Back to Journals » Eye and Brain » Volume 15
A Neuro-Ophthalmologist’s Guide to Advances in Intracranial Pressure Measurements
Authors Mollan SP , Momin SN, Khatkar PS , Grech O, Sinclair AJ , Tsermoulas G
Received 18 July 2023
Accepted for publication 15 September 2023
Published 27 September 2023 Volume 2023:15 Pages 113—124
DOI https://doi.org/10.2147/EB.S404642
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Margaret Wong-Riley
Susan P Mollan,1,2 Sehrish NA Momin,3 Pavan S Khatkar,4 Olivia Grech,2 Alex J Sinclair,2,5 Georgios Tsermoulas2,6
1Birmingham Neuro-Ophthalmology, Queen Elizabeth Hospital, University Hospitals Birmingham, Birmingham, UK; 2Translational Brain Science, Institute of Metabolism and Systems Research; Birmingham, University of Birmingham, Birmingham, UK; 3Ophthalmology Department, The Aga Khan University Hospital, Karachi, Pakistan; 4Medical School, Imperial College London, London, UK; 5Department of Neurology, Queen Elizabeth Hospital, University Hospitals Birmingham, Birmingham, UK; 6Department of Neurosurgery, Queen Elizabeth Hospital, University Hospitals Birmingham, Birmingham, UK
Correspondence: Susan P Mollan, Birmingham Neuro-Ophthalmology, Queen Elizabeth Hospital, Birmingham, UK, Tel/Fax +44 121 3716912, Email [email protected]
Abstract: Cerebrospinal fluid disorders have a wide-ranging impact on vision, headache, cognition and a person’s quality of life. Due to advances in technology and accessibility, intracranial pressure measurement and monitoring, usually managed by neurosurgeons, are being employed more widely in clinical practice. These developments are of direct importance for Ophthalmologists and Neurologists because the ability to readily measure intracranial pressure can aide management decisions. The aim of this review is to present the emerging evidence for intracranial pressure measurement methods and interpretation that is relevant to Neuro-ophthalmologists.
Keywords: cerebrospinal fluid, intracranial pressure, intraparenchymal intracranial pressure sensors, lumbar puncture opening pressure, neuro-ophthalmology, non-invasive, telemetric intracranial pressure monitor, pseudotumour cerebri, waveform
Introduction
Understanding the physiology of intracranial pressure (ICP) is essential in conditions where the cerebrospinal fluid (CSF) dynamics are altered. The majority of techniques to measure ICP are invasive and for ethical reasons there is a scarcity of literature on normal physiological ICP conditions. This gap in knowledge results in debate over normal, threshold and abnormal values of all ICP parameters, and clinical correlation has yet to be fully determined.
There are many variables to be considered when measuring ICP. Fundamentally, the majority of CSF is produced from the choroid plexus, and flows into the ventricles and flows to the subarachnoid space. Movement is influenced by many factors including the cardiac and respiratory cycles.1 ICP measurements are known to be different when measured in the cranium or spine,2 and are known to change when measured in different postures.3–5 There are also alterations observed between children and adults and ICP measures continue to change throughout life.6,7 Lumbar puncture remains the most common way to measure ICP, providing a single reading. Other direct ICP measurements are more invasive, befitting directed inpatient care, and resulting in continuous data regarding the CSF system.3
CSF circulatory disorders have a wide-ranging impact. Vision can be at risk; headaches and cognition can directly affect quality of life.8–12 Due to the multidisciplinary nature of outcomes in people affected by CSF disorders, Ophthalmologists and Neurologists require contemporary knowledge of the advancing field of ICP measurement. ICP measurements have become primary clinical trial outcomes and are now used routinely in clinical practice utilising intraparenchymal monitors and ICP monitors in line with CSF shunts in some centres.3,13,14 Thus, the aim of this review was to assess the literature and apprise neuro-ophthalmologists in the current practice of ICP measurement and monitoring, relevant to their practice.
Materials and Methods
To support the aims of this narrative review, a detailed search of the scientific literature included all English language paper from three databases (PubMed, clinicaltrials.gov and Cochrane Library) were searched for relevant literature published from inception until May 30th, 2023. In brief, search terms were organized into three main domains: 1) Normal ICP; 2) Invasive ICP measurement and; 3) Non-invasive ICP measurement. The Boolean operator “OR” was used to separate each term within each concept, and each concept was concatenated by the operator “AND”. When possible, Medical Subject Heading terms were used to expand the search language. The search strategy was identical in all databases, with minor adaptation to the coding to suit individual database settings. Key words included Cerebrospinal fluid; Compliance; Idiopathic Intracranial Hypertension; Intracranial pressure; Intracranial pressure monitoring; Intracranial pressure waveform; Lumbar puncture; Obstructive hydrocephalus; Papilloedema; Pseudotumor cerebri, and Telemetric intracranial pressure monitor.
