Back to Journals » Therapeutics and Clinical Risk Management » Volume 14
A multicentric pharmacovigilance study: collection and analysis of adverse drug reactions in relapsing-remitting multiple sclerosis patients
Authors Gugliandolo A, Longo F, Marrosu MG, Mancardi GL, Gandoglia I , Melis M, Lo Giudice F, Bramanti P, Mazzon E
Received 21 May 2018
Accepted for publication 15 July 2018
Published 26 September 2018 Volume 2018:14 Pages 1765—1788
DOI https://doi.org/10.2147/TCRM.S174864
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Garry Walsh
Agnese Gugliandolo,1 Federica Longo,1 Maria Giovanna Marrosu,2 Giovanni Luigi Mancardi,3,4 Ilaria Gandoglia,3 Maurizio Melis,5 Fabrizio Lo Giudice,1 Placido Bramanti,1 Emanuela Mazzon1
1Department of Experimental Neurology, IRCCS Centro Neurolesi “Bonino Pulejo”, Messina, Italy; 2Department of Medical Sciences and Public Health, University of Cagliari, Cagliari, Italy; 3Department of Neurosciences, Rehabilitation, Ophthalmology, Genetics, Maternal and Child Health (DINOGMI), University of Genoa, Genoa, Italy; 4IRCCS Azienda Ospedaliera Universitaria San Martino-IST, Genoa, Italy; 5SC Neurologia e Stroke Unit, Azienda Ospedaliera G Brotzu, Cagliari, Italy
Purpose: We performed a pharmacovigilance study of 10 drugs used in patients with relapsing-remitting multiple sclerosis (RR-MS). Our aim was to provide an overview of the safety of these drugs by the evaluation of reported expected and unexpected adverse reactions.
Patients and methods: We collected and analyzed adverse drug reactions from RR-MS patients belonging to four hospitals in three Italian regions, for a period of 24 months.
Results: We received a total of 411 adverse reactions, of which 84.18% were expected and only 15.82% were unexpected. We found no correlation between the number of reported adverse reactions and the route of administration (injectable/intravenous drugs N=224, oral drugs N=187). However, oral agents have caused a greater number of unexpected moderate-to-severe adverse reactions while, in injectable and infusion therapies, they have been evaluated as mild–moderate adverse reactions.
Conclusion: Our results underscore the importance of monitoring the safety profile of multiple sclerosis therapies, with particular attention to oral agents that have been introduced later in the clinical practice.
Keywords: pharmacovigilance, relapsing-remitting multiple sclerosis, adverse drug reaction, disease-modifying therapy, safety
Introduction
Multiple sclerosis (MS) is a chronic inflammatory autoimmune demyelinating disease of the central nervous system (CNS), affecting more than 2.3 million people in the world, with a predominance in women (female:male ratio 2:1, but some reports also indicated 3:1).1–3 This disease is associated with different symptoms, depending on the location of the areas of demyelination in the CNS. The most frequent symptoms are as follows: fatigue, pain, bladder and bowel dysfunctions, visual problems, depression, spasticity, sexual dysfunctions, and movement and coordination problems.
Relapsing-remitting MS (RR-MS) is the most common MS form. About 85% of people have initially this form, characterized by symptomatic attacks (relapses) followed by partial or total recovery (remissions).2 In most patients, the disease develops gradually into a chronic progressive phase, characterized by continuous accumulation of neurological impairments. Disease-modifying therapies (DMTs) are used with the purpose to prevent or reduce the number of relapses occurring in RR-MS and delay the progression of the disease.
DMTs include injectable drugs (interferon β-1a Avonex® [IFN β-1a-A], interferon β-1a Rebif® [IFN β-1a-R], interferon β-1b Betaferon® [IFN β-1b-B], interferon β-1b Extavia® [IFN β-1b-E], peginterferon β-1a [PEG IFN β-1a], and glatiramer acetate [GA]), intravenous therapies (natalizumab [NAT]), and oral agents (fingolimod [FTY720], teriflunomide, and dimethyl fumarate [DMF]).
All of these drugs showed immunomodulatory and anti-inflammatory properties, although using various mechanisms of action (MOAs). Interferons (IFNs) β exert their effect by promoting the anti-inflammatory cytokines, the inhibition of T-cell proliferation, and the reduction of inflammatory cell migration across the blood–brain barrier (BBB).4
The monoclonal antibody NAT inhibits leukocyte migration into the CNS, attenuating inflammation.5
GA is composed of synthetic peptides that simulate sequences of myelin, preventing the attack by immune system against this protein.6
Teriflunomide reduces the proliferation of T and B cells.7 Similarly, FTY720 is also able to reduce the number of circulating lymphocytes and to inhibit their migration into the CNS.8 DMF protects neuronal cells reducing oxidative stress and activating Nrf2.9 The characteristics and MOAs of each drug are summarized in Table 1.
Based on their marketing authorization and their individual risk–benefit profile, MS drugs can be categorized into first- and second-line treatments. IFNs β and GA, as well as DMF and teriflunomide, are adopted as first-line therapies. In case of unsatisfactory response or safety issues with these drugs, the treatment can turn to second-line therapies, such as NAT and FTY720.10 This distinction does not coincide perfectly in different countries. For example, FTY720 is approved as a first-line treatment in the United States, while it is labeled as a second-line drug in the European Union.11
The efficacy and the safety profile of these therapies vary from a moderate efficacy and high safety of first-line drugs to a higher efficacy closely associated with an increased safety risk of second-line treatments. In a previous study, both oral and injectable DMTs were reported to be well tolerated.12 Among the oral agents, FTY720 presents more safety issues, requiring the monitoring of infections, cancers, lymphocyte, and levels of liver enzymes.13
In this scenario, pharmacovigilance becomes a fundamental source for the acquisition of new data, in order to deepen and constantly update the drug safety profile. Indeed, safety problems of some therapies only became evident during extension and postmarketing studies. This means that a more precise benefit–risk ratio is possible when a postmarketing surveillance is performed.
This postmarketing observational study shows the results of a multicentric pharmacovigilance program, sponsored by the Italian Drug Agency (AIFA). The aim of this study was to analyze expected and unexpected adverse drug reactions in RR-MS patients treated with DMTs, in order to evaluate their safety profile.
Patients and methods
We performed a multicentric study involving three Italian regions (Liguria, Sicily, and Sardinia) for a duration of 24 months. Specifically, we collected adverse drug reaction reports from RR-MS patients belonging to four hospitals, of which two hospitals are located in Cagliari, one hospital is located in Messina, and one hospital is located in Genoa.
