Back to Journals » Therapeutics and Clinical Risk Management » Volume 19
A Multi-Centric Comparative Study Between Endoscopy-Assisted Laparoscopic Surgery (EALS) vs Laparoscopic Surgery for the Treatment of Gastric Duplication Cysts in Children
Authors Luo Y, Liu J, Jiang Z, Yang X , Lin S, Mao X
Received 19 June 2023
Accepted for publication 20 September 2023
Published 11 October 2023 Volume 2023:19 Pages 801—810
DOI https://doi.org/10.2147/TCRM.S426691
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Deyun Wang
Yanmei Luo,1,* Jie Liu,2,* Zhihui Jiang,3,* Xinghai Yang,1 Song Lin,1 Xiaowen Mao1
1Department of Pediatric Surgery, Maternal and Child Health Hospital of Hubei, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, People’s Republic of China; 2Department of Pediatric Surgery, Yijishan Hospital of Wannan Medical College, Wannan Medical College, Wuhu, People’s Republic of China; 3Department of General Surgery, Qingdao Women and Children’s Hospital, Qingdao, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Xiaowen Mao; Song Lin, Department of Pediatric Surgery, Maternal and Child Health Hospital of Hubei, Tongji Medical College, Huazhong University of Science and Technology, No. 58 Guanggu Third Road, Donghu New Technology Development Zone, Wuhan City, 430070, Hubei Province, People’s Republic of China, Email [email protected]; [email protected]
Purpose: To compare and analyze the therapeutic effects of endoscopy-assisted laparoscopic surgery (EALS) and laparoscopic surgery (LS) in the treatment of gastric duplication cysts (GDCs).
Patients and Methods: We reviewed the clinical data of children with GDCs who underwent surgical treatment at Hubei Maternal and Child Health Hospital, Yijishan Hospital of Wannan Medical College, and Qingdao Women and Children’s Medical Center from September 2014 to November 2022.
Results: The study comprised 29 children with GDCs, including 14 in the EALS group and 15 in the LS group. There was no significant difference between the two groups in terms of age, sex, weight, and cyst size characteristics. There was a significant difference between the two groups in terms of average surgical time (P> 0.05), which was 1.100 ± 0.833 hours in the EALS group and 1.933 ± 0.159 hours in the LS group. There was a significant difference between the two groups (P< 0.05) in average intraoperative blood loss, which was 7.93 ± 3.81 milliliters in the EALS group and 11.80 ± 2.72 milliliters in the LS group. There was a significant difference between the two groups (P< 0.05) in average postoperative fasting time, which was 73.79 ± 8.36 hours in the EALS group and 114.1 ± 9.24 hours in the LS group. There was a significant difference between the two groups (P< 0.05) in average postoperative hospital stay, which was 10.21 ± 4.25 days in the EALS group and 14.47 ± 4.36 days in the LS group.
Conclusion: EALS technology can not only shorten surgical time, accurately locate GDCs, reduce injuries, and decrease the probability of complications but also achieve treatment goals safely and reliably.
Keywords: gastric duplication cyst, laparoscopic, endoscopic, surgery, children
Introduction
Gastric duplication cysts (GDCs) are relatively rare in clinical practice, accounting for only 3~5% of all digestive tract duplications.1 The clinical manifestations of GDCs are different, and in most cases of GDC, there are no typical clinical manifestations. The clinical manifestations of some GDCs are often cyst infection, perforation, gastrointestinal bleeding, and surrounds found unintentionally during prenatal examination or physical examination. Ultrasound, computed tomography (CT) and magnetic resonance imaging (MRI) often indicate cystic space-occupying lesions, which are confirmed by tissue compression, obstruction, vomiting, etc.2 In some patients with large cysts, the abdominal mass can be palpated in the upper abdomen.3
The research report states that GDCs are mostly benign, but malignant lesions may also occur.4 Most scholars advocate for early surgical treatment before serious complications occur.5,6 The surgical methods include open surgery and laparoscopic surgery. However, due to the different growth sites of GDCs, it is sometimes difficult to achieve precise positioning with traditional open surgery and laparoscopic surgery, which may lead to excessive resection of gastric tissue and postoperative gastric abnormalities.7 In addition, if the lump is located near the pylorus, the pyloric ring should be appropriately preserved during the surgery to avoid postoperative narrowing of the gastric outlet.