Results
Normal Physiological Conditions
Up to 10% of the cranial cavity is occupied by CSF, with 80% of the CSF being produced by the choroid plexus at a rate of approximately 0.35 mL per minute.15 This maintains a continuous volume of between 125–150mls, the fluid being replaced 3–4 times in any given 24-hour period.15 ICP is the pressure inside the skull generated primarily by the arterial pulsatile waves and is typically regarded as a mean ICP. CSF pressure reflects ICP. Under normal physiological conditions, CSF pressure and ICP are not static but dynamic with the pulsations driven by the cardiac cycle. Postural changes and body movements cause a hydrodynamic shift in the direction of gravitational force that affects the craniospinal system. This shift results in CSF redistribution, cerebrovascular changes, alterations in cerebral venous pressure and fluctuations in ICP. CSF pressure and ICP have been found to be dependent on the following factors: systolic pulse wave, respiratory cycle, central venous pressure, intraabdominal pressure, physical activity, and body position.16,17
Normal ICP
ICP values are not absolute but represent measurements of a dynamic system that is linked to the cardiac cycle and has a mean (or static) pressure and a pulsatile pressure. The mean ICP is more widely used in clinical practice, but an understanding of the pulsatile ICP and its importance has increased with recent advances in ICP measurement. The mean ICP value is a relative pressure value that represents the pressure gradient between inside and outside of the skull and can be expressed as mmHg, cmH20 or mbar. The zero level reference pressure is typically set at the foramen of Monro. There are no reference values existing for normal ICP in either adults or children because it would be unethical to invasively measure ICP in normal people. Normative values for ICP have been established from measurement of people with neurological conditions (Table 1), typically taken from people who have suffered from a severe traumatic brain injury.18 These ranges, traditionally accepted to be pseudonormal, with emerging evidence cause fresh debate. Overall, supine mean ICP is likely to be much lower than previously thought.19–21 While the ICP level is known to differ through life (Table 1), it appears that children have a higher mean ICP than adults.22 There is an accepted age decline of between 0.69 and 1.00 mmHg per decade of life.19,22,23 Most clinicians agree that abnormal thresholds for ICP, such as that of the diagnostic threshold for idiopathic intracranial hypertension (IIH),24 or for treatment of traumatic brain injury,25 are much higher than these traditional ranges of normal.
 |
Table 1 Intracranial Pressure Measures (Supine Position) |
The pulsatile ICP represents fluctuations of the pressure caused mainly by variation in the blood pressure during the cardiac cycle and is generated primarily by the large arteries in the cranial subarachnoid space. Respiratory and vasomotor-induced fluctuations in pressure have a smaller effect and traditionally the pulsatile ICP value was considered to be the cardiac-induced pulsatility.26 The measure of the pulsatile ICP is the amplitude of the pulse wave, ie, the difference between the peak and the trough of the ICP during the cardiac cycle. While mean ICP is distributed evenly within the CSF system, the pulsatile ICP is higher in the intracranial compartment close to the large arteries, and gradually reduces as the pulsatile wave travels along the spinal subarachnoid channels towards the lumbar theca. Pulsatile ICP is a measure of intracranial compliance and higher values are associated with reduced compensatory reserve. A pulsatile ICP value of >4mmHg measured at the lumbar level is considered by some to be abnormally elevated.27–29 The typical disease that displays increased CSF pulsatility is normal pressure hydrocephalus which is characterized by normal mean ICP, but elevated pulsatile pressure due to stiffness of the wall of the intracranial arteries that do not absorb much energy of the arterial pulse wave.
Factors That Affect Intracranial Pressure Measurement
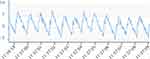
Changes in body position significantly affect the measurement of ICP30 (Figure 1). Movement from lying down (supine) to standing (upright) causes the mean ICP to fall by just over 50% (Figure 1). The mean ICP is similar in the standing (upright) position to sitting (upright) position.4 ICP measured in the left lateral decubitus position that is recommended for lumbar puncture opening pressure measurements is observed to increase by approximately 13% over that of supine ICP measurement.4,31–34 Neck flexion and hip flexion in combination have been shown to have a significant effect on increasing ICP in the left lateral decubitus position.5 These findings have important implications for conditions such as IIH, where the diagnostic criteria stipulate that the lumbar puncture should be measured in the left lateral decubitus position with the hips flexed at 90°.35 Most studies have documented a rise in ICP in the supine position for a prolonged duration of time.4,22 There is still debate regarding whether ICP changes due to the sleep-wake and circadian rhythm. A recent mechanistic study has reported increased CSF secretion that is likely influenced by circadian rhythm and not the sleep-wake cycle.36 ICP is acutely affected by Valsalva manoeuvres like coughing and bending (Figures 2 and 3). In such actions, ICP increases substantially and returns to baseline levels within seconds.4
Understanding the ICP Waveform
ICP waveform contains valuable information about the physiology of CSF dynamics and the cerebrospinal pathophysiology (Table 2 and Figure 1). In general, there is a positive correlation between mean ICP and pulsatile ICP, with higher values indicating lower brain compliance and vice versa. Fluctuations in the pulsatile ICP also occur with postural changes, with higher values observed in the upright compared to the supine position.19,34,37 It is challenging to explain this observation from the mechanistic perspective given that compliance is considered lower in the supine position, and fine volume-pressure regulations at the glia–neuro-vascular interface may be of particular significance here.38
 |
Table 2 Waveform Terms |
Methods of Invasive ICP Measurement
There are currently many methods of measuring ICP both invasively and non-invasively. Intraventricular ICP monitoring with an external ventricular drain is considered a gold standard measurement.3,39 Invasive ICP monitoring is accurate with the ability for continuous monitoring but has known clinical complications of misplacement, risk of hemorrhage and a cumulative risk of infection.40 There is not a universally accepted practice for setting the reference level and hence, in some cases, institutional data lacks standardization for confidentially comparison.