We monitored the following drugs: IFN β-1a-A, IFN β-1a-R, IFN β-1b-B, IFN β-1b-E, PEG IFN β-1a, GA, NAT, FTY720, teriflunomide, and DMF.
For each patient reporting adverse drug reactions, we collected personal data (age and sex) and medical history. Specifically, we recorded the presence of any comorbidities, previous MS therapies, and the number of doses taken before the adverse reaction onset.
All the reports received during our study were analyzed in order to evaluate the expected and unexpected adverse reactions occurred. In particular, an adverse drug reaction refers to an injury caused by taking a drug and we considered as expected any adverse effect already listed on the summary of product characteristics available on European Medicines Agency (EMA) and AIFA websites.14–23 On the contrary, we assessed an event as unexpected if it was not consistent with the drug product information.
Finally, all the adverse reactions were classified in relation to the degree of intensity. We decided to consider “severe” any serious interference with the daily activity performance that required medical intervention/therapy and may need hospitalization; “moderate” any mild or moderate interference with the daily activity performance that could need a minimal medical intervention/therapy; and “mild” each event causing no interference with the daily activity performance and requiring no medical intervention/therapy.
Approval for this study is not required because it was authorized by AIFA and supported by funds from the pharmacovigilance multiregional project. Nevertheless, the privacy of all patients was guaranteed.
Statistical analysis
Statistical analysis of the data was performed using the GraphPad Prism Version 6.0 program (GraphPad Software, Inc., La Jolla, CA, USA). Statistical significance was measured by chi-squared test. P-value <0.05 was considered statistically significant.
Results
The cohort examined in the four hospitals participating in this study was composed of 6,039 RR-MS patients. We received 265 adverse drug reaction reports from RR-MS patients (205 women and 60 men; average age 42.06±10.33 years). The distribution of RR-MS patients reporting adverse reactions in relation to age and sex is reported in Figures 1 and 2. Many subjects reported more than one adverse event, and for this reason, we have collected 411 reactions. Among these, 346 reactions have already been associated with the monitored drugs (Table 2 and Figure 3), while 65 reactions were unexpected (Table 3 and Figure 3). We observed that the choice to discontinue the therapy was mainly driven by the adverse reaction severity, even when the treatment was effective in improving the patient condition.
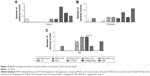
  | Figure 2 (A–E) Distribution of RR-MS population reporting adverse reactions in relation to age and sex. |
Some of the patients had received previously other therapies, as indicated in Tables 2 and 3. We can underline that the previous therapies included IFNs in most cases. On the contrary, almost all patients of our cohort treated with IFNs did not receive previous therapies, except for PEG IFN β-1a, in line with the fact that IFNs represent first-line treatment. Looking at the different therapies used, we can observe that the patients treated with three or more drugs showed mostly adverse events of moderate intensity. However, it was not possible to evaluate the influence of the sequence of different administered drugs on the appearance of the adverse reactions, because we had a small cohort of patients and dividing them on the bases of the sequence of drugs used caused a further fragmentation of patients in little groups, making difficult to reach a significant and valid result.
IFNs
In our study, about 42% of adverse drug reaction reports were attributed to IFNs. Specifically, we collected 154 adverse effects, among which only about 9% were unexpected. In relation to the severity degree, 5.84% were recorded as severe events.
IFN β-1a
We received 23 reports of IFN β-1a-A, involving patients in 30.43% of cases suffered from comorbidities. For the 23 reports received, 21 reported only expected adverse events (Table 2), one reported only unexpected reactions (Table 3), while the other one showed both expected and unexpected events (Tables 2 and 3). We recorded 30 adverse effects related to IFN β-1a-A (1.3 adverse drug reactions per report, Figure 3), of which two were unexpected (Figures 4 and 5). Specifically, these reactions were a moderate irritable mood and a severe erectile dysfunction (Table 3).
In regard of expected adverse events, for IFN β-1a-A, the value was significantly higher compared to the expected adverse events with NAT and FTY720. In particular, we received a significantly lower number of expected adverse events of moderate grade compared to IFN β-1a-R, IFN β-1b-B, PEG IFN β-1a, GA, FTY720, teriflunomide, and DMF. On the contrary, the number of those of mild grade was significantly higher compared to the mild adverse events recorded for IFN β-1a-R, IFN β-1b-B, GA, FTY720, and teriflunomide (Figure 4).
Generally, the most common adverse effects during IFN β-1a-A therapy are flu-like symptoms and injection site reactions. Flu-like syndrome has also been frequently reported in our study (N=10) mainly of mild severity, and only in three cases, it was necessary to stop the drug administration. Injection site reactions were less common and recorded as a severe necrosis (N=1) leading to drug discontinuation and a moderate bruising (N=1).
General disorders induced by IFN β-1a-A were also collected, such as pyrexia (N=2), malaise (N=1), and pain (N=1), as well as musculoskeletal symptoms such as myalgia (N=2) and arthralgia (N=2). Other expected adverse events were as follows: vomiting (N=1), leukopenia (N=1), pruritus (N=1), diarrhea (N=1), tachycardia (N=1), mixed anxiety-depressive disorder (N=1), and hepatic transaminases increased (N=1). Finally, we recorded a severe nephrotic syndrome (N=1) reported by a 44-year-old female patient who had undergone 104 administrations before the reaction onset. This event is considered among the expected but rare adverse effect, and it resulted in the suspension of IFN β-1a-A.
From the reports received, it emerged that IFN β-1a-A was permanently discontinued in 34.78% of cases due to the adverse reactions, while in 4.35%, it was decided to suspend the treatment only temporarily. On the contrary, patients continued the drug administration in 60.87% of reports.
For IFN β-1a-R, we received 31 adverse drug reaction reports. Looking at expected adverse events, IFN β-1a-R showed a significantly higher number of reactions compared to NAT and FTY720. We observed only one expected adverse reaction of severe grade, showing a statistical significance compared to FTY720. The number of expected adverse reactions of moderate grade for IFN β-1a-R was significant compared to those for DMF and IFN β-1a-A. Those of mild grade were significantly higher compared to GA but lower compared to IFN β-1a-A (Figure 4).
In 48.38% of cases, patients reporting adverse events (Figure 1B) were affected by concomitant diseases (Tables 2 and 3).
We collected 36 adverse reactions related to IFN β-1a-R (1.2 adverse drug reactions per report), of which 32 adverse reactions were expected and four adverse reactions were unexpected (Figure 3). The most common adverse effects observed were flu-like symptoms (N=8) and headache (N=7). Injection site reactions were signaled in 16.12% of reports. In particular, these effects were reported as abscess (N=1), pain (N=1), redness (N=1), erythema (N=1), and necrosis (N=1) of mild–moderate intensity.