Endoscopy-assisted laparoscopic surgery (EALS), a dual-mirror combined technique, can maximize the protection of normal gastric wall tissue and provides a new method for the surgical treatment of submucosal gastric tumors.7 It is currently widely used in adults. At present, there is only one report on the application of EALS in children.7 However, due to the limited number of cases of GDCs, most of them are mainly reported in medical records. Further research is needed to determine whether EALS has important advantages compared to laparoscopic surgery. This study is a retrospective analysis of the clinical data of 29 children with GDCs admitted to three pediatric surgery centers in China from September 2014 to November 2022. Comparing the treatment effects of two different surgical methods is expected to provide some diagnostic and therapeutic experience for the management of this disease.
Patients and Methods
Patients and Clinical Parameters
In this study, the clinical data of children with GDC who underwent surgical treatment in Hubei Maternal and Child Health Hospital, Yijishan Hospital of Wannan Medical College, and Qingdao Women and Children’s Medical Center from September 2014 to November 2022 were reviewed. All patients were examined by abdominal B-ultrasound and CT or MRI, which suggested the diagnosis of GDC. GDCs are treated surgically as soon as they are detected. The clinical and follow-up data of the patients were obtained by consulting the electronic medical records and follow-up records. To reduce study bias, children with other diseases or malformations requiring concurrent surgical management were not included in this study. This study was reviewed and approved by the Ethics Committee of Hubei Maternal and Child Health Hospital (2022IEC017), and all the legal guardians of children involved in this study signed the informed consent form.
Treatment Methods
Preoperative Preparation
A complete preoperative examination was performed, surgical indications were clarified, and surgical contraindications, fasting, fluid replacement, and correct water and electrolyte disorders were eliminated before the operation.
EALS
After general anesthesia, a gastroscope was inserted orally. The surgeon’s left hand held the endoscopic control section, and the upper and lower left and right knobs were adjusted with the thumb. The mirror was held in the surgeon’s right hand; the end of the mirror was aimed at the base of the patient’s tongue and inserted into the back wall of the pharynx through the dental pad, then into the esophagus through the base of the tongue along the left pear-shaped recess and through the cardia into the antrum and pylorus. The four walls of the antrum were observed with the pylorus as the center. The anterior and upper walls of the duodenal bulb were observed, and the posterior and lower walls of the duodenal bulb were observed through the pylorus. Next, the angle was adjusted upward to the right, and the mirror was rotated clockwise into the descending part of the duodenum. Exiting the pylorus, the operating part was rotated clockwise, and the greater curvature of the antrum and the posterior wall of the stomach were observed. The angle knob was adjusted upward to observe the vertical part of the stomach and the stomach fundus. The angle knob was twisted to the maximum to rotate it 180 degrees clockwise and observe the cardiac region from the front. After the observation, the lens was extracted, allowing air into the stomach, and the lower, middle and upper esophagus were observed. Intraoperatively, the cyst site was explored, the protrusion was clamped with a hemostatic metal clip, and a guide mark (or light source location) was made. Assisted laparoscopy was performed to remove the gastric duplication deformity.
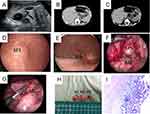
The protrusion was clamped with a hemostatic metal clip, and a guide mark (or light source location) was made. The child was transferred to the supine position, and the focus was still observed directly under the digestive endoscope. The skin was routinely disinfected and then covered with towels and sheets. A small incision was made at the umbilicus, a 5 mm trocar was placed, an endoscope lens was placed, an artificial pneumoperitoneum was established to maintain 6~8 mmHg (1 mmHg=0.133 kPa). A 3 mm and a 5 mm trocar was placed at the outer edge of the rectus abdominis on the left and right sides of the horizontal line of the umbilicus, respectively, and nondestructive forceps were placed. Under the guidance of the digestive endoscope, we explored the mass, explored the location and size of the cyst, exposed the operation field, electrocoagulated and separated along the edge of the mass under laparoscopy, and gradually peeled off the mass completely along the edge. After we trimmed the serous layer on the surface, the serous layer was sutured intermittently under the endoscope once the wound was completely hemostatic, and the whole operation process was completed under the monitoring of the digestive endoscope. Abdominal cavity exploration and gastroscope monitoring showed no bleeding. After removing the digestive endoscope and counting the gauze and instruments, we used 5–0 absorbable sutures to suture the incisions of the abdominal wall layer by layer. Medical tissue glue was applied to the skin incision. The pathological specimens were sent to the pathology room for examination (Figure 1).
