Back to Journals » Clinical Ophthalmology » Volume 16
A New and Easier Approach to Preserflo MicroShunt Implantation
Authors Fea AM , Ghilardi A, Bovone D, Reibaldi M, Rossi A, Craven ER
Received 3 December 2021
Accepted for publication 17 February 2022
Published 27 April 2022 Volume 2022:16 Pages 1281—1288
DOI https://doi.org/10.2147/OPTH.S307835
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
Supplementary video of "Preserflo MicroShunt implantation made easier" [ID 307835].
Views: 2867
Antonio M Fea,1 Andrea Ghilardi,1 Davide Bovone,1 Michele Reibaldi,1 Alessandro Rossi,1 Earl R Craven2
1Dipartimento di Scienze Oftalmologiche-Universita’ di Torino, Torino, Italy; 2Glaucoma Center of Excellence, The Johns Hopkins Wilmer Eye Institute, Baltimore, Maryland, USA
Correspondence: Antonio M Fea, Tel +39 3495601674, Email [email protected]
Abstract: PRESERFLO™ MicroShunt is a new minimally invasive glaucoma surgical (MIGS) device, implanted with an ab externo approach, which drains the aqueous humor to the subconjunctival space. It has been designed as a safer and less invasive approach for treating medically uncontrolled primary open-angle glaucoma (POAG) patients. The classic way of MicroShunt implantation involves different key steps, which includes creating a small scleral pocket with a 1mm blade; passing a 25-gauge (25G) needle through the scleral pocket into the anterior chamber (AC); and subsequently flushing the stent with a 23-gauge (23G) thin-wall cannula. However, sliding the needle into the scleral pocket can create false passages, thus making the device’s threading more difficult. The purpose of the current paper is to propose a simplified implantation approach. Our method proposes to make the scleral tunnel by using directly the 25G needle and, at the limbus, this 25G needle is used to slightly depress the sclera and enter into the AC. The MicroShunt is subsequently assembled on a 23G cannula mounted on a 1mL syringe. The syringe can then be used to flush the device. Outflow can thus be confirmed immediately by seeing drops of aqueous humor leaking from the external opening of the stent. This new approach may have different potential advantages, such as better control of the site of entry, avoids wrong passages, reduces or eliminates the risk of aqueous humor sideway flow, facilitates a parallel path to the iris plane, and it is faster.
Keywords: MIGS, open-angle glaucoma, Preserflo, MicroShunt, glaucoma surgery, subconjunctival filtration surgery
Introduction
Over the last several years, the field of glaucoma surgery has seen the introduction of micro- or minimally invasive surgeries (MIGS).1–5 These MIGS devices have been developed for the treatment of medically uncontrolled primary open-angle glaucoma (POAG) patients, in an attempt to improve safety, while maintaining the efficacy in terms of lowering intraocular pressure (IOP).1–5 MIGS devices can be divided in: trabecular, suprachoroidal, and subconjunctival.1,3 The subconjunctival outflow mimics the mechanism of trabeculectomy. It provides low post-operative IOP while simultaneously conferring a standardized procedure and a better safety profile as compared to trabeculectomy.1–5 All the subconjunctival devices rely on the implant of a small tube. The lumen size of these devices has been approximated using the Hagen-Poiseuille equation for laminar flow.1 In general, the lumen is chosen to prevent chronic hypotony and to be sufficiently large to avoid clogging.
Although there is some debate about considering the MicroShunt as a MIGS, for the purposes of this document, the term MIGS will apply to it. The PreserfloTM MicroShunt implant has been recently introduced.6 This shunt consists of a poly-styrene-block-isobutylene-block-styrene polymer that has been previously used as a coronary stent because it provokes minimal inflammation and encapsulation.7,8 The device has a length of 8.5 mm and an internal lumen of 70 μm allowing for controlled flow and maintenance of an IOP above 5 mm Hg assuming average aqueous production.8 The length of the device allows for a more posterior aqueous outflow, and for this reason, a wide posterior dissection is recommended.
In general, the oblique quadrants are the preferred implantation areas because this will avoid proximity to the superior rectus muscle. The mitomycin-C (MMC) concentration and exposure time have changed according to risk factors or the surgeon’s experience.9–16
This short communication aims to outline a further modification of the procedure, allowing for a faster and easier implant of the MicroShunt.
Methods
The Ethic Committee of the University of Torino approved the review of the medical charts. Since it was a retrospective revision of the medical charts, the Ethic Committee waived the need of written inform consent to participate in the study. Nevertheless, written informed consent was provided by all the participants before surgery.
In order to ensure patients confidentiality, their information was anonymized by using unique identifiers. The study protocol adhered to the tenets of the Declaration of Helsinki and the Good Clinical Practice/International Council for Harmonization Guidelines.