IFN β-1a-R is frequently associated with hepatic dysfunction, but in our study, transaminases’ elevation was reported a few times (N=3), as a moderate reaction never leads to drug discontinuation. General disorders were also observed, such as pyrexia (N=1) and asthenia (N=2), as well as musculoskeletal reactions of arthralgia (N=1), musculoskeletal stiffness (N=1), and pain in extremity (N=1). Moreover, we recorded mild insomnia (N=2) and severe urticaria (N=1).
Even a patient treated with IFN β-1a-R experienced an erectile dysfunction and following this adverse unexpected event, the drug was discontinued. The other recorded unexpected effects were all moderate and related to tachycardia (N=1), panic attacks (N=1), and nervousness (N=1).
Our evaluation showed that IFN β-1a-R was definitively discontinued in 35.48% of reports. The total number of unexpected adverse drug reactions for both IFN β-1a-A and IFN β-1a-R was significantly lower compared to NAT and FTY720 (Figure 3).
IFN β-1b
A total of 15 reports involving IFN β-1b-B were received in 24 months, 40% of which referred to subjects suffering from comorbidities (Tables 2 and 3).
We collected 21 adverse effects that were all expected (1.4 adverse drug reactions per report; Figure 3), a significantly higher number compared with expected adverse reactions for IFN β-1b-E, NAT, and FTY720. The most common referred to injection site reactions, found in 60% of reports, and described as pain (N=5), mass (N=2), bruising (N=1), skin reaction (N=1), and abscess (N=1) of mild–moderate intensity. Additionally, ~33.3% of cases were related to general disorders, such as flu-like symptoms (N=1), pyrexia (N=2), asthenia (N=1), and malaise (N=1). Moreover, headache (N=2), myasthenia (N=1), paresthesia (N=1), lymphopenia (N=1), and γ-glutamyl-transferase increased (N=1) were also recorded. Looking at the severity, only one severe expected reaction was received, with a significant difference compared to FTY720. Moderate grade expected reactions were significantly higher compared to those for IFN β-1a-A, while the number of those of mild grade was significantly lower compared to the same drug (Table 2, Figure 4).
Among the reports analyzed, 73.3% resolved to IFN β-1b-B discontinuation due to the adverse reaction onset.
IFN β-1b-E showed the lowest number of expected adverse events, and in particular only one of mild grade, a data statistically significant compared to GA. During our project, we received only two reports involving IFN β-1b-E. The first case was related to a subject with no concurrent disease who experienced a moderate abdominal pain and an unexpected moderate rachialgia, because of which the drug was discontinued. The other report involved a patient who suffered from depression and disk herniation as comorbidities and experienced an unexpected urticaria of mild severity not leading to drug discontinuation (Tables 2 and 3).
IFN β-1b-E showed a significant elevated number of unexpected adverse drug reactions compared to IFN β-1b-B (Figure 3).
PEG IFN β-1a
Among the different IFNs β, the highest number of reports we received was attributed to PEG IFN β-1a (N=42). The number of expected adverse events was significantly higher compared to those for NAT and FTY720. Regarding the severity grade, PEG IFN β-1a showed a significantly lower number of severe expected adverse events compared to those with GA and FTY720 but a significantly higher number of expected reactions of mild grade compared to the same therapies. The number of expected moderate reactions was significantly higher compared to IFN β-1a-A but lower compared to GA (Figure 4).
Patients reporting adverse effects suffered from concomitant diseases in 38% of cases (Tables 2 and 3).
We collected 64 adverse reactions (1.5 adverse drug reactions per report, Figure 3), of which 57 adverse reactions have previously been associated with PEG IFN β-1a (Figures 3 and 4 and Table 2) and seven adverse reactions were unexpected (Figures 3 and 5 and Table 3). Specifically, we noticed flu-like syndrome (N=17) in 40.47% of reports, with an intensity ranging from mild to severe. An equal percentage was related to mild–moderate injection site reactions, described as redness (N=9), rash (N=5), erythema (N=2), and necrosis (N=1). General disorders of pyrexia (N=6) and asthenia (N=2) also commonly occurred, as well as myalgia (N=4), arthralgia (N=3), urticaria (N=3), lymphopenia (N=3), and thrombocytopenia (N=1). Moreover, a moderate elevation of hepatic transaminases (N=1) was recorded.
All the reported unexpected reactions had a mild–moderate severity and referred to oral aphthae (N=1), eyelid edema (N=1), localized erythema (N=1), unmotivated anger (N=1), confusional state (N=1), diarrhea (N=1), and abdominal pain (N=1).
As a consequence of the adverse reaction onset, it was decided PEG IFN β-1a discontinuation in 26.83% of reports.
GA
A significantly higher number of expected adverse events were reported for GA compared to NAT and FTY720. The majority were of moderate grade. In particular, the number of moderate grade expected adverse reactions was significantly higher compared to those received with IFN β-1a-A, PEG IFN β-1a, and DMF. The number of mild reactions was significant compared to the received mild expected events for IFN β-1b-E and NAT and significantly lower compared to IFN β-1a-A, IFN β-1a-R, PEG IFN β-1a, teriflunomide, and DMF. The number of expected adverse reactions of severe grade for GA was significantly higher compared to PEG β-1a (Figure 4).
We received 30 reports involving GA-treated patients who suffered from concurrent diseases in 46.6% of cases (Tables 2 and 3).
We counted an amount of 59 adverse reactions attributed to GA (two adverse drug reactions per report), 13.56% of which were unexpected (Table 3 and Figure 3). Particularly, ~33% of reports referred to reactions of moderate–severe grade, which occurred immediately after GA administration and mostly resolved within a day, such as rash (N=5), dyspnea (N=4), tachycardia (N=3), pyrexia (N=2), flushing (N=2), chest pain (N=2), choking sensation (N=2), anxiety (N=2), palpitation (N=1), syncope (N=1), vomiting (N=2), depression (N=1), nausea (N=1), confusional state (N=1), and visual field defect (N=1). Injection site reactions of mild–moderate severity were also common (30% of reports), and they manifested as mass (N=5), rash (N=3), pruritus (N=1), pain (N=1), and redness (N=1). In addition, moderate or severe hypersensitivity reactions (N=3) occurred after GA administration and, in all of these cases, the drug discontinuation was decided. The remaining expected events recorded were mixed anxiety-depressive disorder (N=1), influenza (N=2), urticaria (N=2), skin disorder of unspecified nature (N=1), and motor dysfunction (N=1).