The surgical steps for laparoscopic surgery were basically the same as those of EALS. Laparoscopic surgery is a direct laparoscopic procedure without the use of digestive endoscopy during the surgery. The cyst was carefully separated along the boundaries of the duplicated cyst with an ultrasonic knife, and then the cyst was removed completely. To compare the advantages and disadvantages of the two surgical methods, we collected relevant information during and after surgery. We generally use a volumetric method to measure the amount of intraoperative blood loss. In short, we measure the volume of fluid in the suction bag first, and then subtract the flushing amount from the total amount of fluid in the suction bag to calculate the amount of blood loss. For accurate calculations, we use syringes to measure. To accurately assess the recovery of gastrointestinal function, we determine the time for postoperative fasting based on the amount of gastric tube drainage after surgery. Because patients in this study were younger and pain scores may not be accurate, we did not compare postoperative pain.
Statistical Analysis
All statistical analyses in this study were carried out using the R programming language, version 3.6.0 (R Foundation), in which a P value < 0.05 was considered statistically significant.
Results
A total of 31 children with GDC underwent surgical treatment in this study, while laparotomy was chosen for 2 newborns due to other deformities, resulting in them not being included in this study. Ultimately, 29 children underwent laparoscopic treatment (Figure 2, Table 1), 14 patients in the EALS group and 15 patients in the laparoscopic surgery group. The EALS group consisted of 8 boys and 6 girls, with an average age of 15.57 ± 4.234 months. The laparoscopic surgery group included 6 boys and 9 girls. The average age was 16.33 ± 2.318 months. There was no significant difference between the two groups in terms of age and sex (P>0.05) (Table 2). Among all patients, 11 cases were complicated with other systemic malformations, one case involved multiple gastric duplication (3 gastric duplication cysts at different positions), and the remaining 15 cases were simple gastric duplication. The average weight of the children in the EALS group was 12.29 ± 1.21 kilograms, while the average weight of the children in the laparoscopic surgery group was 12.50 ± 1.38 kilograms. There was no significant difference between the two groups (P>0.05) (Table 2). The average size of cysts in the EALS group was 4.314 ± 0.036 square centimeters, while in the laparoscopic surgery group, it was 4.350 ± 0.048 square centimeters. There was no significant difference between the two groups (P>0.05) (Table 2).
 |
Table 1 Demographic Data of the LECS and LS Groups Before Operation |
 |
Table 2 Demographic Data of the LECS and LG Groups Before Operation |
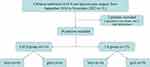 |
Figure 2 The algorithm of patient enrollment. |
The results showed that the surgery was successfully completed in all 29 children, and the final pathological diagnosis was GDC. There were 14 children with gastric duplication in the EALS group, with an average surgical time of 1.100 ± 0.833 hours. The average surgical time was 1.933 ± 0.159 hours in the laparoscopic surgery group; there was a significant difference between the two groups (P<0.05) (Figure 3A, Table 3). The average intraoperative blood loss in the EALS group was 7.93 ± 3.81 milliliters, while in the laparoscopic surgery group, it was 11.80 ± 2.72 milliliters; there was a significant difference between the two groups (P<0.05) (Figure 3B, Table 3). The average postoperative fasting time in the EALS group was 73.79 ± 8.36 hours, while in the laparoscopic surgery group, it was 114.1 ± 9.24 hours; there was a significant difference between the two groups (P<0.05) (Figure 3C, Table 3). The average postoperative hospitalization time in the EALS group was 10.21 ± 4.25 days, while in the laparoscopic surgery group, it was 14.47 ± 4.36 days; there was a significant difference between the two groups (P<0.05) (Figure 3D, Table 3). There was one case of incision infection in each of the EALS and laparoscopic surgery groups, indicating no significant difference between the two groups in this regard (P>0.05) (Table 3).