Lumbar Puncture
Lumbar puncture is the most common method for assessing CSF pressure. There is debate regarding the normal range. In one study, the 95% reference interval for CSF opening pressure was 10 to 25 cm CSF, and they noted that body mass index had a small impact on the CSF opening pressure.41 Few studies have investigated normal populations.42–44 In the largest population study to date, investigating aging, the 95% reference interval was found to be 82–242 mmH2O.42 Lumbar puncture has been found to be safe with rare long-term complications.45 Common complications are post lumbar puncture headache and back pain.45,46 Very rare complications include cranial neuropathies, nerve root irritation, infections, cerebral herniation, and bleeding complications.45,47 Guidelines help minimise the risks and discomfort.48
Lumbar puncture is relied on as a diagnostic criterion for idiopathic intracranial hypertension and idiopathic intracranial hypertension without papilloedema.35,49 There have been detailed discussions on the threshold for the diagnosis of in both adults and children.24,35,50 In the United Kingdom, consensus amongst neurologists, neuro-ophthalmologists and neurosurgeons who manage idiopathic intracranial hypertension suggest a “grey zone” between an opening pressure of 25 and 30cmH20, and with an increased opening pressure pathology more likely.24 A further factor for those living with obesity and idiopathic intracranial hypertension is the influence of body mass index, where it is well documented that the CSF pressure has been found to be increased with body mass index.42,44,51 In a paediatric study, CSF opening pressure increased by 3cmH20 for every 10 unit increase in body mass index.52 Lumbar puncture closing pressure is often quoted; however, there is little clinical significance of this measure, unless it is extremely low. Serial lumbar puncture for therapeutic reasons in idiopathic intracranial hypertension is no longer recommended, primarily because the entire volume of CSF is replaced every 6–7 hours.46,53,54 However, in pregnancy with increasing papilloedema in the first trimester, serial lumbar puncture has been recommended as a temporising measure.55,56
ICP Monitoring Devices
Microtransducer ICP monitoring devices are becoming more frequently used. These have the ability to monitor ICP in various locations such as the parenchyma, epidural space, subdural space, subarachnoid space and ventricles. They can be characterized by their method of measurement using fiber optic devices (CaminoTM ICP monitor (Integra LifeSciences, Plainsboro, NJ, USA)), strain gauge devices (CodmanTM Microsensor (Codman and Shurtleff Inc., Raynham, MA, USA), RaumedicTM Neurovent P-Senor (Raumedic AG, Helmbrechts, Germany)) and PressioTM (Sophysa, Orsay, France), and pneumatic sensor (SpiegelbergTM (Spiegelberg GmbH & Co KG, Hamburg, Germany)). The M.scio (MiethkeTM, Potsdam, Germany) is a telesensor that has a measuring cell housed within a reservoir that can also be integrated into a shunt. The systems have sampling frequencies 5–200 Hz and have been shown to be accurate with low incidences of complications.18,57–60
All the ICP monitors have a safety profile that includes known complications of misplacement, intracerebral hemorrhage and infection.40 Another challenge is zero level drift, especially with intraparenchymal monitors secondary to gliosis around the probe over time, that may affect the accuracy of the ICP reading. Not all ICP monitors are compatible with magnetic resonance imaging (MRI) as they contain ferromagnetic components (CaminoTM and PressioTM sensor), whereas the Codman MicroSensor, Neurovent-p-TelTM, MiethkeTM M.scio and the Spiegelberg sensor are MRI safe.
These wireless telemetric devices are becoming increasingly used in research and clinical practice.61 The ability to interrogate the ICP without having to perform additional procedures (ie, repeated lumbar punctures) has been particularly beneficial in research where the CSF pressure has been used as the primary outcome.13,62 In clinical practice, having a telemetric ICP monitor has been instrumental in understanding how the ICP changes as a person when idiopathic intracranial hypertension becomes fulminant.63 ICP telemetry also is of particular importance in IIH headache. The differentiation between phenotypes of the raised ICP headache and non-pressure related headaches such as migrainous headaches or medication over-use headache is a challenge and can lead to over investigation.64,65
The ICP monitors used in line with a ventricular shunt, such as M.scio telesensor (Aesculap-Miethke, Germany), have clear clinical utility in avoiding excessive valve adjustment where symptoms of over- and under-drainage are reported; in exclusion of shunt malfunction; and to reassure the patient who presents with exacerbation of headache.14,66 While the number of CSF diversion surgeries for idiopathic intracranial hypertension has declined over the last decade,67 the COVID-19 pandemic saw an acute rise in people with IIH presenting with sight threatening disease.68 This leads in one neuroscience centre to an opportunity to evaluate the M.scio telesensor (Aesculap-Miethke, Germany) in a large cohort of people with idiopathic intracranial hypertension requiring surgery for sight threatening papilloedema.37 In the cohort of fifty-seven people, 37 had the proGAV2.0 set at 15 cmH2O and 20 had the proGAV2.0 set at 10 cmH2O. The average value of mean sitting ICP was −2.7 mmHg versus −5.6 mmHg (p = 0.08), and mean supine ICP was 18.4 mmHg versus 12.9 mmHg (p = 0.001) at the valve settings of 15 cmH2O versus 10 cmH2O, respectively.