Study Participants
The current study included consecutive POAG patients ≥ 18 years and a medically treated preoperative IOP ≥23 mm Hg who underwent a standalone MicroShunt implant procedure.
Surgical Procedure
The PRESERFLOTM MicroShunt (Santen former Innfocus, Miami, FL, USA) is provided in a sterile packaged kit containing a 3-mm scleral marker, a 1-mm triangular-blade knife, 3 LASIK ShieldsTM (EYETEC, Antwerp, Belgium), a marker pen, and a 25-gauge (25G) needle.
Before using the MicroShunt, it is advised by the manufacturer to prime it with a 23G cannula, which is not included in the set.
All the procedures have been performed under local anesthesia by an experienced surgeon (AMF).
The surgical technique suggested by the manufacturer consists of 10 steps (see Table 1).
 |
Table 1 Overview of the MicroShunt Device Surgical Procedure Recommended by the Manufacturer |
Rationale of the New Surgical Technique
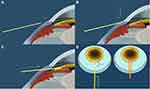
Although, as a favorable point, the classical procedure is familiar to glaucoma surgeons implanting tubes, some steps may pose a challenge. In particular, while sliding the 25G needle, its tip can create wrong/false passages on a different plane or enter the anterior chamber before reaching the apex of the scleral tunnel. Controlling the path of the 25G needle is indeed extremely difficult as the space within the scleral tunnel is virtual or, in any case, extremely thin (See Figure 1).
In some cases, this issue can make it difficult to threading the MicroShunt into the anterior chamber (AC), since its tip gets blocked within the tunnel. Additionally, this maneuver could be even more complex in eyes with abnormal limbal anatomy.
Moreover, if the second attempt continues to fail, the surgeon may be forced to implant the device more superiorly. This location, due to the presence of the superior rectus insertion, is more prone to subsequent scarring.
In order to avoid this problem, one option is to enter into the AC with the tip of the micro knife used to create the scleral pocket. Although this technique may save time and prevent the creation of wrong passages, it may be difficult to judge the length of the entry into the AC. Additionally, the triangular shape of the blade determines a larger path and, therefore, a side-flow in the early postoperative period. According to the Poiseuille’s law, a side-flow would also nullify the attempt to create a predetermined aqueous humor outflow from the AC, which may favor the incidence of hypotony.
New Surgical Technique
Our surgical technique makes two improvements to the traditional surgical procedure. The first one is to perform the tunnel directly with the 25G needle. As second improvement, our technique proposes to attach a 23G cannula, which is commonly used to aspirate silicon oil, to the posterior end of the MicroShunt. That way, the surgeon has the possibility of flushing the device directly when it is threaded.
To use the 25G needle for making the tunnel simplifies the surgical procedure, as it eliminates the need to make the scleral pocket, while significantly reducing the scleral area involved in the procedure. Additionally, this improvement also facilitates the entry in a more parallel plane to the iris, by depressing the sclera when close to the limbus, which minimizes the potential endothelial cell damage in the long term (See Figure 1 and Supplementary video.
The second improvement proposed by the new technique is to use a 23 G cannula, similar to those commonly used to aspirate silicone oil. This 23G cannula holds perfectly the MicroShunt and facilitates its flushing. Furthermore, the fluid injected into the AC will also increase the pressure allowing an aqueous humor flow through the distal end of the device (See Figure 1 and Supplemental video).
Clinical Experience
Our clinical experience consists of 15 eyes from 15 OAG patients, who have undergone a standalone MicroShunt, with a follow-up of 3 months. Although data about IOP lowering and reduction of ocular hypotensive medications are presented, our main purpose was focused on the early post-operative complications.
All the patients were Caucasians, with a median (Interquartile range, IqR) age of 76.0 (71.8 to 84.3) years, and 6 (40.0%) were women. Main demographic and clinical characteristics are summarized in Table 2.
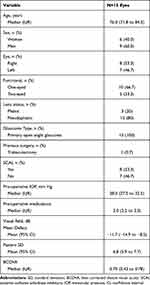 |
Table 2 Main Demographic and Clinical Characteristics of the Study Population |
Median (IqR) IOP decreased from 28.0 (27.0 to 32.5) mm Hg at baseline to 11.0 (10.0 to 12.0) mm Hg at month 3 (Hodges-Lehmann median difference: −18.0 mm Hg, 95% confidence interval: −22.0 to −14.0 mm Hg, p=0.0010) (Figure 2). Similarly, the number of ocular hypotensive medications was significantly reduced from 3.0 (2.2 to 3.0) drugs at baseline to 0.0 (0.0 to 0.12) drugs at month 3 (Hodges-Lehmann median difference: −2.5 drugs, 95% confidence interval: −3.0 to −2.0 drugs, p=0.0007). At month 3 no patient was on systemic IOP lowering medications.