 |
Table 3 Comparison of Surgical Related Characteristic Information Between the Two Groups of Patients |
Discussion
The cause of GDC may be related to the formation of a duplicate stomach when a group of cells detach from the main body when the foregut differentiates into the stomach in the embryonic stage.8 Most GDCs are found in prenatal examinations and physical examinations of newborns and children, and some are found in adulthood.9 Their clinical manifestations are different. The mucosa of the duplicate stomach has the function of secreting part of the gastric juice, and the cyst can gradually become enlarged with the secretion of gastric juice. Initially, the cyst is small, there are no typical clinical manifestations. However, with the growth and development of the cyst, the secretion of gastric juice continues to increase, and the tension increases.10 Given these factors in combination with the size and location of the cyst, abdominal pain, vomiting, gastrointestinal bleeding, perforation, surrounding tissue compression, obstruction and other symptoms may be manifested, or an abdominal mass may be presented on medical examination.11 A GDC is often monitored by ultrasound, CT and MRI to provide the basis for diagnosis and is finally confirmed by surgery and pathology.12
With the rapid development of medical imaging diagnostic technology, most GDCs can be initially diagnosed and differentiated by auxiliary examination before delivery or in the neonatal period.13 Ultrasound examination is convenient and affordable and is often the first choice for preliminary screening and follow-up monitoring.14 It can clarify the nature of the GDC and explore the size of the cyst, the thickness of the cyst wall, the layered structure of the cyst wall, the adjacent cyst, the blood supply of the cyst, and the relationship with the gastric wall.2,14 Ultrasonic examination has limitations, however, and is often affected by gastrointestinal gas, infection, perforation and bleeding, which are easily missed. In addition, ultrasound has some limitations in the diagnosis of GDC of tubular lesions, which is highly dependent on the experience of doctors; the rate of missed diagnosis or misdiagnosis is relatively high. The location, shape, size, density, and thickness of the cyst wall and its surrounding area can be established by CT and MRI, which are often used as examination means for further diagnosis, cyst location and qualitative diagnosis.15,16 Upper gastrointestinal radiography is often used for preoperative selective examination. When the cyst is large or connected with the gastric cavity, the filling defect of the gastric wall can be seen. Tubular gastric duplication can be shown as a niche shadow of the gastric wall.15 In the process of clinical work-up, if the stomach is unwell or a mass connected with the stomach is found in physical examination, clinicians often choose digestive endoscopy to make a clear diagnosis. Therefore, preoperative digestive endoscopy is also often used as one of the diagnostic examination methods for gastric duplication; it can be used to determine the location and shape of the mass, further clarify the diagnosis and classification, and differentiate it from gastric ulcer and diverticulum.
The gold standard for the diagnosis of gastric duplication is postoperative pathological examination, and the current reference, as follows, is still the diagnostic standard proposed by Rowling in 1959:17 ① the cyst wall is adjacent to the stomach wall; ② the smooth muscle of the cyst is continuous with the smooth muscle of the gastric wall; ③ the cyst is covered by digestive tract epithelium. In the preoperative diagnosis, according to the location, nature and type of gastric duplication cyst, it is necessary to be vigilant to differentiate it from spleen cyst, intestinal duplication, mesenteric cyst, neuroblastoma, lymphangioma, etc., as well as from gastric diverticulum, gastric ulcer, gastric tumor, etc. There have been cases of gastric adenomyoma reported as gastric duplication, so it is necessary to pay attention to differentiation to prevent missed diagnosis and misdiagnosis.18