The unexpected adverse reactions were significant compared to those received for NAT and FTY720. From our evaluation, we found that the unexpected events were mainly of moderate–severe degree and referred to panic attacks (N=3), tachypnea (N=1), and irritable mood (N=1). In addition, a patient reported vertigo and postural instability of moderate intensity, as well as a slight hemiparesis for ~5 months, which however did not result in drug discontinuation. Among the reports analyzed, 53.33% resulted in GA discontinuation due to the adverse reaction onset.
NAT
For NAT, we received a significantly lower number of expected adverse reactions compared to those recorded for IFN β-1a-A, IFN β-1a-R, IFN β-1b-B, PEG IFN β-1a, GA, teriflunomide, and DMF. In addition, the number of mild grade expected reactions was significant compared to those for GA (Figure 4).
We received seven reports from NAT-treated patients who have undergone 3–109 administrations (one infusion/month) at the time of adverse reaction onset. In particular, only two reports belonged to subjects who suffered from concomitant diseases: the first one was affected by diabetes and the other one showed mixed anxiety-depressive disorder and conversion disorder.
About 86% of reports included patients who received NAT for >24 months, a period after which the risk of developing progressive multifocal leukoencephalopathy (PML) increases. Nevertheless, no case of this infection was reported.
We collected 11 adverse drug reactions, mainly of mild–moderate severity (1.6 adverse drug reactions per report), 63.64% of which were unexpected (Figures 3 and 5 and Table 3). Specifically, we highlighted pyrexia (N=3) and chest pain (N=1) as expected reactions. Furthermore, we recorded unexpected events described as cough (N=2), femoral fracture (N=1), paresthesia (N=1), hyposthenia (N=1), diarrhea (N=1), and bronchopneumonia (N=1). The number of unexpected drug reactions was significantly higher compared to IFN β-1a-A, IFN β-1a-R, and IFN β-1b-B. A significant difference was also recorded compared to GA and DMF. In particular, the mild ones were higher compared to FTY720 (Figure 5).
Finally, our analysis underlined that only two of the seven cases received have discontinued NAT following the adverse reaction onset.
Fingolimod
For FTY720, we received a significantly lower number of expected adverse reactions compared to IFN β-1a-A, IFN β-1a-R, IFN β-1b-B, PEG IFN β-1a, GA, teriflunomide, and DMF. In particular, the number of those of severe grade was significant compared to DMF, and it was significantly higher compared to those of teriflunomide, IFN β-1a-R, IFN β-1b-B, and PEG IFN β-1a. The reactions of moderate grade were significantly higher compared to IFN β-1a-A. On the contrary, the reactions of mild grade were significantly lower compared to IFN β-1a-A, PEG IFN β-1a, and DMF (Figure 4).
We received 19 reports involving FTY720-treated patients. A total of 57.89% of cases suffered from concomitant diseases (Tables 2 and 3).
We collected an amount of 31 adverse drug reactions attributed to FTY720 (1.6 adverse drug reactions per report), 45.16% of which were unexpected (Table 3 and Figure 3).
During FTY720 treatment, the elevation of hepatic enzymes is common and often causes drug discontinuation. In our study, increase in liver transaminases was also recorded (N=4), for which the temporary suspension of therapy was decided until these values have returned to the normal range.
The immunosuppressive effects of FTY720 may increase the risk of infections too, especially in reference to opportunistic ones. In our analysis, the reported infections were candidiasis (N=1), influenza (N=1), and urinary tract infection (N=1). Moreover, it is well known that FTY720 commonly reduces the number of circulating lymphocytes due to its MOA, but in our study, only one patient reported a moderate lymphopenia without leading to FTY720 discontinuation. However, other reactions related to the blood and lymphatic system have been observed, represented by leukopenia (N=1) and peripheral edema (N=1).
In clinical trials and in real-world practice, basal-cell carcinoma has been observed in FTY720-treated patients; for this reason, vigilance for skin lesions is strongly recommended. In our study, a case of this skin cancer was reported by a 48-year-old female patient treated with FTY720 for 15 months. This event was classified as moderate and led to drug discontinuation.
Finally, the remaining expected reactions recorded were headache (N=3), asthenia (N=1), alopecia (N=1), and pruritus (N=1).
The reported unexpected effects were mainly of moderate–severe intensity. The number of unexpected drug reactions was significant compared to IFN β-1a-A, IFN β-1a-R, IFN β-1b-B, PEG IFN β-1a, GA, teriflunomide and DMF (Figure 3). In particular, we observed psychiatric disorders, such as insomnia (N=2), behavioral disorder (N=1), and anxiety (N=1), as well as musculoskeletal adverse reactions given by pain in extremity (N=2), muscle spasms (N=1), and arthralgia (N=1). Isolated cases of warts (N=1), acne worsening (N=1), hypothyroidism (N=1), and chest pain (N=1) were also reported. In addition, a severe bilateral ovarian carcinoma and an aortic dissection, which led the patient to death, were noticed.
In 35.29% of reports, it was necessary to stop FTY720 administration permanently, while it was decided only a temporary suspension in 17.65% of reports. On the contrary, 47.06% of reports described the drug continuation even after the adverse reaction onset.
Teriflunomide
The number of expected adverse reactions was significantly higher compared to those for NAT and FTY720. The number of those of severe grade was significantly lower compared to FTY720. Reactions of moderate grade were significantly higher compared to IFN β-1a-A. The expected reactions of mild grade were higher compared to GA but lower compared to IFN β-1a-A (Figure 4). We received 38 reports involving teriflunomide-treated patients, 52.63% of which suffered from comorbidities (Tables 2 and 3).
Our analysis led to the registration of 61 adverse reactions (1.6 adverse drug reactions per report), 11.48% of which were unexpected (Table 3).
As already well known from the information available on this drug, gastrointestinal disorders are the most frequent adverse reactions reported by the majority of patients receiving teriflunomide. In our study, such events were recorded in 42.10% of reports and all of them had a mild–moderate severity grade. In particular, these subjects experienced diarrhea (N=8), abdominal pain upper (N=5), nausea (N=3), gastroenteritis (N=3), and pancreatitis (N=2).
Generally, teriflunomide has also been associated with the elevation of blood pressure and a decrease in leukocytes, as well as an increase in hepatic enzymes. For this reason, special precautions must be performed before and after teriflunomide treatment, which consist of assessing and monitoring of the following parameters: blood pressure, blood cells’ count, and hepatic function. In our analysis, we also observed hypertension (N=7), hepatic transaminases increased (N=4), leukopenia (N=1), and neutropenia (N=1). Alopecia (N=9) was another effect commonly reported in our project, and it was described mainly as a mild–moderate event. The remaining expected reactions recorded were headache (N=2), weight decrease (N=1), musculoskeletal pain (N=2), arthralgia (N=1), asthenia (N=1), pain (N=2), respiratory tract infection (N=1), and rash (N=1).
