated the good clinical applicability of the nomogram in predicting SGIB in critically ill patients with ICH because the ranges of threshold probabilities were wide and practical. The results are shown in Figure 4.
Discussion
In the present study, we focused on the critically ill patients with ICH and found that 28.0% of the patients with severe ICH developed SGIB during hospitalization. We presented a nomogram based upon gastric pH at admission, ICH volume and sepsis to predict the probability of SGIB after ICH. The discrimination and calibration of the nomogram was confirmed to be satisfactory.
There are limited data on prediction models for the occurrence of SGIB in ICH, especially for critically ill patients. The previous models or scores are limited by the use of dichotomization/categorization of predictors, in which predictive accuracy may decrease due to categorizing continuous variables into several risk groups. Comparatively, a nomogram is generally performed as a statistical model with the ability to generate an individual probability of a clinical event by integrating diverse prognostic and determinant variables, which is useful for achieving personalized medication.17 Therefore, we constructed a nomogram comprising gastric pH at admission, ICH volume and sepsis.
The important predictors for SGIB included in the nomogram were gastric pH at admission and hematoma volume. This meant patients with low gastric pH or large hematoma volume were susceptible to the occurrence of SGIB. In accordance with previous reports, hematoma volume could predict well stress ulcer for ICH patients.1,2 In addition, we took gastric pH at admission into consideration. As a result, gastric pH at admission was demonstrated to be a predictor for SGIB in ICH patients. From the point of view of the mechanism, a large hematoma may result in an increase of intracranial pressure when ICH occurs. The raised intracranial pressure may cause vagal hyperactivity, leading subsequently to mucosal ischemia and increased gastric acid secretion.18,19 Of note, the primary pathophysiologic mechanism leading to SGIB is ischemia and reperfusion injury, although the formation of stress ulcer was multifactorial.20 To our knowledge, this is the first study where gastric pH at admission as a potential risk factor for SGIB is considered and evaluated.
Sepsis was another well-known predictor of SGIB after ICH. Patients with ICH, especially in severe cases, often tend to be complicated by infection, which in turn can lead to sepsis. The incidence of sepsis in the present study was 30.2%. For critically ill patients in intensive care unit, several studies have highlighted the importance of septicemia in producing SGIB.10,21 Likewise, in our study sepsis played a very vital role in inducing stress ulcer after severe ICH. One possible reason was that sepsis could trigger a massive release of inflammatory cytokines, which could exacerbate gastrointestinal mucosal ischemia caused by brain damage, thereby promoting the development of stress ulcer.22,23
Then, we assessed the performance of the nomogram. Compared with the previously reported results, the nomogram composition of gastric pH at admission, ICH volume and sepsis in this study showed good discrimination. The calibration suggested a general consistency between the predicted and actual risk of SGIB. The DCA proved its clinical utility. Thus, the nomogram was a reliable model to evaluate the risk of SGIB in ICH. For those patients with ICH at high risk for SGIB, we can take a relatively aggressive implementation of pharmacological prophylaxis because those patients might benefit from more aggressive prophylaxis. Compared with the previous prediction models,1,10 we made comprehensive evaluation on the performance and clinical benefits of the nomogram and underlined the value of its clinical utility. Comparatively, an important novelty was that we identified gastric pH at admission as a potential risk factor for SGIB.
In fact, recent evidence has highlighted the controversy of SUP due to the increased adverse reactions of acid-suppressive therapy and the perceived decline in occurrence of stress ulcer.24,25 Adverse effects associated with PPIs, including mainly the risk of pneumonia and Clostridium difficile infection, may partly counterbalance their potential benefits.26–29 Therefore, it was quite important for clinicians to identify those patients with ICH at high risk for SGIB. Now, we built a nomogram with satisfactory discrimination and calibration. This nomogram model can help clinicians to identify those patients precisely at high risk for SGIB. Accurate prediction of the risk of SGIB in patients with ICH helps with appropriate treatment decisions, avoiding lack of treatment and over-treatment.