The treatment of gastric duplication can be classified by type as endoscopic treatment or surgical treatment.5,19,20 Surgical treatment mainly includes traditional laparotomy and endoscopic surgery.5,20–22 Digestive endoscopic surgery mainly includes endoscopic fenestration of intragastric cysts and cyst dissection. The main types of laparotomy are repeated gastrectomy, partial gastrectomy or gastric mucosal exfoliation. With the promotion and maturity of endoscopic minimally invasive technology, many experts at home and abroad have reported the promotion of minimally invasive surgery, namely, laparoscopic resection of gastric duplication.5,22 Krishna Kumar et al23 reported on a 10-month-old female infant who was diagnosed with nonbiliary vomiting before surgery. Surgeons performed traditional open surgery to remove the 8*6 cm cyst located on the greater curvature of the stomach. The child recovered well after surgery and was discharged from the hospital. Sasaki et al24 reported for the first time on laparoscopic treatment of gastric duplication cysts and found that minimally invasive surgery has the characteristics of beauty and minimal trauma, making it more suitable for minimally invasive treatment of gastric duplication cysts. Ren et al25 reported 5 cases of neonatal gastric duplication cysts treated with laparoscopy, believing that compared with traditional open surgery, laparoscopy has the characteristics of less trauma and faster postoperative recovery, making it feasible for the diagnosis and treatment of neonatal gastric duplication cysts. Hattori et al7 applied the EALS technique to a 9-year-old boy with a gastric duplication cyst in the anterior pyloric region. During the surgery, EALS was successfully performed to remove the mass and preserve the patient’s pyloric ring. However, due to the rarity of cases, no large sample of cases has been reported, the operation has not been reported and promoted, and its adaptability and feasibility have not been verified.
The results of this study indicate that laparoscopic surgery is much less invasive than open surgery and has important advantages in the diagnosis and treatment of gastric duplication. However, for some gastric duplication cysts in special locations, exploration in laparoscopic surgery is sometimes difficult, and the surgical time is too long. Compared with laparoscopic surgery, using EALS may not only offer all the advantages of endoscopy but also shorten the exploration time of cysts and enable them to be accurately located under the guidance of gastroscope monitoring during the operation. This helps to identify tissue layers, reduce side effects, protect the integrity of the gastric mucosa barrier, reduce the operation time, reduce the amount of bleeding during the operation, shorten the fasting time after the operation, and achieve rapid recovery. In fact, preoperative gastroscopy localization can provide more accurate diagnosis and classification. For submucosal gastric replication, direct gastroscopy surgery can be used to avoid abdominal surgery and gastric resection. The use of natural lumen surgery in the human body can reduce abdominal surgical trauma. Before surgery, if it is difficult to remove the top of the cyst or peel off the cyst under gastroscopy or if there is perforation or vascular damage during the surgery, double endoscopy preparation can also be carried out through endoscopy in a timely manner to ensure surgical safety.5
In the course of clinical diagnosis and treatment, when stomach pain, vomiting, upper gastrointestinal bleeding or a mass connected to the stomach is found in physical examination, gastroscopy is routinely performed to make a clear diagnosis. Therefore, gastroscopy is also often used as a diagnostic method for gastric duplication. Applying EALS can not only reduce the pain of preoperative diagnostic gastroscopy and multiple anesthesia but also reduce the difficulty of surgical exploration, reduce the damage to the gastric mucosal barrier, make the resection more accurate, reduce the probability of intraoperative and postoperative complications, reduce the postoperative fasting pain of children, and achieve a rapid recovery effect. The risk of aspiration by gastroscopy under general anesthesia intubation is low, and the operation is relatively safe.26 Therefore, in the diagnosis and treatment of gastric duplication, the double-mirror combined technique is worth trying and recommending. Of course, the selection of surgical methods for children with different types and different positions of gastric duplication and different complications needs to be comprehensively evaluated; selection must be made according to the actual conditions of the disease and the requirements of the children and their families.27
This study has some limitations. First, due to the low incidence rate of gastric duplication, the number of cases in this study was not large, and the total number of cases in three large hospitals was only 29. Second, due to the absence of neonatal surgeons in this study, the number of cases of gastric duplication in newborns was not determined. Third, although all surgeries are performed by senior pediatric surgeons, the surgical abilities of surgeons in various medical centers cannot be guaranteed to be the same, so there are also certain errors. We will avoid these limitations in future large-scale, multicenter studies.