Finally, we noticed depression (N=1), chest pain (N=1), herpes zoster (N=1), tachycardia (N=2), and pruritus (N=1) as unexpected events, with an intensity grade ranging from moderate to severe. Strangely, a severe case of PML was also found, an infection that has not been associated with teriflunomide administration yet. No mild grade events were received.
In 39.47% of reports we received, it was decided definitively to discontinue teriflunomide; in 7.9%, the treatment was interrupted temporarily, whereas 52.63% described the continuation of this drug despite the adverse reaction onset.
DMF
For DMF, we received the highest number of expected adverse reactions, with a significant difference compared to NAT and FTY720. The number of those of severe grade was significant compared to FTY720. The moderate grade expected adverse reactions reached a statistical significance compared to IFN β-1a-A, IFN β-1a-R and GA. The mild adverse reactions were higher compared to GA and FTY720 (Figure 4). We received 58 reports attributed to DMF, involving patients who were affected by comorbidities in 37.93% of cases (Tables 2 and 3). We collected a total of 95 adverse effects (1.6 adverse drug reactions per report), of which 84.21% have already been associated with DMF (Figures 3 and 4 and Table 2) and 15.79% were unexpected (Figures 3 and 5 and Table 3).
Generally, a well-known manifestation during DMF treatment is flushing, which was also registered in our study in ~56.89% of reports (N=33), with a mild or moderate severity. Gastrointestinal adverse reactions also commonly occurred in 43.10% of cases and reported as diarrhea (N=6), abdominal pain and abdominal pain upper (N=12), nausea (N=2), gastrointestinal disorders (N=8), and gastroenteritis (N=1). Several cases of rash (N=4), pruritus (N=6), and lymphopenia (N=3) were recorded too. In addition, a few patients experienced hepatic disorders during DMF treatment, related to elevation of transaminases (N=2) and a severe drug-induced liver injury (N=1). The remaining expected effects reported were asthenia (N=1) and burning sensation on a patient trunk (N=1).
The number of unexpected adverse events was significant compared to those recorded for NAT and FTY720 (Figure 3).
The unexpected effects we noticed referred to thrombocytosis (N=1), lymphadenopathy (N=1), respiratory failure (N=1), rhinitis (N=1), dyspnea (N=1), headache (N=2), vertigo (N=2), insomnia (N=1), pain (N=1), vaginal discharge (N=1), weight decrease (N=1), and chest pain (N=1). In addition, we recorded omphalitis (N=1), an infection of the umbilical stump.
Finally, our analysis showed that DMF was permanently discontinued in 36.21% of reports due to the adverse reactions, whereas in 3.45%, it was decided to suspend the treatment only temporarily; 60.34% reported the continuation of DMF administration.
Comparison of adverse drug reactions occurred in DMTs with different routes of administration
DMTs may be administered through different routes. In particular, IFN β-1a-R, IFN β-1b, PEG IFN β-1a, and GA are injected subcutaneously; IFN β-1a-A is administered by intramuscular injection, while NAT is an intravenous infusion therapy. FTY720, teriflunomide, and DMF are oral agents.
We compared drugs administered through injection/infusion with those given orally.
For these two groups, we put together the collected data to perform a comparative analysis. Starting from 265 reports related to DMTs treated patients, we analyzed an amount of 411 adverse drug reactions, of which 224 adverse drug reactions were attributed to injectable/intravenous agents and 187 adverse drug reactions were recorded during oral drugs, corresponding to 54.5 and 45.5%, respectively. Specifically, we collected 195 expected (16 severe, 116 moderate, 62 mild, and one unavailable intensity) and 29 unexpected adverse reactions (three severe, 16 moderate, nine mild, and one unavailable intensity) related to injectable or infusion therapies. The percentages of expected and unexpected events were 87.05 and 12.95, respectively. In addition, 151 expected (15 severe, 82 moderate, and 54 mild) and 36 unexpected (10 severe, 23 moderate, and three mild) adverse effects were reported during oral therapies. A total of 80.75% of the reactions recorded in oral agents have already been associated with these drugs, whereas 19.25% were unexpected.
Discussion
MS is a chronic disease of the CNS primarily characterized by demyelination and axonal loss caused by autoreactive T cells. Although its etiology has not been completely understood yet, over the last 20 years, numerous therapies have been made available to patients in order to improve their quality of life. Just for this purpose, the expansion of treatment options for MS has led to the introduction of DMTs with the aim of improving the clinical course of this disease and reducing long-term disability.
The first consideration that we can deduce from this study is that only a small portion of RR-MS patients show the onset of adverse events during the therapeutic treatment. Indeed, only 265 patients on a total of 6,039 showed at least one adverse drug reaction.
We observed that in most cases, IFNs were administered as first therapy. Furthermore, in the majority of patients treated with previous treatments, the first drugs administered were IFNs. However, it was not possible to properly consider the importance of the sequence of the drugs used on the onset of adverse events, because of the wide variety of the different combinations of therapies used and the small cohort of this study that caused an excessive fragmentation in small groups of patients.
During our program, we monitored five different IFNs. The whole group has been involved in a high number of adverse drug reactions that were almost completely expected and of mild-to-moderate intensity. Very few events were unexpected, and among these, only one was of a severe grade. The latter referred to a case of erectile dysfunction in a 53-year-old male patient, who had undergone 308 IFN β-1a-A administrations before the adverse reaction onset.
These data led us to confirm the safety profile of these drugs, similar to what Zettl et al24 affirmed in their review, in which they have defined the IFNs related adverse reactions as mild, manageable, and reversible.
The highest amount of reported adverse effects was attributed to PEG IFN β-1a and IFN β-1a-R. On the contrary, IFN β-1b-E collected the lowest number of adverse events among those attributed to IFNs during our program. It is important to evaluate when the adverse reaction appeared during the course of therapy, given that adverse events appearing later could be due to accumulated toxicity. Interestingly, in most cases, all IFNs, except PEG IFN β-1a, caused adverse reactions after the administration of an elevated number of doses. On the contrary, PEG IFN β-1a-associated adverse events appeared earlier.
GA showed a comparable trend to PEG IFN β-1a: for both drugs, an analog number of reported adverse reactions and a similar severity in unexpected events were recorded; on the contrary, expected reaction intensity tended to be more moderate–severe in GA-treated patients.