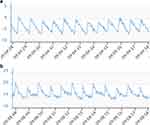
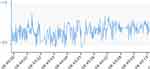
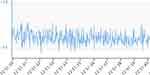
Interpretation of the ICP recording with this technology requires assessment of both mean and pulsatile ICP. The presence of a pulsatile ICP waveform indicates that there is patency of the proximal catheter, and the interpretation is clinically meaningful. A mean ICP and a pulsatile ICP within a range expected in the specific patient population with P1>P2 (Figure 4a and b) are suggestive of a functioning shunt with a compliant intracranial compartment. P1 with ICP values still within the expected range (Figure 2) may indicate low compliance, in which case adjusting the valve to drain more CSF may help, but further research on waveform analysis is needed to explore this assumption. A high mean and pulsatile ICP with P1<P2 (Figure 3) is typical of blockage distal to the telesensor with reduced compliance. Low mean ICP is suggestive of over-drainage and should be assessed with possible postural symptoms. The absence of pulsatility is more challenging to interpret because it can occur in both patent and proximally blocked shunts (Figures 5 and 6), but this scenario is less common. A possible explanation of the absence of pulsatility in patent shunts is intermittent collapse of the ventricular wall on the catheter.37
Surrogate Methods of Measuring Intracranial Pressure
There are many non-invasive methods for measuring ICP that have emerged. Most of the work centres around an ICP level of >20cmH20, as if the ICP is sustained at this level worse outcomes are reported for many intracranial conditions.3 Ultrasound measurement of the optic nerve sheath diameter requires high-quality ultrasound images performed by an expert. As the optic nerve sheath is distensible, CSF pressure variations influence the volume of CSF around the optic nerve particularly in the anterior, retro-bulbar compartment, about 3 mm behind the globe. A linear relationship between peri-optic CSF pressure and ICP has been found.69–71 The threshold to detect ICP >20 cm H20 has been reported to be an optic nerve sheath diameter of ≥5 mm, with a sensitivity of 88% and specificity of 93%.72 Limitations of ultrasound to assess the optic nerve sheath include other hypoechoic artifacts that can be confused with the optic nerve-optic nerve sheath complex, interexamination variability based on sonographer experience, and the small size of the structure relative to the tiny differences differentiating normal vs abnormal optic nerve sheath diameters.71 Transcranial Doppler measures the blood flow characteristics and cerebrovascular hemodynamics within the basal arteries of the brain. The transcranial Doppler waveform can provide a calculated non-invasive measure of ICP. ICP measured by transcranial Doppler has a reported sensitivity of 92.3%, and a specificity of 70%.73 It is highly operator dependent and requires detailed three-dimensional knowledge of cerebrovascular anatomy.
Optical Coherence Tomography (OCT) imaging of the retina and optic nerve has a central role in managing papilloedema and a number of measures have been found to correlate with ICP.24,74 A positive association between the optic nerve head volume OCT measures and ICP has been found. Surrogacy analysis demonstrated its ability to inform ICP changes, at 12 months reported decrease in central thickness of 50 μm was associated with a decrease in ICP of 5 cmH2O.74 Very minor changes can be detected on OCT, particularly in the presence of optic atrophy that can help guide management.75 Assessment of the peripapillary Bruch’s membrane angle and the optic nerve head height has been found to predict elevated mean ICP wave amplitude representing pulsatile ICP and mean ICP in another study.76 OCT imaging has also been used for the sole for detection of raised ICP utilising the infrared videos documenting spontaneous venous pulsations.77 While OCT imaging is ubiquitous in eye clinics all over the world, the majority of devices are table top with only a few flexible enough for supine measurements.78 Care must also be taken that the images are segmented properly before readings are taken, as proprietary software is prone to artefact at the optic nerve head.79
Neuro-imaging provides recognised features of raised ICP such as changes in venous sinuses calibre, stenosis of transverse sinus, empty or partially empty sella turcica, and volumetric alterations of the optic nerve, including optic nerve tortuosity, optic nerve sheath distension and posterior optic globe-sclera flattening; MRI is now being used to predict ICP.80,81 MRI-ICP is an advanced quantitative MRI technique that enables calculation of compliance: it utilises phase-contrast sequences to measure flow of cerebrospinal fluid (CSF), internal carotid arteries, vertebral arteries, and internal jugular veins. This information is then used to compute intracranial volume and pressure changes, ie, compliance.82 This has recently been evaluated in IIH and found to replicate well-established physiological relations with opening pressure. The technique also showed a biologically plausible compliance reduction in IIH which might contribute to headache disability. This study also found that compliance improved with successful treatment.83 While further work is required to evaluate these findings in IIH, MRI remains a relative inaccessible method for routine measures of ICP.
Conclusion
Neuro-ophthalmologists need to be aware of the increasing sophistication of the technologies that are being used to measure ICP, and understand the clinical utility of the wireless ICP monitors. Future work will focus on interpretation of the morphology of the ICP waveform and understanding brain compliance in neuro-ophthalmic disorders affected by CSF disorders.