The present study has the following limitations. First, this was a retrospective study, so some potential confounders that might have some impact on the risk of SGIB after ICH were neglected. Second, this study was single-centered and demographic characteristics were not the absolute same as those of the general population, causing a selection bias. Finally, the clinical effect of this nomogram in actual application might be less accurate owing to the limited samples. A multicenter validation study is still needed to confirm the performance of our nomogram before its application into clinical practice. Despite these limitations, to our knowledge, this study is the first attempt to establish a predictive nomogram for SGIB in critically ill patients with ICH.
Conclusion
We proposed and validated a nomogram model that included gastric pH at admission, ICH volume and sepsis, which can provide an individual and precise prediction of the risk probability of SGIB following hemorrhagic stroke in Chinese patients.
Acknowledgments
This work was partly supported by the Science and Technology Department of Hunan Province (2021JJ40477).
Disclosure
The authors declare no conflicts of interest in this work.
References
1. Misra UK, Kalita J, Pandey S, Mandal SK. Predictors of gastrointestinal bleeding in acute intracerebral haemorrhage. J Neurol Sci. 2003;208(1–2):25–29. doi:10.1016/S0022-510X(02)00415-X
2. Liu BL, Li B, Zhang X, et al. A randomized controlled study comparing omeprazole and cimetidine for the prophylaxis of stress-related upper gastrointestinal bleeding in patients with intracerebral hemorrhage. J Neurosurg. 2013;118:115–120. doi:10.3171/2012.9.JNS12170
3. Misra UK, Kalita J, Pandey S, Mandal SK, Srivastava M. A randomized placebo controlled trial of ranitidine versus sucralfate in patients with spontaneous intracerebral hemorrhage for prevention of gastric hemorrhage. J Neurol Sci. 2005;239(1):5–10. doi:10.1016/j.jns.2005.07.011
4. Madsen KR, Lorentzen K, Clausen N, et al. Guideline for stress ulcer prophylaxis in the intensive care unit. Dan Med J. 2014;61:C4811.
5. Rhodes A, Evans LE, Alhazzani W, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock: 2016. Intensive Care Med. 2017;43:304–377.
6. Krag M, Perner A, Wetterslev J, et al. Prevalence and outcome of gastrointestinal bleeding and use of acid suppressants in acutely ill adult intensive care patients. Intensive Care Med. 2015;41:833–845. doi:10.1007/s00134-015-3725-1
7. Barletta JF, Kanji S, MacLaren R, Lat I, Erstad BL. American-Canadian consortium for intensive care drug utilization I. Pharmacoepidemiology of stress ulcer prophylaxis in the United States and Canada. J Crit Care. 2014;29(6):955–960. doi:10.1016/j.jcrc.2014.06.025
8. Pappas M, Jolly S, Vijan S. Defining appropriate use of proton-pump inhibitors among medical inpatients. J Gen Intern Med. 2016;31(4):364–371. doi:10.1007/s11606-015-3536-7
9. Cook DJ, Fuller HD, Guyatt GH, et al. Risk factors for gastrointestinal bleeding in critically ill patients. Canadian critical care trials group. N Engl J Med. 1994;330(6):377–381. doi:10.1056/NEJM199402103300601
10. Yang TC, Li JG
11. Jehi L, Yardi R, Chagin K, et al. Development and validation of nomograms to provide individualised predictions of seizure outcomes after epilepsy surgery: a retrospective analysis. Lancet Neurol. 2015;14(3):283–290. doi:10.1016/S1474-4422(14)70325-4