For IFNs and GA, injection site reactions were the most common. In particular, we highlighted pain, redness, pruritus, mass, and skin manifestations for both of these drugs, while IFNs were also related to abscess, necrosis, and bruising. Furthermore, only IFNs have been strongly associated with flu-like symptoms. These data are in agreement with Moses and Brandes,25 who obtained similar information after performing a search on the MEDLINE and EMBASE databases to analyze the studies published between 1985 and 2007.
NAT was one of the drugs with the lowest number of reported adverse reactions, despite being a strongly monitored agent due to the drug–PML risk correlation. PML is a demyelinating brain disease caused by John Cunningham virus (JCV) infection that occurs in immunocompromised patients but also associated with specific immunotherapies. As of December 7, 2017, there have been 756 confirmed cases of NAT-associated PML.26 In the STRATA MS study, 14 confirmed cases of PML were detected on a total of 1,094 patients. All these patients had detectable anti-JCV antibodies at least 6 months before diagnosis and after at least 2 years of NAT exposure.27 However, no report of such infection correlated with this drug was detected in our program, but it is important to notice that it may be due to the low number of reports received for NAT.
Among the recorded NAT-related adverse reactions, we observed more unexpected than expected events; however, almost all had a mild-to-moderate intensity and they did not result in NAT discontinuation. Our results are in line with another pharmacovigilance program on NAT conducted in Sicily region, which showed a similar severity grade in the reactions they analyzed.28
For years, only the DMTs requiring parenteral administration had been available. More recently, a great goal has been achieved in the MS therapeutic scenario thanks to the introduction of oral DMTs, which should ensure a higher adherence due to their easier route of administration. In this context, pharmacovigilance is essential for monitoring the safety profile of these agents and detecting any adverse reactions not shown in clinical trials.
Considering oral DMTs, DMF recorded the highest number of adverse events, both for expected and for unexpected effects. The expected reactions were mostly of mild–moderate intensity, while among the unexpected moderate severity prevailed. Teriflunomide was the second for the number of reported reactions, in which moderate expected events dominated. However, unexpected effects were the lowest compared to the other oral therapies, but none of them was mild.
Finally, FTY720 was the oral agent reporting the lowest number of adverse events, but it recorded the highest level of severe unexpected reactions in the entire sample of the examined drugs.
Both teriflunomide and DMF have been commonly associated with gastrointestinal disorders, described mainly as diarrhea, abdominal pain, nausea, and gastroenteritis. Together with these adverse effects, the most frequent event associated with DMF was flushing, while alopecia and hypertension have been reported by several teriflunomide-treated patients. All this information is in accordance with data from TEMSO and DEFINE studies, two Phase III clinical trials describing an increased incidence of the reactions mentioned earlier in patients receiving the active treatment compared to the placebo group.29,30
A common point highlighted during our analysis in all the oral DMTs was an increase in liver enzymes, an event already described in Phase III clinical trials. In particular, elevations in hepatic enzyme levels were common findings in TEMSO and FREEDOMS studies, with a percentage of 14.2 and 15.8, respectively.8,29 This adverse reaction was also associated with DMF in DEFINE trial, but with a lower percentage (6%).30 Also, in our study, among patients reporting oral DMTs related adverse reactions, a higher percentage was associated with FTY720 (21%) and teriflunomide (10.5%), while a lower number of cases was reported for DMF (3.4%).
In order to verify if the recorded unexpected reactions had already been highlighted, we evaluated them compared to what is reported in literature.
One FTY720-treated patient died because of an aortic dissection. The subject in question was a 65-year-old man treated with FTY720 for more than a year and without concomitant disease related to the cause of death. No other cases of aortic dissection during FTY720 therapy had previously been described in the literature. Anyhow, this agent has already been associated with cardiovascular events, such as hypertension, T-wave inversion, bradycardia, bradyarrhythmia, myocardial ischemia, and atrioventricular block.21,31
Another unexpected adverse event reported in our study was a bilateral ovarian carcinoma, occurred in a 50-year-old female patient treated with FTY720 for 5 years. From information available in the summary of product characteristics and literature, it is known that this drug has been involved in several cases of cancer, such as Merkel cell carcinoma, Kaposi sarcoma, melanoma, and basal cell carcinoma.31–34 Even in our study, this last skin cancer has been reported in a 48-year-old female patient treated with FTY720 for 15 months.
At the same time, a potential anticancer role of FTY720 has already been shown, due to inhibition of the proto-oncogene sphingosine kinase 1 (SK1).35 In vitro and in vivo studies demonstrate the ability of this agent to induce apoptosis and autophagy in ovarian cancer cells and decrease tumor weight, supporting its potential therapeutic role in this type of cancer.36,37 This evidence is in contrast with our case report of bilateral ovarian carcinoma, questioning the correlation between this drug and the adverse event occurred.
In our study, FTY720 has also been correlated to hypothyroidism. This unexpected event occurred in a 32-year-old woman treated with the drug in question for >2 months. Patients affected by MS show an important risk of autoimmune diseases, such as thyroid dysfunctions;38 FTY720 may increase this susceptibility due to its immunomodulatory MOA. Another similar case was previously described in the literature, strengthening this possible association and the need of additional researches.39
Strangely, a case involving a 36-year-old woman who contracted PML during teriflunomide treatment was reported.40 After a 10-year therapy with IFN β-1a, the patient has undergone NAT for ~3 years (33 infusions). This last agent was discontinued because of a JCV index of 3.6, although the last magnetic resonance imaging (MRI) performed after NAT suspension showed no signs of PML.40 Subsequently, the patient started teriflunomide treatment. After 8 months from NAT discontinuation, the teriflunomide-treated patient underwent a brain MRI, which detected a new lesion highly suggestive of PML. Actually, the occurrence of this infection after only 3 months of teriflunomide administration suggests a long incubation of JCV and the possible involvement of NAT.40
Previously, some PML cases associated with the oral DMF and FTY720 have been described.41 On the contrary, no certain teriflunomide-related PML has been reported yet, although some cases involving its precursor molecule leflunomide were observed.42,43 These results suggest the importance of a more prolonged PML monitoring and the need for increased data related to DMTs–PML correlation.
Considering NAT, an adverse drug reaction case report of bronchopneumonia in a 53-year-old female patient was received. This subject had undergone 34 NAT administrations before the adverse reaction onset. Although this event is unexpected according to the summary of product characteristics, different cases of pulmonary infection during NAT treatment have been described in the literature, such as pulmonary tuberculosis, Mycobacterium intracellulare pneumonia, and a case of Mycobacterium kansasii lung infection.44–46 This evidence suggests a possible role of this agent in opportunistic pulmonary infections, probably due to its immunosuppressive action.
