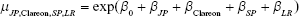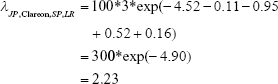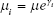Back to Journals » Clinical Ophthalmology » Volume 12
A meta-analysis of Nd:YAG capsulotomy rates for two hydrophobic intraocular lens materials
Authors Von Tress M, Marotta JS, Lane SS , Sarangapani R
Received 3 January 2018
Accepted for publication 24 April 2018
Published 22 June 2018 Volume 2018:12 Pages 1125—1136
DOI https://doi.org/10.2147/OPTH.S161380
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Mark Von Tress,1 James S Marotta,2 Stephen S Lane,3 Ramesh Sarangapani1
1Modeling and Simulation, 2IOL Projects, 3Global Clinical Strategy, Alcon Laboratories, Fort Worth, TX, USA
Purpose: The purpose of this study is to estimate and compare neodymium-doped yttrium aluminum garnet (Nd:YAG) capsulotomy rates for AcrySof® and Clareon® intraocular lens (IOL) materials using historical data from the medical literature and Alcon-sponsored clinical studies.
Methods: Clinical trials that involved the implantation of AcrySof or Clareon monofocal IOLs in subjects with cataract or presbyopia were extracted from the literature and a company repository of clinical studies. The study duration, number of eyes, and cumulative percent of Nd:YAGs for posterior capsule opacification were extracted. Bayesian random effects meta-analyses were conducted to estimate and compare outcomes for the 2 different IOL materials.
Results: A Bayesian random effects, meta-analysis was performed that combined a literature review of published AcrySof Nd:YAG posterior capsulotomy rates and Nd:YAG rates observed in Alcon-sponsored clinical studies of AcrySof and Clareon. Sixteen Alcon studies contained Nd:YAG data suitable for meta-analysis. Three of these Alcon studies contained results for the Clareon material (2 one-year studies, and 1 three-year study). The literature review included 50 papers from 1998 to 2015. In combination, 30,891 eyes were available for analysis and 2040 Nd:YAG procedures were reported in studies with a follow-up duration ranging in length from 4 months to 10 years. The overall probability of performing a Nd:YAG capsulotomy within a year of implant for AcrySof was 1.44% (1.11% to 1.83%) and 0.62% (0.21% to 1.38%) for Clareon. There was small improvement in the probability of Nd:YAG within a year of implant for Clareon lenses of about 0.82% with a 95% credible interval of (0.07% to 1.36%) at 1 year. Results were similar for incidence rates per 100 surgeries in a year: 0.62 (0.21 to 1.40) for Clareon, 1.46 (1.12 to 1.87) for AcrySof, and the difference was 0.84 (0.07 to 1.39) favoring Clareon. At 3 years, the overall probability of performing a Nd:YAG capsulotomy for AcrySof was 4.19% (3.24% to 5.30%) compared with only 1.82% (0.63% to 4.02%) for Clareon.
Conclusion: A meta-analysis of Clareon multi-piece and single-piece clinical data predicts that the cumulative Clareon Nd:YAG probability will be ≤ AcrySof by 2.37% (0.18% to 3.91%) at 3 years. The results indicate that Clareon is likely to perform as well as, and possibly better than, AcrySof in terms of Nd:YAG capsulotomy rates.
Keywords: Nd:YAG, PCO, IOL, AcrySof, Clareon, capsulotomy
Introduction
Posterior capsule opacification (PCO) is one of the most common complications following cataract surgery. PCO occurs when lens epithelial cells proliferate between the posterior capsular bag and the intraocular lens posterior optical surface and has the potential to impact the patient’s visual function. Once PCO significantly reduces visual function, treatment can be provided by using a neodymium-doped yttrium aluminum garnet (Nd:YAG) laser to create an opening in the posterior capsule. In addition to the costs associated with the procedure, Nd:YAG capsulotomy procedures carry their own complications and risks. Hence, as part of the implant selection process, surgeons should be aware of the rate at which intraocular lenses (IOLs) require Nd:YAG capsulotomy. A more detailed review of what is known about the problem may be found in the discussion section.
AcrySof and the new Clareon IOLs are made from 2 different acrylate/methacrylate copolymer hydrophobic materials. This analysis estimates the probability and incidence of Nd:YAG for AcrySof and Clareon IOLs using a meta-analysis of literature reports and Alcon-sponsored studies. Some comparisons are made of Nd:YAG probability and incidence based on statistical model estimates. The results indicate that Clareon is likely to perform as well as, and possibly better than, AcrySof in terms of Nd:YAG rates.