Conclusion
EALS technology can not only shorten surgical time, accurately locate GDCs, reduce injuries, and decrease the probability of complications but also achieve treatment goals safely and reliably. It can also help achieve rapid recovery and alleviate the pain of children after surgery. Furthermore, EALS has the advantages of being minimally invasive and incurring aesthetically pleasing results; therefore, it is worth promoting.
Abbreviations
GDC, Gastric duplication cysts; EALS, endoscopy assisted laparoscopic surgery.
Data Sharing Statement
All data is contained within the manuscript and its additional files.
Ethics Approval and Consent to Participate
The present study was in accordance with the Declaration of Helsinki and was approved by the Ethics Committee of Hubei Maternal and Child Health Hospital (2022IEC017), and all the legal guardians of children involved in this study signed the informed consent form.
Acknowledgments
Yanmei Luo, Jie Liu and Zhihui Jiang are co-first authors for this study.
Author Contributions
YL, JL and ZJ contributed equally to this paper. YL and XM designed the research, analyzed the data, and wrote the manuscript; JL, ZJ, XY and SL analyzed and interpreted the data; XM designed the research, analyzed the data, and corrected the manuscript. All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This study was funded by the Research project of Hubei Maternal and Child Health Hospital (Grant No. 2021SFYM024), the Talent Introduction Fund of Yijishan Hospital of Wannan Medical College (Grant No. YR202203), the Scientific Research Project of Wannan Medical College (Grant No. WK2022ZF08), the Teaching quality and teaching reform project of Wannan Medical College (Grant No. 2022xnfz09), and Anhui provincial Department of Education university research project (Grant No. 2023AH051763). The funders had no role in the design of the study and collection, analysis, and interpretation of data and in writing the manuscript.
Disclosure
All authors declare that they have no conflicts of interest in this work.
References
1. Blakley C, Ruiz-Elizalde A, Yu Z, Palle S. Gastric duplication cyst. J Pediatr Gastroenterol Nutr. 2022;75(5):e106–7. doi:10.1097/MPG.0000000000003539
2. Massidda M, Rocchi C, Tomassini G, et al. Gastric duplication cyst: a challenging EUS differential diagnosis between subepithelial gastric lesion and exophytic pancreatic cystic neoplasm-a case report and a literature review. Clin J Gastroenterol. 2022;15(3):560–567. doi:10.1007/s12328-022-01619-3
3. Bennani A, Miry A, Kamaoui I, Harroudi T. Gastric duplication cyst in an adult with autoimmune hemolytic anemia: a case report and review of the literature. J Med Case Rep. 2018;12(1):380. doi:10.1186/s13256-018-1895-5
4. Gagne A, Sazonova O, Marceau S, Perigny M, Joubert P. A foregut duplication cyst of the stomach in association with a gastrointestinal stromal tumor and a leiomyoma: a case report. Case Rep Pathol. 2016;2016:1537240. doi:10.1155/2016/1537240
5. Zhao H, Zhai Y, Guo R, Xu H, Lv L, Zhang S. Case report: joint diagnosis and treatment of intrathoracic gastric duplication with a gastric communication in a child by laparoscopy and gastroscopy. Front Pediatr. 2023;11:1143741. doi:10.3389/fped.2023.1143741
6. Lee S, Uno K, Fujishima F, et al. Gastric duplication cyst with occult GIST component. ACG Case Rep J. 2020;7(2):e260. doi:10.14309/crj.0000000000000260
7. Hattori K, Takamizawa S. Laparoscopic-endoscopic cooperative surgery for a gastric duplication cyst in the prepyloric region to preserve the pylorus ring in a child: a case report. Asian J Endosc Surg. 2020;13(4):596–599. doi:10.1111/ases.12794