Disclosure
Professor Alexandra Sinclair reports personal fees from Invex therapeutics in her role as Director with stock holdings. Honoria from Allergan, Novartis, Chiesi and Amgen outside the submitted work. Professor Susan Mollan has received honoraria for speaker events from AbbVie, Heidelberg engineering, Chugai-Roche Ltd and Teva. Honoraria for advisory boards for Invex Therapeutics, Gensight and ocular therapeutix. Consultancy fees from the Velux foundation, Neurodiem and Invex Therapeutics. She also reports grants from National institute of health research and UK space agency. Ms Olivia Grech reports scientific consultancy fee from Index Therapeutics. Professor Alex Sinclair owns shares from Invex Therapeutics. Dr Georgios Tsermoulas was an invited speaker to a “lunch symposium” sponsored by Braun in the Hydrocephalus 2022 meeting in Gothenburg on Mon 12th Sep 2022 and presented on “Intracranial pressure recordings in shunted patients with Idiopathic Intracranial Hypertension”. However, he did not receive any remuneration or any other benefits in relation to this presentation. All declared interested are outside the area of this submitted work. All other authors declare no competing interests in this work.
References
1. Bothwell SW, Janigro D, Patabendige A. Cerebrospinal fluid dynamics and intracranial pressure elevation in neurological diseases. Fluids Barriers CNS. 2019;16:9.
2. Loman J, Myerson A, Goldman D. Effects of alterations in posture on the cerebrospinal fluid pressure. Arch Neur Psych. 1935;33:1279–1295. doi:10.1001/archneurpsyc.1935.02250180138007
3. Czosnyka M, Pickard JD. Monitoring and interpretation of intracranial pressure. J Neurol Neurosurg Psychiatry. 2004;75:813–821. doi:10.1136/jnnp.2003.033126
4. Mitchell JL, Buckham R, Lyons H, et al. Evaluation of diurnal and postural intracranial pressure employing telemetric monitoring in idiopathic intracranial hypertension. Fluids Barriers CNS. 2022;19:85. doi:10.1186/s12987-022-00384-2
5. Pedersen SH, Andresen M, Lilja-Cyron A, et al. Lumbar puncture position influences intracranial pressure. Acta Neurochir. 2021;163:1997–2004. doi:10.1007/s00701-021-04813-3
6. Avery RA. Interpretation of lumbar puncture opening pressure measurements in children. J Neuroophthalmol. 2014;34:284–287. doi:10.1097/WNO.0000000000000154
7. Pedersen SSH, Jørgensen AL, Andresen M, et al. Differences in intracranial pressure seen in children and adults could be caused by age differences. Fluids Barriers CNS. 2015;12(Suppl 1):O21. doi:10.1186/2045-8118-12-S1-O21
8. Thaller M, Homer V, Hyder Y, et al. The idiopathic intracranial hypertension prospective cohort study: evaluation of prognostic factors and outcomes. J Neurol. 2023;270:851–863. doi:10.1007/s00415-022-11402-6
9. Hyder YF, Homer V, Thaller M, et al. Defining the phenotype and prognosis of people with idiopathic intracranial hypertension after cerebrospinal fluid diversion surgery. Am J Ophthalmol. 2023;250:70–81. doi:10.1016/j.ajo.2023.01.016
10. Mollan SP, Wakerley BR, Alimajstorovic Z, et al. Intracranial pressure directly predicts headache morbidity in idiopathic intracranial hypertension. J Headache Pain. 2021;22:118. doi:10.1186/s10194-021-01321-8
11. Grech O, Clouter A, Mitchell JL, et al. Cognitive performance in idiopathic intracranial hypertension and relevance of intracranial pressure. Brain Commun. 2021;3(3):fcab202. doi:10.1093/braincomms/fcab202
12. Mulla Y, Markey KA, Woolley RL, et al. Headache determines quality of life in idiopathic intracranial hypertension. J Headache Pain. 2015;16:521. doi:10.1186/s10194-015-0521-9
13. Mollan SP, Sinclair AJ. Outcomes measures in idiopathic intracranial hypertension. Expert Rev Neurother. 2021;21:687–700. doi:10.1080/14737175.2021.1931127
14. Tsermoulas G, Thant KZ, Byrne ME, et al. The Birmingham standardized idiopathic intracranial hypertension shunt protocol: technical note. World Neurosurg. 2022;167:147–151. doi:10.1016/j.wneu.2022.08.154
15. Sakka L, Coll G, Chazal J. Anatomy and physiology of cerebrospinal fluid. Eur Ann Otorhinolaryngol Head Neck Dis. 2011;128:309–316. doi:10.1016/j.anorl.2011.03.002
16. Wilson MH. Monro-Kellie 2.0: the dynamic vascular and venous pathophysiological components of intracranial pressure. J Cereb Blood Flow Metab. 2016;36:1338–1350. doi:10.1177/0271678X16648711
17. Sagirov AF, Sergeev TV, Shabrov AV, et al. Postural influence on intracranial fluid dynamics: an overview. J Physiol Anthropol. 2023;42:5. doi:10.1186/s40101-023-00323-6
18. Raboel P, Bartek J, Andresen M, et al. Intracranial pressure monitoring: invasive versus non-invasive methods—a review. Crit Care Res Pract. 2012;2012:1–14. doi:10.1155/2012/950393
19. Chari A, Dasgupta D, Smedley A, et al. Intraparenchymal intracranial pressure monitoring for hydrocephalus and cerebrospinal fluid disorders. Acta Neurochir. 2017;159:1967–1978. doi:10.1007/s00701-017-3281-2
20. Andresen M, Hadi A, Juhler M. Evaluation of intracranial pressure in different body postures and disease entities. Acta Neurochir Suppl. 2016;122:45–47.