As compared to preoperative values, visual acuity was significantly lower at day-1, week-1, and month-1, but it was recovered and stabilized from month-2 (Figure 3).
Regarding safety, two (13.3%) eyes had hyphema (approximately 1 mm) at the first postoperative day, which disappeared completely within a week. Three (20.0%) eyes experienced a peripheral choroidal detachment, which was successfully resolved with medical therapy within one month. No additional surgery was necessary in any of the patients.
Discussion
The currently available evidence evaluating the effectiveness and safety of MicroShunt has shown promising results, although it is limited.9–16 Surgeon’s experience and the clinical outcomes are crucial for improving and simplifying surgical techniques.
In this paper, we aimed to show a faster, more consistent, and straightforward technique to implant this device. Clinical data of this technique have been presented looking for early complication associated potentially with the technique, rather than with the purpose of analyzing its effectiveness.
The device has two lateral fins whose theoretical function is to prevent a possible side flow and the movement of the MicroShunt.6,8 The traditional technique recommends to make a shallow scleral pocket 3 mm posterior to and towards the limbus with a triangular-bladed knife, with the purpose of accommodating these lateral fins. However, its length and the fact that the scleral pocket starts 3 mm from the limbus make the device protrude significantly into the anterior chamber. Due to this fact, when using the classical technique, we seldom implanted the device with the fins under the scleral pocket for preventing an excessive length of the device in the anterior chamber.
Using our technique, the stent is potentially free to move and dislocate as the fins are accessible under the Tenon’s capsule. Nevertheless, it should be highlighted that no dislocation occurred in our sample.
Using a needle for constructing a scleral tunnel for implanting a drainage device is not new. Albis-Donado et al17 reported good clinical outcomes in patients who underwent an Ahmed glaucoma valve implantation through a needle-generated scleral tunnel, without a tube-covering patch.
In our technique, we used a 25G with an external diameter of 0.515mm with a track of 3 to 4 mm in length, which was sufficient to hold firmly in place the device. Considering that the external diameter of the MicroShunt is 0.35 mm, potentially, the use of a smaller needle may determine an even more stable grip and a lesser side-flow. Either a 26 (0.466), a 27G (0.413), or even a 28G (0.362) needle may be used, but we do not have experience with these smaller diameter needles. Further mid- long-term researches evaluating these options need to be performed.
Another potential issue that arises with the present technique would be scleral erosion. It should be noted, nevertheless, that a similar technique using a 20G microvitreoretinal blade18 or a much bigger 22–23G needles17 has been reported for Molteno implant without migration nor erosion18 and Ahmed tubes with a minimal rate of tube retraction (4/186).17
As compared to the traditional graft technique, the needle technique was associated with several advantages, such as faster surgery, and a flatter transition between conjunctiva and cornea, with a lower incidence of Dellen and painful blebs.17,18 Additionally, both studies suggested that the absence of erosion is related to a tighter fit between the tube and the tunnel, determining fewer micromovements and abrasion.17,18
Regarding safety, the incidence of postoperative complications seemed to be slightly greater than that reported by other papers, although it should be mentioned that we paid special attention to report even uneventful complications in this paper and that none of them, nevertheless, was clinically significant.
Although the incidence of false passages has not been reported in previous studies,9–16 this intraoperative complication can occur and forces the creation of another tunnel on the side, thus increasing the risk of anterior chamber hemorrhages and consuming space, potentially moving the path of the micro shunt in a less favorable position.
This short-report has several limitations that need to be mentioned. Among them, the limited sample size, the short length of follow-up, and the lack of a control group are the most important ones. Nevertheless, the current paper described a method that significantly improves the insertion of the MicroShunt with similar rates of intra-operative and early post-operative complications than the ones reported with the traditional technique.9–16
In summary, the use of the needle to create an intrascleral path has shown promising results in this small cohort of patients. Its use may be beneficial specially in those cases when the presence of other devices limits the space. Further studies are needed to determine the long-term stability of this technique and the potential benefit of smaller needles.
Acknowledgments
Medical writing and Editorial assistant services have been provided by Antonio Martínez (MD) of Ciencia y Deporte S.L. and covered by an unrestricted Grant from the University of Turin.
The authors also wanted to express their gratitude to A Mazzoleni, L Guazzone, C Caiafa, E Suozzo, M Pallotta and M Grindi for their collaboration during the study.
Funding
There is no funding to report.
Disclosure
Dr Antonio M Fea is consultant for Glaukos, Ivantis, iSTAR, EyeD, and personal fees from and consultant for AbbVie, outside the submitted work. Dr Earl R Craven is currently an employee for AbbVie and reports personal fees from Santen, outside the submitted work. The authors report no other conflicts of interest in this work.