Methods
Materials and study conduct
All lenses and lens materials in this paper, except those in Table 1, are registered trademark products of Alcon Laboratories (Fort Worth, TX, USA). This research followed the guidelines of the Declaration of Helsinki. The studies used in this systematic review were conducted under the ethical approval of the appropriate IRB for each study.
  | Table 1 Published 3-year Nd:YAG capsulotomy rates of different IOLs |
Bibliographic research
All Alcon-sponsored IOL studies dating from 1990 to 2015 were examined for available data for this analysis. The list of studies was obtained from the Alcon Clinical Data Management group that maintains a list of all company-sponsored studies. Sixty-three studies were identified as IOL studies, which included data on monofocal lens models. Sixteen studies had Nd:YAG data available for this meta-analysis, 3 involving Clareon and 14 with AcrySof. The number of Nd:YAG procedures, the number of eyes, and the study follow-up length were extracted from the clinical study reports. The studies and data used in this analysis are listed in Table 2. A summary of the studies is given in the following outline.
- Clinical data from 3 Alcon-sponsored studies that used Clareon monofocal lens models
- Study 15, multi-piece, non-Japanese study (3-year follow-up). This study also had an AcrySof control arm
- Study 5, single-piece, non-Japanese study (1.25-year follow-up)
- Study 7, single-piece, Japanese study (1.17-year follow-up)
- Clinical data from 14 Alcon-sponsored studies that used AcrySof monofocal lens models
- Studies, protocol initiation dates ranged from 1999 to 2014
- Single piece and multi-piece
- All had “sharp” edge designs
- Studies ranged in follow-up length from 4 months to 3 years
- Number of eyes per lens model ranged from 20 to 456
- Studies were also categorized as being Japanese or non-Japanese studies
A literature review was also performed using a PubMed search of publications available before 2016. The search terms were “YAG AcrySof” or “Posterior capsulotomy AcrySof”. The first search term identified 84 papers and the second 131. Of these, 50 were found to provide sufficient information on model, study location, Nd:YAG capsulotomy rates, and follow-up duration for inclusion in the analysis.1–50 Studies were not required to be double masked or randomized comparisons. Due to the large percentage of studies conducted in Japan and the potential for cultural differences in Nd:YAG capsulotomy treatment, an analysis of Nd:YAG rates for Japanese vs non-Japanese studies was conducted. A summary of the studies is given in the following outline.
- PubMed search for papers having AcrySof monofocal lenses and Nd:YAG rates.
- 50 studies from 1998 to 2016 were found for monofocal lenses
- 12 AcrySof lens models were found (8 multi-piece and 4 single piece)
- All had “sharp” edge designs
- Study follow-up ranged in length from 1 to 10 years
- Number of eyes per lens model ranged from 19 to 6,575
- Studies were also categorized as being Japanese or non-Japanese studies.
Data from the literature search provided cumulative rates at each year. Some papers provided >1 time point per study. To calculate the number of Nd:YAG procedures for each time point, the number enrolled in the study was multiplied by the rate at that time point. Alcon studies only reported the rate at the end of the study. The studies and extracted are listed in Table 3.
Statistical methods
The Nd:YAG frequencies were assumed to follow a random effects generalized linear Poisson-normal model. For individual studies, the frequency of Nd:YAG procedures was assumed to be a Poisson random variable. The Poisson distribution is often used for the analysis of adverse event frequencies. However, because of the variation in study design, the means from each study were assumed to vary according to a log-normal distribution. The log-normal distribution has approximately the same shape as a gamma mixture distribution often used for modeling incidence heterogeneity. Bayesian methods were used to estimate the model parameters and random effects. The log-normal assumption addresses heterogeneity, as discussed in general methods for Cochrane Reviews section 9.5.51 Credible intervals are used for inference since they account for variability between and within studies. Credible intervals are marked in parentheses. The parameters and random effects were assumed to have normal prior distributions. The prior parameters were estimated by maximum likelihood to reduce bias in the estimates. The final model parameters and random effects were estimated using PROC MCMC of SAS version 9.2 software (SAS Institute, Cary, NC, USA).
The mean varied according to where the study was conducted (Japan or not), the lens material (Clareon or AcrySof), lens design (single-piece or multi-piece), and data source (literature review or Alcon-sponsored study). The indicator variable for Japan or not allows us to examine the possibility that there are different responses to the lens materials in Asian vs non-Asian eyes and to help confirm the combinability of the AcrySof and Clareon studies. The 4 indicator variables generate a list of 16 possible means, 1 for each combination of the 4 factors in this study. As an example, the incidence rate for non-Japanese, AcrySof, multi-piece, Alcon study is simply 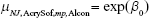 since all of the indicator variables are 0 for this combination. Similarly, the incidence rate for a Japanese, Clareon, single-piece, literature review study may be estimated by
since all of the indicator variables are 0 for this combination. Similarly, the incidence rate for a Japanese, Clareon, single-piece, literature review study may be estimated by
|
since the values of all of the indicator variables in this combination are 1. Note that this dataset will not contain any studies with this combination, however, the mean for this combination may be estimated using this combination of parameters.