21. Andresen M, Juhler M. Intracranial pressure following complete removal of a small demarcated brain tumor: a model for normal intracranial pressure in humans. J Neurosurg. 2014;121:797–801. doi:10.3171/2014.2.JNS132209
22. Pedersen SH, Lilja-Cyron A, Andresen M, et al. The relationship between intracranial pressure and age—chasing age-related reference values. World Neurosurg. 2017;110:e119–23. doi:10.1016/j.wneu.2017.10.086
23. Saehle T, Eide PK. Intracranial pressure monitoring in pediatric and adult patients with hydrocephalus and tentative shunt failure: a single-center experience over 10 years in 146 patients. J Neurosurg. 2015;122:1076–1086. doi:10.3171/2014.12.JNS141029
24. Mollan SP, Davies B, Silver NC, et al. Idiopathic intracranial hypertension: consensus guidelines on management. J Neurol Neurosurg Psychiatry. 2018;89:1088–1100. doi:10.1136/jnnp-2017-317440
25. Kochanek PM, Tasker RC, Carney N, et al. Guidelines for the management of pediatric severe traumatic brain injury, third edition. Pediatr Crit Care Med. 2019;20:S1–82. doi:10.1097/PCC.0000000000001735
26. Wagshul ME, Eide PK, Madsen JR. The pulsating brain: a review of experimental and clinical studies of intracranial pulsatility. Fluids Barriers CNS. 2011;8:5. doi:10.1186/2045-8118-8-5
27. Eide PK, Sorteberg W. Diagnostic intracranial pressure monitoring and surgical management in idiopathic normal pressure hydrocephalus: a 6-year review of 214 patients. Neurosurgery. 2010;66:80–91. doi:10.1227/01.NEU.0000363408.69856.B8
28. Saehle T, Eide PK. Characteristics of intracranial pressure (ICP) waves and ICP in children with treatment-responsive hydrocephalus. Acta Neurochir. 2015;157:1003–1014. doi:10.1007/s00701-015-2410-z
29. Czosnyka Z, Czosnyka M. Long-term monitoring of intracranial pressure in normal pressure hydrocephalus and other CSF disorders. Acta Neurochir. 2017;159:1979–1980. doi:10.1007/s00701-017-3282-1
30. Magnaes B. Body position and cerebrospinal fluid pressure. Part 1: clinical studies on the effect of rapid postural changes. J Neurosurg. 1976;44:687–697. doi:10.3171/jns.1976.44.6.0687
31. Andresen M, Hadi A, Petersen LG, et al. Effect of postural changes on ICP in healthy and ill subjects. Acta Neurochir. 2015;157:109–113. doi:10.1007/s00701-014-2250-2
32. Petersen LG, Petersen JCG, Andresen M, et al. Postural influence on intracranial and cerebral perfusion pressure in ambulatory neurosurgical patients. Am J Physiol Regul Integr Comp Physiol. 2016;310:R100–4. doi:10.1152/ajpregu.00302.2015
33. Eklund A, Jóhannesson G, Johansson E, et al. The pressure difference between eye and brain changes with posture. Ann Neurol. 2016;80:269–276. doi:10.1002/ana.24713
34. D’Antona L, Craven CL, Bremner F, et al. Effect of position on intracranial pressure and compliance: a cross-sectional study including 101 patients. J Neurosurg. 2021;136:1781–1789. doi:10.3171/2021.6.JNS203573
35. Friedman DI, Liu GT, Digre KB. Revised diagnostic criteria for the pseudotumor cerebri syndrome in adults and children. Neurology. 2013;81:1159–1165. doi:10.1212/WNL.0b013e3182a55f17
36. Steffensen AB, Edelbo BL, Barbuskaite D, et al. Nocturnal increase in cerebrospinal fluid secretion as a circadian regulator of intracranial pressure. Fluids Barriers CNS. 2023;20:49. doi:10.1186/s12987-023-00451-2
37. Afshari FT, Samara M, Thant KZ, et al. Interpretation of telemetric intracranial pressure recordings in people with idiopathic intracranial hypertension after shunt implantation. Acta Neurochir. 2023;165:1523–1531. doi:10.1007/s00701-023-05572-z
38. Eide PK. Abnormal intracranial pulse pressure amplitude despite normalized static intracranial pressure in idiopathic intracranial hypertension refractory to conservative medical therapy. Life. 2021;11:537. doi:10.3390/life11060537
39. Fried HI, Nathan BR, Rowe AS, et al. The insertion and management of external ventricular drains: an evidence-based consensus statement: a statement for healthcare professionals from the neurocritical care society. Neurocrit Care. 2016;24:61–81. doi:10.1007/s12028-015-0224-8
40. Tavakoli S, Peitz G, Ares W, et al. Complications of invasive intracranial pressure monitoring devices in neurocritical care. Neurosurg Focus. 2017;43:E6. doi:10.3171/2017.8.FOCUS17450
41. Whiteley W, Al-Shahi R, Warlow CP, et al. CSF opening pressure: reference interval and the effect of body mass index. Neurology. 2006;67:1690–1691.