References
1. Ansari E. An update on implants for Minimally Invasive Glaucoma Surgery (MIGS). Ophthalmol Ther. 2017;6(2):233–241. doi:10.1007/s40123-017-0098-2
2. Bar-David L, Blumenthal EZ. Evolution of glaucoma surgery in the last 25Years. Rambam Maimonides Med J. 2018;9(3):e0024. doi:10.5041/RMMJ.10345.
3. Mathew DJ, Buys YM. Minimally invasive glaucoma surgery: a critical appraisal of the literature. Annu Rev Vis Sci. 2020;6:47–89. doi:10.1146/annurev-vision-121219-081737
4. Vinod K, Gedde SJ. Safety profile of minimally invasive glaucoma surgery. Curr Opin Ophthalmol. 2021;32(2):160–168. doi:10.1097/ICU.0000000000000731
5. Pereira ICF, van de Wijdeven R, Wyss HM, et al. Conventional glaucoma implants and the new MIGS devices: a comprehensive review of current options and future directions. Eye. 2021;35(12):3202–3221. doi:10.1038/s41433-021-01595-x
6. Lee RMH, Bouremel Y, Eames I, Brocchini S, Khaw PT. Translating minimally invasive glaucoma surgery devices. Clin Transl Sci. 2020;13(1):14–25. doi:10.1111/cts.12660
7. Pinchuk L, Wilson GJ, Barry JJ, et al. Medical applications of poly (styrene-block-isobutylene-block-styrene) (“SIBS”). Biomaterials. 2008;29(4):448–460. doi:10.1016/j.biomaterials.2007.09.041
8. Beckers JM, Pinchuk L. Minimally invasive glaucoma surgery with a new Ab-externo subconjunctival bypass – current status and review of literature. Eur Ophthal Rev. 2019;13(1):27–30. doi:10.17925/EOR.2019.13.1.27
9. Riss I, Batlle J, Pinchuk L, et al. [One-year results on the safety and efficacy of the InnFocus MicroShunt™ depending on placement and concentration of mitomycin C]. J Fr Ophtalmol. 2015;38(9):855–860. French. doi:10.1016/j.jfo.2015.05.005
10. Batlle JF, Fantes F, Riss I, et al. Three-year follow-up of a novel aqueous humor MicroShunt. J Glaucoma. 2016;25(2):e58–65. doi:10.1097/IJG.0000000000000368
11. Scheres LMJ, Kujovic-Aleksov S, Ramdas WD, et al. XEN® gel stent compared to PRESERFLO™ MicroShunt implantation for primary open-angle glaucoma: two-year results. Acta Ophthalmol. 2021;99(3):e433–e440. doi:10.1111/aos.14602
12. Batlle JF, Corona A, Albuquerque R. Long-term results of the PRESERFLO®MicroShunt in patients with primary open-angle glaucoma from a single-center non-randomized study. J Glaucoma. 2021;30(3):281–286. doi:10.1097/IJG.0000000000001734
13. Beckers H, Aptel F, Webers C, et al. Two-year results of a multicentre study assessing the MicroShunt in patients with primary open-angle glaucoma: 0.4 mg/mL mitomycin C outcomes.
14. Schlenker MB, Durr GM, Michaelov E, Ahmed IIK. Intermediate outcomes of a novel standalone ab externo SIBS microshunt with mitomycin C. Am J Ophthalmol. 2020;215:141–153. doi:10.1016/j.ajo.2020.02.020
15. Baker ND, Barnebey HS, Moster MR, et al.; INN005 Study Group. Ab-externomicroshunt versus trabeculectomy in primary open-angle glaucoma: one-year results from a 2-year randomized, Multicenter Study. Ophthalmology. 2021;128(12):1710–1721. doi:10.1016/j.ophtha.2021.05.023
16. Fea AM, Laffi GL, Martini E, et al. Effectiveness of MicroShunt in primary open-angle and pseudoexfoliative glaucoma patients: a retrospective European multicenter study. Ophthalmol Glaucoma. 2021. doi:10.1016/j.ogla.2021.08.005
17. Albis-Donado O, Gil-Carrasco F, Romero-Quijada R, Thomas R. Evaluation of Ahmed glaucoma valve implantation through a needle-generated scleral tunnel in Mexican children with glaucoma. Indian J Ophthalmol. 2010;58(5):365–373. doi:10.4103/0301-4738.67039
18. Leong JK, McCluskey P, Lightman S, Towler HM. Outcome of graft free Molteno tube insertion. Br J Ophthalmol. 2006;90(4):501–505. doi:10.1136/bjo.2005.079087
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.