The means are also called incidence rates when multiplied by 100 because they represent the number of Nd:YAG procedures expected per year for 100 surgeries. The incidence of Nd:YAG for n surgeries over t years is λ = n*t*μ, and the probability of x Nd:YAG’s is P(X = x/n, t) = exp(−λ)λx/x!. One of the advantages of using the Poisson distribution is that the incidence rate is constant over time, so the number of cases can be predicted to accumulate on a constant annual basis. This makes extrapolation reasonable, especially if there are observations from other groups across the same time frame.
As an example, the expected number of events in a Japanese, Clareon, single-piece, literature review study of 100 patients for 3 years is estimated by
|
where the numbers in parentheses in the first line come from Table 4. The value 100 represents the number of patients and 3 is the number of years. The exp() factor is the rate per person per year, or incidence rate. All of the 16 combinations of factors can be estimated from other combinations of the parameters. Hence, expected event frequencies may be estimated indirectly for combination factors that may not have been observed.
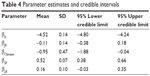  | Table 4 Parameter estimates and credible intervals |
Note that because the degree of heterogeneity among the different studies varies in terms of length, study design/execution, and population, many of the assumptions required for simple descriptive statistics are invalidated. That is, the usual assumption of identical distributions for the different study parameters is not valid for this combination of studies. Consequently, because of the variation in study design, the means from each study were assumed to vary according to a log-normal distribution, and Bayesian meta-analysis methods were used to account for study heterogeneity. The mean for study i is expressed mathematically as
|
where γi is a normally distributed random variable with a mean of zero. This expression addresses heterogeneity of studies by multiplying the study mean by a heterogeneity factor, or equivalently, by adding a random effect for study variation to the log of the study mean.
Results
Sixteen Alcon studies contained Nd:YAG data suitable for meta-analysis. Three of these Alcon studies contained results for the Clareon material (2 one-year studies, and 1 three-year study). The literature review included 50 papers from 1998 to 2015. In combination, 30,891 eyes were available for analysis and 2040 Nd:YAG capsulotomy procedures were reported in studies ranging in length from 4 months to 10 years. The probability for the occurrence of a Nd:YAG capsulotomy is estimated in Figure 1 with 95% credible intervals. The 3 lines are from the literature search (brown), AcrySof clinical studies (blue), and Clareon studies (black). The dashed lines indicate where estimates are extrapolated from the observed data. It should be noted that the AcrySof and literature search results for AcrySof are close, which helps to validate the extrapolation for Alcon clinical studies. Also, the asymmetry in the Clareon data range in Figure 1 may result from using logarithms to estimate incidence rates and from the Clareon study durations (1 and 3 years).
  | Figure 1 Combined probability of a Nd:YAG by time for literature review, Alcon AcrySof and Clareon studies. |
At 3 years, Clareon and AcrySof probabilities of an Nd:YAG differed by 2.37% with a 95% credible interval of 0.18% to 3.91% favoring Clareon. The probability of an Nd:YAG by 3 years was 4.19% (3.24% to 5.30%) for the AcrySof lens compared with 1.82% (0.63% to 4.02%) for the Clareon lens (Table 5).
The credible interval for incidence per 100 surgeries per year was 0.62 (0.21 to 2.21) per year for Clareon, and 1.46 (1.12 to 1.87) per year for AcrySof. The difference in predicted incidence was 0.84 cases per year with a prediction interval of (0.07 to 1.39) cases per year.
Sensitivity analyses
Sensitivity to the design factor differences was examined in terms of the 3-year probability of a Nd:YAG (Table 5) and in terms of incidence (Table 6). Differences between lens materials and between factors were found to be negligible with respect to the data source (literature vs internal) and study location (Japan vs non-Japan) for the 3-year probabilities of a Nd:YAG. The credible intervals did not contain zero for the comparisons of multi-piece to single-piece lens designs. However, the credible intervals were short indicating that a conclusion of non-inferiority of multi- to single-piece lens models may be more appropriate instead of a conclusion of superiority. This non-inferiority result supports the combination data from the multi- and single-piece lenses. These results may be attributed to the large sample size available for analysis rather than a true difference. This conclusion is also supported in other studies.52,53
  | Table 6 Average number of cases expected per 100 surgeries per year (incidence) |
Figures 2 and 3 are forest plots of the analysis results. The figures clearly demonstrate the study heterogeneity. Many of the simple estimates of probability of an event per person-year fall outside of the credible regions. The combination of these outliers with other studies using Bayesian methods suppressed the influence of these studies in the overall analysis.