12. Cappellari M, Mangiafico S, Saia V, et al. Ier-sich nomogram to predict symptomatic intracerebral hemorrhage after thrombectomy for stroke. Stroke. 2019;50(4):909–916. doi:10.1161/STROKEAHA.118.023316
13. Divani AA, Majidi S, Luo X, et al. The ABCs of accurate volumetric measurement of cerebral hematoma. Stroke. 2011;42(6):1569–1574. doi:10.1161/STROKEAHA.110.607861
14. Singer M, Deutschman CS, Seymour CW, et al. The third international consensus definitions for sepsis and septic shock (sepsis-3). JAMA. 2016;315(8):801–810. doi:10.1001/jama.2016.0287
15. Cook D, Guyatt G, Longo DL. Prophylaxis against upper gastrointestinal bleeding in hospitalized patients. N Engl J Med. 2018;378(26):2506–2516. doi:10.1056/NEJMra1605507
16. Pencina MJ, D’Agostino RB. Overall c as a measure of discrimination in survival analysis: model specific population value and confidence interval estimation. Stat Med. 2004;23:2109–2123. doi:10.1002/sim.1802
17. Balachandran VP, Gonen M, Smith JJ, DeMatteo RP. Nomograms in oncology: more than meets the eye. Lancet Oncol. 2015;16(4):e173–e180. doi:10.1016/S1470-2045(14)71116-7
18. Cheung LY. Thomas g orr memorial lecture. Pathogenesis, prophylaxis, and treatment of stress gastritis. Am J Surg. 1988;156(6):437–440. doi:10.1016/S0002-9610(88)80522-1
19. Maynard N, Bihari D, Beale R, et al. Assessment of splanchnic oxygenation by gastric tonometry in patients with acute circulatory failure. JAMA. 1993;270:1203–1210. doi:10.1001/jama.1993.03510100053032
20. Barletta JF, Mangram AJ, Sucher JF, Zach V. Stress ulcer prophylaxis in neurocritical care. Neurocrit Care. 2017;29:344–357. doi:10.1007/s12028-017-0447-y
21. Sasabuchi Y, Matsui H, Lefor AK, Fushimi K, Yasunaga H. Risks and benefits of stress ulcer prophylaxis for patients with severe sepsis. Crit Care Med. 2016;44(7):e464–e469. doi:10.1097/CCM.0000000000001667
22. Overhaus M, Tögel S, Pezzone MA, Bauer AJ. Mechanisms of polymicrobial sepsis-induced ileus. Am J Physiol Gastrointest Liver Physiol. 2004;287(3):G685–G694. doi:10.1152/ajpgi.00359.2003
23. Pastores SM, Katz DP, Kvetan V. Splanchnic ischemia and gut mucosal injury in sepsis and the multiple organ dysfunction syndrome. Am J Gastroenterol. 1996;91:1697–1710.
24. Alshamsi F, Belley-Cote E, Cook D, et al. Efficacy and safety of proton pump inhibitors for stress ulcer prophylaxis in critically ill patients: a systematic review and meta-analysis of randomized trials. Crit Care. 2016;20(1):120. doi:10.1186/s13054-016-1305-6
25. Krag M, Marker S, Perner A, et al. Pantoprazole in patients at risk for gastrointestinal bleeding in the ICU. N Engl J Med. 2018;379:2199–2208.
26. Freedberg DE, Lebwohl B, Abrams JA. The impact of proton pump inhibitors on the human gastrointestinal microbiome. Clin Lab Med. 2014;34:771–785. doi:10.1016/j.cll.2014.08.008
27. MacLaren R, Reynolds PM, Allen RR. Histamine-2 receptor antagonists vs proton pump inhibitors on gastrointestinal tract hemorrhage and infectious complications in the intensive care unit. JAMA Intern Med. 2014;174(4):564–574. doi:10.1001/jamainternmed.2013.14673
28. Barletta JF, Sclar DA. Proton pump inhibitors increase the risk for hospital-acquired clostridium difficile infection in critically ill patients. Crit Care. 2014;18(6):714. doi:10.1186/s13054-014-0714-7
29. Buendgens L, Bruensing J, Matthes M, et al. Administration of proton pump inhibitors in critically ill medical patients is associated with increased risk of developing clostridium difficile-associated diarrhea. J Crit Care. 2014;29:696 e611–695.
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.