42. Wang F, Lesser ER, Cutsforth-Gregory JK, et al. Population-based evaluation of lumbar puncture opening pressures. Front Neurol. 2019;10:899. doi:10.3389/fneur.2019.00899
43. Gilland O, Tourtellotte WW, O’Tauma L, Henderson WG. Normal cerebrospinal fluid pressure. J Neurosurg. 1974;40:587–593. doi:10.3171/jns.1974.40.5.0587
44. Malm J, Jacobsson J, Birgander R, Eklund A. Reference values for CSF outflow resistance and intracranial pressure in healthy elderly. Neurology. 2011;76(10):903–909. doi:10.1212/WNL.0b013e31820f2dd0
45. Duits FH, Martinez-Lage P, Paquet C, et al. The multicenter lumbar puncture feasibility study. Alzheimers Dement. 2016;12:154–163. doi:10.1016/j.jalz.2015.08.003
46. Yiangou A, Mitchell J, Markey KA, et al. Therapeutic lumbar puncture for headache in idiopathic intracranial hypertension: minimal gain, is it worth the pain? Cephalalgia. 2019;39:245–253. doi:10.1177/0333102418782192
47. Lyons HS, Ramalingam S, Mitchell JL, et al. Multiple lumbar punctures aiming to relieve headache results in iatrogenic spinal hematoma: a case report. J Med Case Rep. 2022;16:464. doi:10.1186/s13256-022-03687-y
48. Engelborghs S, Niemantsverdriet E, Struyfs H, et al. Consensus guidelines for lumbar puncture in patients with neurological diseases. Alzheimers Dement. 2017;8:111–126. doi:10.1016/j.dadm.2017.04.007
49. Mollan SP, Chong YJ, Grech O, et al. Current perspectives on idiopathic intracranial hypertension without papilloedema. Life. 2024;11:472.
50. Lyons HS, Mollan SP, Liu GT, et al. Different characteristics of pre-pubertal and post-pubertal idiopathic intracranial hypertension: a narrative review. Neuroophthalmology. 2022;47:63–74. doi:10.1080/01658107.2022.2153874
51. Fleischman D, Berdahl JP, Zaydlarova J, et al. Cerebrospinal fluid pressure decreases with older age. PLoS One. 2012;7:e52664. doi:10.1371/journal.pone.0052664
52. Seiden JA, Huh JW, Boswinkel J, et al. Reference range for cerebrospinal fluid opening pressure in children. N Engl J Med. 2010;363:891–893. doi:10.1056/NEJMc1004957
53. Scotton WJ, Mollan SP, Walters T, et al. Characterising the patient experience of diagnostic lumbar puncture in idiopathic intracranial hypertension: a cross-sectional online survey. BMJ Open. 2018;8:e020445. doi:10.1136/bmjopen-2017-020445
54. Hoffmann J, Mollan SP, Paemeleire K, et al. European headache federation guideline on idiopathic intracranial hypertension. J Headache Pain. 2018;19:93. doi:10.1186/s10194-018-0919-2
55. Thaller M, Homer V, Mollan SP, et al. Disease course and long-term outcomes in pregnant women with idiopathic intracranial hypertension: the iih prospective maternal health study. Neurology. 2023;100:e1598–e1610. doi:10.1212/WNL.0000000000206854
56. Thaller M, Wakerley BR, Abbott S, et al. Managing idiopathic intracranial hypertension in pregnancy: practical advice. Pract Neurol. 2022;22:295–300. doi:10.1136/practneurol-2021-003152
57. Gelabert-González M, Ginesta-Galan V, Sernamito-García R, et al. The Camino intracranial pressure device in clinical practice. Assessment in a 1000 cases. Acta Neurochirurgica. 2006;148:435–441. doi:10.1007/s00701-005-0683-3
58. Bekar A, Dogan S, Abas F, et al. Risk factors and complications of intracranial pressure monitoring with a fiberoptic device. J Clin Neurosci. 2009;16(2):236–240. doi:10.1016/j.jocn.2008.02.008
59. Koskinen LOD, Olivecrona M. Clinical experience with the intraparenchymal intracranial pressure monitoring Codman microsensor system. Neurosurgery. 2005;56(4):693–698. doi:10.1227/01.NEU.0000156609.95596.24
60. Bratton SL, Chesnut RM, Ghajar J, et al. Guidelines for the management of severe traumatic brain injury. VII. Intracranial pressure monitoring technology. J Neurotrauma. 2007;24(supplement 1):S45–S54. doi:10.1089/neu.2007.9989
61. Mitchell JL, Mollan SP, Vijay V, et al. Novel advances in monitoring and therapeutic approaches in idiopathic intracranial hypertension. Curr Opin Neurol. 2019;32:422–431. doi:10.1097/WCO.0000000000000690
62. Mitchell JL, Lyons HS, Walker JK, et al. The effect of GLP-1RA exenatide on idiopathic intracranial hypertension: a randomized clinical trial. Brain. 2023;146(5):1821–1830. doi:10.1093/brain/awad003
63. Mitchell JL, Mollan SP, Tsermoulas G, et al. Telemetric monitoring in idiopathic intracranial hypertension demonstrates intracranial pressure in a case with sight-threatening disease. Acta Neurochir. 2021;163:725–731. doi:10.1007/s00701-020-04640-y
64. Mollan SP, Grech O, Sinclair AJ. Headache attributed to idiopathic intracranial hypertension and persistent post-idiopathic intracranial hypertension headache: a narrative review. Headache. 2021;61:808–816. doi:10.1111/head.14125
65. Mollan SP, Spitzer D, Nicholl DJ. Raised intracranial pressure in those presenting with headache. BMJ. 2018;363:k3252. doi:10.1136/bmj.k3252
66. Galloway L, Karia K, White AM, et al. Cerebrospinal fluid shunting protocol for idiopathic intracranial hypertension for an improved revision rate. J Neurosurg. 2021;136:1790–1795. doi:10.3171/2021.5.JNS21821
67. Mollan SP, Mytton J, Tsermoulas G, et al. Idiopathic intracranial hypertension: evaluation of admissions and emergency readmissions through the hospital episode statistic dataset between 2002–2020. Life. 2021;5:417.