  | Figure 2 Forest plot comparing probability per person-year for Clareon and AcrySof studies from the Alcon internal clinical database (top 3 bars are Clareon data). |
  | Figure 3 Forest plot comparing probability per person-year for internal Clareon studies and published literature studies on AcrySof (top 3 bars are Clareon data). |
Table 4 contains the parameter estimates and 95% credible intervals.
The parameter estimate for Clareon was negative and its credible interval did not include zero, which suggests a statistically significant reduction in Nd:YAG incidence. Similarly, single-piece lens designs had a slightly greater incidence of Nd:YAG since its parameter estimate was positive and the credible interval did not include zero. However, these statistically significant differences did not translate into large changes in incidence rates as reported in Table 6. The heterogeneity factors were log-normally distributed with a mean of 1.40, a SD of 1.21, and a 95% credible interval of (0.16 to 4.63).
Discussion
A meta-analysis was performed to estimate Nd:YAG capsulotomy probability and incidence for intraocular lenses made of either Clareon or AcrySof material. The analysis included data from 16 internal Alcon clinical studies, and 50 studies taken from the literature. Results for AcrySof lenses from the literature review and those conducted by Alcon were consistent suggesting that future literature results for Clareon may be similar to those of the 3 internal Clareon studies. The 3 internal Clareon studies were single arm. Under ideal conditions for a meta-analysis, all of the studies would be randomized, double-masked, comparative studies if the purpose of this paper had been to compare Nd:YAG capsulotomy rates. Consequently, inference regarding material comparisons from this meta-analysis must be tempered by the fact that multiple study designs, study lengths, study periods, and lens designs were combined to estimate and compare rates. Statistical methods were used to help compensate for the heterogeneity of the combined studies.
This analysis combined results from both multi-piece and single-piece lenses into a single database for each lens material. Despite the differences in lens design, this combination of data is relevant because of the sensitivity analysis findings that the Nd:YAG capsulotomy rates are non-inferior. It is also supported by the conclusion of a 2010 Cochrane Review on PCO, which found that there was no significant difference in the Nd:YAG capsulotomy rate when comparing single-piece and multi-piece acrylic lenses.55 In addition, several published clinical studies have concluded that there is no difference in the PCO or Nd:YAG rate between multi-piece and single-piece lenses made from the same material (AcrySof). Bender et al, reviewed a series of 131 cataract patients and concluded that there was statistically significant difference in the percentage area of PCO between the AcrySof single-piece and multi-piece lenses when followed for 1 year.17 Zemaitienė et al conducted a randomized study of 74 eyes unilaterally implanted with either an AcrySof single-piece or multi-piece lenses and concluded that with 2-year follow-up, there was no difference in the PCO development of the 2 lens designs.3 Sacu et al conducted a bilateral randomized study where an AcrySof multi-piece lens was implanted in one eye followed by implantation of an AcrySof single-piece lens implanted in the other eye of 52 patients.54 After 2 years, the authors concluded that there was no statistically significant difference in PCO for the 2 lens models. Leydolt et al published the 5-year follow-up from the same patients that were randomized in the Sacu et al publication and reported that there was no significant difference in both the PCO and Nd:YAG rates between the 2 lens models.38,54 Duman et al reported on a retrospective review of 4,970 eyes followed for a mean time of 84 months and also concluded that there was no difference in the PCO rate of single-piece and multi-piece lenses made from the AcrySof material.2 Similarly, this meta-analysis also concludes that the probability of an Nd:YAG being performed with single-piece and multi-piece lenses made from the same material is similar.