8. Ye X, Wang M, Wang Y, Lin D, Wang X. Gastric duplication cyst with ectopic pancreas in a teenager successfully resected by endoscopic submucosal dissection. BMC SURG. 2022;22(1):381. doi:10.1186/s12893-022-01837-z
9. Eom JS, Kim GH, Song GA, et al. Gastric duplication cyst removed by endoscopic submucosal dissection. Korean J Gastroenterol. 2011;58(6):346–349. doi:10.4166/kjg.2011.58.6.346
10. Belghith C, Armi S, Najar S, et al. A case of neonatal gastrointestinal duplication. Pan Afr Med J. 2021;38:353. doi:10.11604/pamj.2021.38.353.28385
11. Sharma D, Bharany RP, Mapshekhar RV. Duplication cyst of pyloric canal: a rare cause of pediatric gastric outlet obstruction: rare case report. INDIAN J SURG. 2013;75(S1):322–325. doi:10.1007/s12262-012-0697-z
12. Gale HI, Gee MS, Westra SJ, Nimkin K. Abdominal ultrasonography of the pediatric gastrointestinal tract. World J Radiol. 2016;8(7):656–667. doi:10.4329/wjr.v8.i7.656
13. Losefsky QP, Cho E, Jeyarajah DR. Adenocarcinoma arising in a gastric duplication cyst. J GASTROINTEST SURG. 2022;26(6):1336–1337. doi:10.1007/s11605-022-05261-9
14. Wang B, Hunter WJ, Bin-Sagheer S, Bewtra C. Rare potential pitfall in endoscopic ultrasound-guided fine needle aspiration biopsy in gastric duplication cyst: a case report. Acta Cytol. 2009;53(2):219–222. doi:10.1159/000325129
15. Laskowska K, Galazka P, Daniluk-Matras I, Leszczynski W, Serafin Z. Use of diagnostic imaging in the evaluation of gastrointestinal tract duplications. Pol J Radiol. 2014;79:243–250. doi:10.12659/PJR.890443
16. Namdaroglu OB, Argon A, Aydogan S, et al. Gastric duplication cyst in adult: challenge for surgeons. J MINIM ACCESS SURG. 2017;13(1):57–59. doi:10.4103/0972-9941.181772
17. Rowling JT. Some observations on gastric cysts. Br J Surg. 1959;46(199):441–445. doi:10.1002/bjs.18004619904
18. Min SH, Kim HY, Kim SH, et al. Gastric adenomyoma mimicking gastric duplication cyst in a 5-year-old girl. J PEDIATR SURG. 2012;47(5):1019–1022. doi:10.1016/j.jpedsurg.2012.02.010
19. Shi Z, Zheng Q, Zhao L, Liu B. Endoscopic septum incision for gastric duplication cyst: first clinical practice. Am J Gastroenterol. 2022;117(4):536. doi:10.14309/ajg.0000000000001653
20. Perek A, Perek S, Kapan M, Goksoy E. Gastric duplication cyst. Dig Surg. 2000;17(6):634–636. doi:10.1159/000051975
21. Pachl M, Patel K, Bowen C, Parikh D. Retroperitoneal gastric duplication cyst: a case report and literature review. Pediatr Surg Int. 2012;28(1):103–105. doi:10.1007/s00383-011-3036-8
22. Gupta V, Javaid U, Jaber G, Mohd D, AlMarzouqi M. Laparoscopic resection of isolated retroperitoneal gastric duplication cyst in an infant. J Coll Physicians Surg Pak. 2019;29:S141–3. doi:10.29271/jcpsp.2019.12.S141
23. Krishna Kumar G. Gastric duplication cyst in an infant presenting with non-bilious vomiting. Malays J Med Sci. 2012;19(1):76–78.
24. Sasaki T, Shimura H, Ryu S, Matsuo K, Ikeda S. Laparoscopic treatment of a gastric duplication cyst: report of a case. Int Surg. 2003;88(2):68–71.
25. Ren HX, Duan LQ, Wu XX, Zhao BH, Jin YY. Laparoscopic resection of gastric duplication cysts in newborns: a report of five cases. BMC Surg. 2017;17(1):37. doi:10.1186/s12893-017-0234-x
26. Zhang H, Chen J, Chen C. Comparison of laparoscopy combined with gastroscopy positioning and open resection for gastric stromal tumours: a meta-analysis. J Minim Access Surg. 2020;16(4):298–307. doi:10.4103/jmas.JMAS_269_19
27. Zhang H, Chen J, Chen C. The efficacy and safety of laparoscopy combined with gastroscopy positioning in treating gastric stromal tumours: a systematic review and meta-analysis. J Minim Access Surg. 2021;17(2):147–152. doi:10.4103/jmas.JMAS_294_19
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.