68. Thaller M, Tsermoulas G, Sun R, et al. Negative impact of COVID-19 lockdown on papilloedema and idiopathic intracranial hypertension. J Neurol Neurosurg Psychiatry. 2021;92:795–797. doi:10.1136/jnnp-2020-325519
69. Newman W, Hollman A, Dutton G, et al. Measurement of optic nerve sheath diameter by ultrasound: a means of detecting acute raised intracranial pressure in hydrocephalus. Br J Ophthalmol. 2002;86:1109–1113. doi:10.1136/bjo.86.10.1109
70. Rajajee V, Vanaman M, Fletcher JJ, et al. Optic nerve ultrasound for the detection of raised intracranial pressure. Neurocrit Care. 2011;15:506–515. doi:10.1007/s12028-011-9606-8
71. Hansen H-C, Helmke K. Validation of the optic nerve sheath response to changing cerebrospinal fluid pressure: ultrasound findings during intrathecal infusion tests. J Neurosurg. 1997;87:34–40. doi:10.3171/jns.1997.87.1.0034
72. Kimberly HH, Shah S, Marill K, et al. Correlation of optic nerve sheath diameter with direct measurement of intracranial pressure. Acad Emerg Med. 2008;15:201–204. doi:10.1111/j.1553-2712.2007.00031.x
73. Dokponou YCH, Badirou OBA, Agada KN, et al. Transcranial Doppler in the non-invasive estimation of intracranial pressure in traumatic brain injury compared to other non-invasive methods in lower-middle income countries: systematic review and meta-analysis. J Clin Neurosci. 2023;113:70–76. doi:10.1016/j.jocn.2023.05.010
74. Vijay V, Mollan SP, Mitchell JL, et al. Using optical coherence tomography as a surrogate of measurements of intracranial pressure in idiopathic intracranial hypertension. JAMA Ophthalmol. 2020;138(12):1264–1271. doi:10.1001/jamaophthalmol.2020.4242
75. Qureshi A, Virdee J, Tsermoulas G, et al. Optical coherence tomography confirms shunt malfunction and recurrence of raised intracranial pressure in optic atrophy. Br J Neurosurg. 2022;36:185–191. doi:10.1080/02688697.2020.1844146
76. Jacobsen HH, Jørstad ØK, Moe MC, et al. Noninvasive estimation of pulsatile and static intracranial pressure by optical coherence tomography. Transl Vis Sci Technol. 2022;11(1):31. doi:10.1167/tvst.11.1.31
77. D’Antona L, McHugh JA, Ricciardi F, et al. Association of intracranial pressure and spontaneous retinal venous pulsation. JAMA Neurol. 2019;76:1502–1505. doi:10.1001/jamaneurol.2019.2935
78. Liu X, Kale AU, Capewell N, et al. Optical coherence tomography (OCT) in unconscious and systemically unwell patients using a mobile OCT device: a pilot study. BMJ Open. 2019;9:e030882. doi:10.1136/bmjopen-2019-030882
79. Aojula A, Mollan SP, Horsburgh J, et al. Segmentation error in spectral domain optical coherence tomography measures of the retinal nerve fibre layer thickness in idiopathic intracranial hypertension. BMC Ophthalmol. 2018;17:257. doi:10.1186/s12886-017-0652-7
80. Witsberger EM, Huston J 3rd, Cutsforth-Gregory JK, et al. Population-based evaluation of indirect signs of increased intracranial pressure. J Neuroophthalmol. 2022;42:e63–e69. doi:10.1097/WNO.0000000000001329
81. Barkatullah AF, Leishangthem L, Moss HE, et al. MRI findings as markers of idiopathic intracranial hypertension. Curr Opin Neurol. 2021;34:75–83. doi:10.1097/WCO.0000000000000885
82. Alperin NJ, Lee SH, Loth F, et al. MR-intracranial pressure (ICP): a method to measure intracranial elastance and pressure noninvasively by means of MR imaging: baboon and human study. Radiology. 2000;217:877–885. doi:10.1148/radiology.217.3.r00dc42877
83. Sassini M, Lyons H, Yiangou A, et al. Non-invasive assessment of brain compliance in idiopathic intracranial hypertension: an MRI-ICP study. Eye. 2023;37:132–138. doi:10.1038/s41433-021-01838-x
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.