In our study, we analyzed a case of omphalitis in a 24-year-old male patient treated with DMF for 5 months.47 Omphalitis is an infection of the umbilical stump that mainly affects newborns and only rarely adults. No case of omphalitis during DMF treatment has been reported in the literature yet. However, the recovery of the infection after DMF discontinuation and the reappearance of the event following the restart of this agent suggest a connection between the treatment and the development of omphalitis.
Two cases of headache occurring during DMF therapy were also reported. The patients in question were a 37-year-old woman and a 27-year-old man treated with this drug for 3 months and 1 month, respectively. We considered these events as unexpected because headache is not mentioned in the summary of product characteristics.23 However, a multicentric study in RR-MS patients has reported it as a common adverse reaction in their cohort during DMF treatment, with a dose-related pattern.48
Finally, we received a report involving a 54-year-old woman in therapy with DMF from 1 month who showed widespread pain and asthenia. The patient presented fibromyalgia as concomitant disease. Pain is not an expected effect of this agent; however, musculoskeletal pain and articular pain occurring during DMF therapy have recently been described.49 Despite this new evidence, we cannot exclude fibromyalgia among the causes of this adverse event.
By examining the route of administration of DMTs and comparing the data collected for injectable/intravenous drugs and oral agents, we observed that the trend in these two groups was rather similar, even if a higher percentage of unexpected events were detected during oral therapies, especially in reference to those of a severe intensity. These data reflect an important knowledge deficit in the long-term safety of oral DMTs, which have been approved in our country only a few years ago, starting in 2011 with FTY720.
Conclusion
In our study, we observed that these treatments seem safe, given that only a small portion of RR-MS patients developed adverse drug events. Furthermore, the most frequently reported adverse reactions occurred during DMTs were expected. However, the higher number of unexpected events and the magnitude of some serious effects reported for oral therapies reflect the lack of data for these treatments that were introduced later in the clinical practice and the importance of postmarketing safety studies to improve knowledge on the subject. Another additional explanation to justify the higher number of unexpected reactions for oral drugs could be the greater attention of health professionals toward these treatments that are still little known compared to the others. Both teriflunomide and FTY720 are in fact under additional monitoring, according to EMA directive.
The limitation of this study was the small number of centers participating, but it encourages and underlines the importance of pharmacovigilance studies, in order to protect the health of patients who already presented a disease of such importance and to better characterize the long-term safety profile of MS therapies.
Acknowledgment
This study was supported by funds of multiregional pharmacovigilance project entitled “Valutazione e riconoscimento delle reazioni avverse in pazienti affetti da sclerosi multipla trattati farmacologicamente” of 2010/2011 of Italian Drug Agency (AIFA).
Author contributions
AG and FL performed the analysis and interpretation of data and wrote the manuscript. MGM, GLM, IG, MM, and PB provided adverse drug reaction reports and critically revised the manuscript for important intellectual content. FLG performed data analysis and designed the figures. EM designed the study and critically revised the manuscript for important intellectual content. All authors contributed toward data analysis, drafting and critically revising the paper and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
Browne P, Chandraratna D, Angood C, et al. Atlas of Multiple Sclerosis 2013: A growing global problem with widespread inequity. Neurology. 2014;83(11):1022–1024. | ||
Ransohoff RM, Hafler DA, Lucchinetti CF. Multiple sclerosis-a quiet revolution. Nat Rev Neurol. 2015;11(3):134–142. | ||
van den Broek HH, Damoiseaux JG, de Baets MH, Hupperts RM. The influence of sex hormones on cytokines in multiple sclerosis and experimental autoimmune encephalomyelitis: a review. Mult Scler. 2005;11(3):349–359. | ||
Dhib-Jalbut S, Marks S. Interferon-beta mechanisms of action in multiple sclerosis. Neurology. 2010;74 Suppl 1:S17–S24. | ||
Hutchinson M. Natalizumab: A new treatment for relapsing remitting multiple sclerosis. Ther Clin Risk Manag. 2007;3(2):259–268. | ||
Ziemssen T, Schrempf W. Glatiramer acetate: mechanisms of action in multiple sclerosis. Int Rev Neurobiol. 2007;79:537–570. | ||
Miller AE. Oral teriflunomide in the treatment of relapsing forms of multiple sclerosis: clinical evidence and long-term experience. Ther Adv Neurol Disord. 2017;10(12):381–396. | ||
Kappos L, Radue EW, O’Connor P, et al. A placebo-controlled trial of oral fingolimod in relapsing multiple sclerosis. N Engl J Med. 2010;362(5):387–401. | ||
Mills EA, Ogrodnik MA, Plave A, Mao-Draayer Y. Emerging Understanding of the Mechanism of Action for Dimethyl Fumarate in the Treatment of Multiple Sclerosis. Front Neurol. 2018;9:5. | ||
Torkildsen Ø, Myhr KM, Bo L. Disease-modifying treatments for multiple sclerosis – a review of approved medications. Eur J Neurol. 2016;23 Suppl 1:18–27. | ||
Gajofatto A, Benedetti MD. Treatment strategies for multiple sclerosis: When to start, when to change, when to stop? World J Clin Cases. 2015;3(7):545–555. | ||
Longbrake EE, Cross AH, Salter A. Efficacy and tolerability of oral versus injectable disease-modifying therapies for multiple sclerosis in clinical practice. Mult Scler J Exp Transl Clin. 2016;2:205521731667786. | ||
Guarnera C, Bramanti P, Mazzon E. Comparison of efficacy and safety of oral agents for the treatment of relapsing-remitting multiple sclerosis. Drug Des Devel Ther. 2017;11:2193–2207. | ||
EMA European Medicines Agency. Interferon β-1a (Avonex®) Summary of Product Characteristics. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000102/WC500029425.pdf. Accessed September 5, 2018. | ||
EMA European Medicines Agency. Interferon β-1a (Rebif®) Summary of Product Characteristics. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000136/WC500048681.pdf. Accessed September 5, 2018. | ||