The ultimate selection of an IOL should be driven not only by its efficacy and safety, but also by an assessment of both the cost of the lens and the subsequent cost of higher Nd:YAG rates associated with implantation of that lens. A 2010 Cochrane Review on PCO concluded that hydrophobic acrylic lenses have significantly lower Nd:YAG capsulotomy rates when compared with hydrophilic lenses.55 The health economic benefit of reduced Nd:YAG capsulotomy rates for cataract patients has been discussed in previous publications.36,56,57 Cullin et al compared the added cost of higher Nd:YAG rates for hydrophilic vs hydrophobic IOLs within their Swedish clinic.36 The authors concluded that the increased risk of capsulotomy for the hydrophilic IOL (17.7% at 2 years) would result in a higher total average costs for cataract surgery. Two other clinical studies tried to expand on the economic risk to patients by combining both the cost of the capsulotomy procedure and the cost of treatment for potential adverse events associated with a Nd:YAG treatment. Smith et al conducted a retrospective review of 1,525 patients implanted with 4 different types of IOLs in order to identify the cost-effectiveness ratio (cost per patient without Nd:YAG laser capsulotomy intervention) for each lens type.57 The costs of Nd:YAG laser treatment and its complications (using the incidence rate of each) were estimated based on data collected by the 4 European investigators. The authors’ analysis showed that hydrophilic IOLs had the highest Nd:YAG rate (31.1% at 3.2 years) among those lenses implanted and subsequently, also had the worst cost-effectiveness ratio in all 4 countries. Hydrophobic acrylic lenses, which had the lowest Nd:YAG rates, were found to be most cost-effective. Boureau et al conducted a retrospective study in order to estimate the cost of Nd:YAG treatment and its complications, which would be paid by the French Social Insurance. Hydrophilic IOLs added the highest cost to cataract surgery; the authors estimated that the cost per patient of a Nd:YAG laser treatment and its complications would be €318.74.56 They concluded that the exclusive use of hydrophilic lenses (which have higher Nd:YAG rates) in France could generate €67.5 to €70.2 million in additional cost to Social Insurance. All 3 of these economic publications have at their center the same message, lenses with lower Nd:YAG capsulotomy rates (like both AcrySof and Clareon) contribute to improved health economics when compared with lenses with higher Nd:YAG rates.
From our analysis, the probability of an Nd:YAG capsulotomy within the first 3 years was estimated to be 4.19% (3.24% to 5.30%) for the AcrySof lens compared with 1.82% (0.63% to 4.02%) for the Clareon lens. These 3-year Nd:YAG capsulotomy rates are substantially lower than those rates, which have been reported for many other IOLs in the clinical literature (Table 1).
In formal statistical language, the results indicate that incidence of Nd:YAG for Clareon is likely to be non-inferior to AcrySof by a non-inferiority margin of 5 cases per 100 surgeries per year. AcrySof is expected to have 1.46 cases per 100 surgeries per year, and Clareon is expected to have 0.62 cases per 100 surgeries per year. These results are similar to the most recent Cochrane Review on this subject, which found low incidence rates over 1–2 years, and no difference between 1-piece and 3-piece hydrophobic acrylic IOLs (odds ratio 1.06 [95% CI: 0.27–4.19]).55 Further long-term clinical studies on Clareon are needed to support these results.
Conclusion
A Bayesian random effects, meta-analysis was performed that combined a literature review of published AcrySof Nd:YAG posterior capsulotomy rates and Alcon-sponsored study Nd:YAG rates for AcrySof and Clareon. In total, 30,891 eyes were available for analysis and 2040 Nd:YAG capsulotomy procedures were reported in studies ranging in length from 4 months to 10 years, though data for Clareon are limited to 3 years. The results indicate that Clareon is likely to perform as well as, and possibly better than, AcrySof in terms of Nd:YAG capsulotomy rates. The probability of an Nd:YAG capsulotomy being performed in the first 3 years after implantation is 4.19% (3.24% to 5.30%) for AcrySof compared with 1.82% (0.63% to 4.02%) for the Clareon lens. Further long-term clinical studies on the Clareon lens are needed to support these results.
Disclosure
All authors are Alcon employees. The authors report no other conflicts of interest in this work.
References
Nishi Y, Ikeda T, Nishi K, Mimura O. Epidemiological evaluation of YAG capsulotomy incidence for posterior capsule opacification in various intraocular lenses in Japanese eyes. Clin Ophthalmol. 2015;9:1613–1617. | ||
Duman R, Karel F, Özyol P, Ateş C. Effect of four different intraocular lenses on posterior capsule opacification. Int J Ophthalmol. 2015;8(1):118–121. | ||
Zemaitienė R, Jašinskas V. Prevention of posterior capsule opacification with 3 intraocular lens models: a prospective, randomized, long-term clinical trial. Medicina (Kaunas). 2011;47(11):595–599. | ||
Lundqvist B, Mönestam E. Ten-year longitudinal visual function and Nd:YAG laser capsulotomy rates in patients less than 65 years at cataract surgery. Am J Ophthalmol. 2010;149(2):238–244. | ||
Ram J, Kumar S, Sukhija J, Severia S. Nd:YAG laser capsulotomy rates following implantation of square-edged intraocular lenses: polymethyl methacrylate versus silicone versus acrylic. Can J Ophthalmol. 2009;44(2):160–164. | ||
Rönbeck M, Zetterström C, Wejde G, Kugelberg M. Comparison of posterior capsule opacification development with 3 intraocular lens types: five-year prospective study. J Cataract Refract Surg. 2009;35(11):1935–1940. | ||
Vock L, Menapace R, Stifter E, Georgopoulos M, Sacu S, Bühl W. Posterior capsule opacification and neodymium:YAG laser capsulotomy rates with a round-edged silicone and a sharp-edged hydrophobic acrylic intraocular lens 10 years after surgery. J Cataract Refract Surg. 2009;35(3):459–465. | ||
Vock L, Crnej A, Findl O, et al. Posterior capsule opacification in silicone and hydrophobic acrylic intraocular lenses with sharp-edge optics six years after surgery. Am J Ophthalmol. 2009;147(4):683–690. | ||
Hayashi K, Yoshida M, Hayashi H. Comparison of posterior capsule opacification between fellow eyes with two types of acrylic intraocular lens. Eye (Lond). 2008;22(1):35–41. | ||
Kohnen T, Fabian E, Gerl R, et al. Optic edge design as long-term factor for posterior capsular opacification rates. Ophthalmology. 2008;115(8):1308–1314. | ||
Hancox J, Spalton D, Heatley C, et al. Fellow-eye comparison of posterior capsule opacification rates after implantation of 1CU accommodating and AcrySof MA30 monofocal intraocular lenses. J Cataract Refract Surg. 2007;33(3):413–417. | ||
Hayashi K, Yoshida M, Hayashi H. Posterior capsule opacification in myopic eyes. J Cataract Refract Surg. 2006;32(4):634–638. | ||
Davison JA. Neodymium:YAG laser posterior capsulotomy after implantation of AcrySof intraocular lenses. J Cataract Refract Surg. 2004;30(7):1492–1500. | ||
Mian SI, Fahim K, Marcovitch A, Gada H, Musch DC, Sugar A. Nd:YAG capsulotomy rates after use of the AcrySof acrylic three piece and one piece intraocular lenses. Br J Ophthalmol. 2005;89(11):1453–1457. | ||
Findl O, Menapace R, Sacu S, Buehl W, Rainer G. Effect of optic material on posterior capsule opacification in intraocular lenses with sharp-edge optics: randomized clinical trial. Ophthalmology. 2005;112(1):67–72. | ||
Auffarth GU, Brezin A, Caporossi A, et al; European PCO Study Group. Comparison of Nd:YAG capsulotomy rates following phacoemulsification with implantation of PMMA, silicone, or acrylic intra-ocular lenses in four European countries. Ophthalmic Epidemiol. 2004;11(4):319–329. | ||
Bender LE, Nimsgern C, Jose R, et al. Effect of 1-piece and 3-piece AcrySof intraocular lenses on the development of posterior capsule opacification after cataract surgery. J Cataract Refract Surg. 2004;30(4):786–789. | ||
Bilge AH, Aykan U, Akin T, Unsal U. The effects of three-piece or single-piece acrylic intraocular lens implantation on posterior capsule opacification. Eur J Ophthalmol. 2004;14(5):375–380. | ||
Mester U, Fabian E, Gerl R, et al. Posterior capsule opacification after implantation of CeeOn Edge 911A, PhacoFlex SI-40NB, and AcrySof MA60BM lenses: one-year results of an intraindividual comparison multicenter study. J Cataract Refract Surg. 2004;30(5):978–985. | ||
Abhilakh Missier KA, Nuijts RM, Tjia KF. Posterior capsule opacification: silicone plate-haptic versus AcrySof intraocular lenses. J Cataract Refract Surg. 2003;29(8):1569–1574. | ||
Wejde G, Kugelberg M, Zetterström C. Posterior capsule opacification: comparison of 3 intraocular lenses of different materials and design. J Cataract Refract Surg. 2003;29(8):1556–1559. | ||