EMA European Medicines Agency. Interferon β-1b (Betaferon®) Summary of Product Characteristics. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000081/WC500053225.pdf. Accessed September 5, 2018. | ||
EMA European Medicines Agency. Interferon β-1b (Extavia®) Summary of Product Characteristics. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000933/WC500034701.pdf. Accessed September 5, 2018. | ||
EMA European Medicines Agency. Peginterferon β-1a (Plegridy®) Summary of Product Characteristics. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/002827/WC500170302.pdf. Accessed September 5, 2018. | ||
AIFA Italian Drug Agency. Glatiramer acetato (Copaxone®) Riassunto delle caratteristiche del prodotto [Glatiramer Acetate Summary of Product Characteristics]. Available from: https://farmaci.agenziafarmaco.gov.it/aifa/servlet/PdfDownloadServlet?pdfFileName=footer_002351_035418_RCP.pdf&retry=0&sys=m0b1l3. Accessed September 5, 2018. | ||
EMA European Medicines Agency. Natalizumab (Tysabri®) Summary of Product Characteristics. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000603/WC500044686.pdf. Accessed September 5, 2018. | ||
EMA European Medicines Agency. Fingolimod (Gilenya®) Summary of Product Characteristics. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/002202/WC500104528.pdf. Accessed September 5, 2018. | ||
EMA European Medicines Agency. Teriflunomide (Aubagio®) Summary of Product Characteristics. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/002514/WC500148682.pdf. Accessed September 5, 2018. | ||
EMA European Medicines Agency. Dimethyl fumarate (Tecfidera®) Summary of Product Characteristics. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/002601/WC500162069.pdf. Accessed September 5, 2018. | ||
Zettl UK, Hecker M, Aktas O, Wagner T, Rommer PS. Interferon β-1a and β-1b for patients with multiple sclerosis: updates to current knowledge. Expert Rev Clin Immunol. 2018;14(2):137–153. | ||
Moses H, Brandes DW. Managing adverse effects of disease-modifying agents used for treatment of multiple sclerosis. Curr Med Res Opin. 2008;24(9):2679–2690. | ||
Biogen Medical Information [webpage on the Internet]. PML Incidence in Patients Receiving Tysabri®. Available from: https://medinfo.biogen.com/. Accessed September 5, 2018. | ||
O’Connor P, Goodman A, Kappos L, et al. Long-term safety and effectiveness of natalizumab redosing and treatment in the STRATA MS Study. Neurology. 2014;83(1):78–86. | ||
Giacoppo S, Ruscica M, Grimaldi LM, Bramanti P, Mazzon E. The Italian Pharmacovigilance Program: An Observational Study of Adverse Effects of Natalizumab in Multiple Sclerosis Therapy. Med Sci Monit. 2017;23:4230–4240. | ||
O’Connor P, Wolinsky JS, Confavreux C, et al. Randomized trial of oral teriflunomide for relapsing multiple sclerosis. N Engl J Med. 2011;365(14):1293–1303. | ||
Gold R, Kappos L, Arnold DL, et al. Placebo-controlled phase 3 study of oral BG-12 for relapsing multiple sclerosis. N Engl J Med. 2012;367(12):1098–1107. | ||
Calabresi PA, Radue EW, Goodin D, et al. Safety and efficacy of fingolimod in patients with relapsing-remitting multiple sclerosis (FREEDOMS II): a double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Neurol. 2014;13(6):545–556. | ||
Mahajan KR, Ko JS, Tetzlaff MT, Hudgens CW, Billings SD, Cohen JA. Merkel cell carcinoma with fingolimod treatment for multiple sclerosis: A case report. Mult Scler Relat Disord. 2017;17:12–14. | ||
Walker S, Brew B. Kaposi sarcoma in a fingolimod-treated patient with multiple sclerosis. J Clin Neurosci. 2016;31:217–218. | ||
Conzett KB, Kolm I, Jelcic I, et al. Melanoma occurring during treatment with fingolimod for multiple sclerosis: a case report. Arch Dermatol. 2011;147(8):991–992. | ||
White C, Alshaker H, Cooper C, Winkler M, Pchejetski D. The emerging role of FTY720 (Fingolimod) in cancer treatment. Oncotarget. 2016;7(17):23106–23127. | ||
Zhang N, Qi Y, Wadham C, et al. FTY720 induces necrotic cell death and autophagy in ovarian cancer cells: a protective role of autophagy. Autophagy. 2010;6(8):1157–1167. | ||
Lee JW, Ryu JY, Yoon G, et al. Sphingosine kinase 1 as a potential therapeutic target in epithelial ovarian cancer. Int J Cancer. 2015;137(1):221–229. | ||
Marrie RA, Horwitz RI. Emerging effects of comorbidities on multiple sclerosis. Lancet Neurol. 2010;9(8):820–828. | ||
Flores J, Rito Y, Torres G, Jung H, Treviño-Frenk I, Corona T. Hypothyroidism in multiple sclerosis patient during fingolimod treatment. J Neurol Sci. 2015;348(1–2):272–273. | ||
Lorefice L, Fenu G, Gerevini S, et al. PML in a person with multiple sclerosis: Is teriflunomide the felon? Neurology. 2018;90(2):83–85. | ||
Berger JR. Classifying PML risk with disease modifying therapies. Mult Scler Relat Disord. 2017;12:59–63. | ||
Rahmlow M, Shuster EA, Dominik J, et al. Leflunomide-associated progressive multifocal leukoencephalopathy. Arch Neurol. 2008;65(11):1538–1539. | ||
Warnatz K, Peter HH, Schumacher M, et al. Infectious CNS disease as a differential diagnosis in systemic rheumatic diseases: three case reports and a review of the literature. Ann Rheum Dis. 2003;62(1):50–57. | ||
Dahdaleh D, Altmann DM, Malik O, Nicholas RS, Breathlessness NRS. Breathlessness, night sweats, and weight loss on natalizumab. Lancet. 2012;380(9843):726–727. | ||
de Masson A, Maillart E, Veziris N, Meyssonnier V, Papeix C, Caumes E. Cavitary pulmonary disease in a patient treated with natalizumab. Presse Med. 2014;43(9):1009–1012. | ||
Hradilek P, Zeman D, Tudik I, Zapletalova O, Ulmann V. Asymptomatic lung disease caused by Mycobacterium kansasii as an opportunistic infection in a patient treated with natalizumab for relapsing-remitting multiple sclerosis. Mult Scler. 2014;20(5):639–640. | ||
Lorefice L, Fenu G, Cabras F, et al. An unusual infection in MS patient treated with dimethyl fumarate: A case report of omphalitis. Mult Scler Relat Disord. 2016;7:65–67. | ||
Kappos L, Gold R, Miller DH, et al. Efficacy and safety of oral fumarate in patients with relapsing-remitting multiple sclerosis: a multicentre, randomised, double-blind, placebo-controlled phase IIb study. Lancet. 2008;372(9648):1463–1472. | ||
Bernardini LR, Zecca C, Clerici VT, Gobbi C, Mantegazza R, Rossi S. Severe articular and musculoskeletal pain: An unexpected side effect of dimethyl-fumarate therapy for multiple sclerosis. J Neurol Sci. 2016;369:139–140. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.