Prosdocimo G, Tassinari G, Sala M, et al. Posterior capsule opacification after phacoemulsification: silicone CeeOn Edge versus acrylate AcrySof intraocular lens. J Cataract Refract Surg. 2003;29(8):1551–1555. | ||
Ernest PH. Posterior capsule opacification and neodymium: YAG capsulotomy rates with AcrySof acrylic and PhacoFlex II silicone intraocular lenses. J Cataract Refract Surg. 2003;29(8):1546–1550. | ||
Stordahl PB, Drolsum L. A comparison of Nd:YAG capsulotomy rate in two different intraocular lenses: AcrySof and Stabibag. Acta Ophthalmol Scand. 2003;81(4):326–330. | ||
Beltrame G, Salvetat ML, Chizzolini M, et al. Posterior capsule opacification and Nd:YAG capsulotomy rates after implantation of silicone, hydrogel and soft acrylic intraocular lenses: a two-year follow-up study. Eur J Ophthalmol. 2002;12(5):388–394. | ||
Javdani SM, Huygens MM, Callebaut F. Neodymium: YAG capsulotomy rates after phacoemulsification with hydrophobic and hydrophilic acrylic intraocular lenses. Bull Soc Belge Ophtalmol. 2002;(283):13–17. | ||
Halpern MT, Covert D, Battista C, Weinstein AJ, Levinson RD, Yan L. Relationship of AcrySof acrylic and PhacoFlex silicone intraocular lenses to visual acuity and posterior capsule opacification. J Cataract Refract Surg. 2002;28(4):662–669. | ||
Pohjalainen T, Vesti E, Uusitalo RJ, Laatikainen L. Posterior capsular opacification in pseudophakic eyes with a silicone or acrylic intraocular lens. Eur J Ophthalmol. 2002;12(3):212–218. | ||
Hayashi K, Hayashi H, Nakao F, Hayashi F. Changes in posterior capsule opacification after poly(methyl methacrylate), silicone, and acrylic intraocular lens implantation. J Cataract Refract Surg. 2001;27(6):817–824. | ||
Scaramuzza A, Fernando GT, Crayford BB. Posterior capsule opacification and lens epithelial cell layer formation: hydroview hydrogel versus AcrySof acrylic intraocular lenses. J Cataract Refract Surg. 2001;27(7):1047–1054. | ||
Küçüksümer Y, Bayraktar S, Sahin S, Yilmaz OF. Posterior capsule opacification 3 years after implantation of an AcrySof and a MemoryLens in fellow eyes. J Cataract Refract Surg. 2000;26(8):1176–1182. | ||
Kobayashi H, Ikeda H, Imamura S, et al. Clinical assessment of long-term safety and efficacy of a widely implanted polyacrylic intraocular lens material. Am J Ophthalmol. 2000;130(3):310–321. | ||
Hollick EJ, Spalton DJ, Ursell PG, et al. The effect of polymethylmethacrylate, silicone, and polyacrylic intraocular lenses on posterior capsular opacification 3 years after cataract surgery. Ophthalmology. 1999;106(1):49–54; discussion 54–55. | ||
Hayashi H, Hayashi K, Nakao F, Hayashi F. Quantitative comparison of posterior capsule opacification after polymethylmethacrylate, silicone, and soft acrylic intraocular lens implantation. Arch Ophthalmol. 1998;116(12):1579–1582. | ||
Chang A, Kugelberg M. Posterior capsule opacification 9 years after phacoemulsification with a hydrophobic and a hydrophilic intraocular lens. Eur J Ophthalmol. 2017;27(2):164–168. | ||
Cullin F, Busch T, Lundström M. Economic considerations related to choice of intraocular lens (IOL) and posterior capsule opacification frequency – a comparison of three different IOLs. Acta Ophthalmol. 2014;92(2):179–183. | ||
Chang A, Behndig A, Rønbeck M, Kugelberg M. Comparison of posterior capsule opacification and glistenings with 2 hydrophobic acrylic intraocular lenses: 5- to 7-year follow-up. J Cataract Refract Surg. 2013;39(5):694–698. | ||
Leydolt C, Davidovic S, Sacu S, et al. Long-term effect of 1-piece and 3-piece hydrophobic acrylic intraocular lens on posterior capsule opacification: a randomized trial. Ophthalmology. 2007;114(9):1663–1669. | ||
Morgan-Warren PJ, Smith JA. Intraocular lens-edge design and material factors contributing to posterior-capsulotomy rates: comparing Hoya FY60aD, PY60aD, and AcrySof SN60WF. Clin Ophthalmol. 2013;7:1661–1667. | ||
Shah GD, Vasavada AR, Praveen MR, Shah AR, Trivedi RH. Incidence and influence of posterior capsule striae on the development of posterior capsule opacification after 1-piece hydrophobic acrylic intraocular lens implantation. J Cataract Refract Surg. 2012;38(2):202–207. | ||
Nanavaty MA, Spalton DJ, Gala KB, Dhital A, Boyce J. Effect of intraocular lens asphericity on posterior capsule opacification between two intraocular lenses with same acrylic material: a fellow-eye study. Acta Ophthalmol. 2012;90(2):e104–e108. | ||
Iwase T, Nishi Y, Oveson BC, Jo YJ. Hydrophobic versus double-square-edged hydrophilic foldable acrylic intraocular lens: effect on posterior capsule opacification. J Cataract Refract Surg. 2011;37(6):1060–1068. | ||
Gangwani V, Hirnschall N, Koshy J, et al. Posterior capsule opacification and capsular bag performance of a microincision intraocular lens. J Cataract Refract Surg. 2011;37(11):1988–1992. | ||
Shah VC, Russo C, Cannon R, Davidson R, Taravella MJ. Incidence of Nd:YAG capsulotomy after implantation of AcrySof multifocal and monofocal intraocular lenses: a case controlled study. J Refract Surg. 2010;26(8):565–568. | ||
Boureau C, Lafuma A, Jeanbat V, Berdeaux G, Smith AF. Incidence of Nd:YAG laser capsulotomies after cataract surgery: comparison of 3 square-edged lenses of different composition. Can J Ophthalmol. 2009;44(2):165–170. | ||
Biber JM, Sandoval HP, Trivedi RH, de Castro LE, French JW, Solomon KD. Comparison of the incidence and visual significance of posterior capsule opacification between multifocal spherical, monofocal spherical, and monofocal aspheric intraocular lenses. J Cataract Refract Surg. 2009;35(7):1234–1238. | ||
Nekolová J, Jirásková N, Pozlerová J, Rozsíval P. Three-year follow-up of posterior capsule opacification after AquaLase and NeoSoniX phacoemulsification. Am J Ophthalmol. 2009;148(3):390–395. | ||
Vasavada AR, Shah A, Raj SM, Praveen MR, Shah GD. Prospective evaluation of posterior capsule opacification in myopic eyes 4 years after implantation of a single-piece acrylic IOL. J Cataract Refract Surg. 2009;35(9):1532–1539. | ||
Kugelberg M, Wejde G, Jayaram H, Zetterström C. Two-year follow-up of posterior capsule opacification after implantation of a hydrophilic or hydrophobic acrylic intraocular lens. Acta Ophthalmol. 2008;86(5):533–536. | ||
Kugelberg M, Wejde G, Jayaram H, Zetterström C. Posterior capsule opacification after implantation of a hydrophilic or a hydrophobic acrylic intraocular lens: one-year follow-up. J Cataract Refract Surg. 2006;32(10):1627–1631. | ||
Higgins J, Thompson S, Spiegelhalter D. A re-evaluation of random effects meta-analysis. J R Stat Soc Ser A Stat Soc. 2009;172(1):137–159. | ||
Buehl W, Findl O. Effect of intraocular lens design on posterior capsule opacification. J Cataract Refract Surg. 2008;34:1976–1985. | ||
Ness P, Werner L, Maddula S, et al. Pathology of 219 human cadaver eyes with 1-piece or 3-piece hydrophobic acrylic intraocular lenses: capsular bag opacification and sites of square-edged barrier breach. J Cataract Refract Surg. 2011;37:923–930. | ||
Sacu S, Findl O, Menapace R, Buehl W, Wirtitsch M. Comparison of posterior capsule opacification between the 1-piece and 3-piece Acrysof intraocular lenses: two-year results of a randomized trial. Ophthalmology. 2004;111(10):1840–1846. | ||
Findl O, Buehl W, Bauer P, Sycha T. Interventions for preventing posterior capsule opacification. Cochrane Database Syst Rev. 2010;(2):CD003738. | ||
Boureau C, Lafuma A, Jeanbat V, Smith AF, Berdeaux G. Cost of cataract surgery after implantation of three intraocular lenses. Clin Ophthalmol. 2009;3:277–285. | ||
Smith AF, Lafuma A, Berdeaux G, et al. Cost-effectiveness analysis of PMMA, silicone, or acrylic intra-ocular lenses in cataract surgery in four European countries. Ophthalmic Epidemiol. 2005;12(5):343–351. | ||
Vasavada AR, Raj SM, Shah A, Shah G, Vasavada V, Vasavada V. Comparison of posterior capsule opacification with hydrophobic acrylic and hydrophilic acrylic intraocular lenses. J Cataract Refract Surg. 2011;37(6):1050–1059. | ||
Leydolt C, Kriechbaum K, Schriefl S, Pachala M, Menapace R. Posterior capsule opacification and neodymium:YAG rates with 2 single-piece hydrophobic acrylic intraocular lenses: three-year results. J Cataract Refract Surg. 2013;39(12):1886–1892. | ||
Kahraman G, Amon M, Ferdinaro C, Nigl K, Walch M. Intraindividual comparative analysis of capsule opacification after implantation of 2 single-piece hydrophobic acrylic intraocular lenses models: three-year follow-up. J Cataract Refract Surg. 2015;41(5):990–996. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.