Back to Journals » Medical Devices: Evidence and Research » Volume 17
A Human Whole Blood Culture System Reveals Detailed Cytokine Release Profiles of Implant Materials
Authors Klimosch SN, Weber M, Caballé-Serrano J, Knorpp T, Munar-Frau A, Schaefer BM, Schmolz M
Received 15 October 2023
Accepted for publication 19 December 2023
Published 5 January 2024 Volume 2024:17 Pages 23—36
DOI https://doi.org/10.2147/MDER.S441403
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Sascha Niclas Klimosch,1,* Marbod Weber,1,* Jordi Caballé-Serrano,2,3 Thomas Knorpp,1 Antonio Munar-Frau,2 Birgit Margareta Schaefer,4 Manfred Schmolz1
1HOT Screen GmbH, Reutlingen, Germany; 2Department of Oral and Maxillofacial Surgery, Universitat Internacional de Catalunya, Barcelona, Spain; 3Department of Periodontology, School of Dental Medicine - University of Bern, Bern, Switzerland; 4Geistlich Pharma AG, Wolhusen, Switzerland
*These authors contributed equally to this work
Correspondence: Manfred Schmolz, HOT Screen GmbH, Aspenhaustr. 25, Reutlingen, 72770, Germany, Tel +49 7121 628705-0, Fax +49 7121 628705-90, Email [email protected]
Introduction: Common in vitro cell culture systems for testing implant material immune compatibility either rely on immortal human leukocyte cell lines or isolated primary cells. Compared to in vivo conditions, this generates an environment of substantially reduced complexity, often lacking important immune cell types, such as neutrophil granulocytes and others. The aim of this study was to establish a reliable test system for in vitro testing of implant materials under in vivo-like conditions.
Methods: Test materials were incubated in closed, CO2-independent, tube-based culture vessels containing a proprietary cell culture medium and human whole blood in either a static or occasionally rotating system. Multiplex cytokine analysis was used to analyze immune cell reactions.
Results: To demonstrate the applicability of the test system to implant materials, three commercially available barrier membranes (polytetrafluoroethylene (PTFE), polycaprolactone (PCL) and collagen) used for dental, trauma and maxillofacial surgery, were investigated for their potential interactions with immune cells. The results showed characteristic differences between the static and rotated incubation methods and in the overall activity profiles with very low immune cell responses to PTFE, intermediate ones to collagen and strong reactions to PCL.
Conclusion: This in vitro human whole blood model, using a complex organotypic matrix, is an excellent, easily standardized tool for categorizing immune cell responses to implant materials. Compared to in vitro cell culture systems used for materials research, this new assay system provides a far more detailed picture of response patterns the immune system can develop when interacting with different types of materials and surfaces.
Keywords: whole blood cultures, in vitro material testing, immune cells, cytokines, barrier membranes
Introduction
One of the mainstays in oral and maxillofacial surgery, as well as other areas of regenerative medicine, is the use of implant materials. These are used either to stabilize/replace fractured or fragile structures,1 support the regeneration of tissues,2 or even form barriers between tissue compartments.3 Depending on the final purpose of materials used in these situations, their characteristics must be optimized in terms of their biological, chemical and physical properties, while biocompatibility must be regarded as the most crucial one.4,5 Although plasma proteins and surrounding tissues are the first to come into contact with the surface of the implant, the body recognizes such “foreign” materials mainly through its sentinels, the cells of the immune system.6 These consists of a variety of types and subtypes of leukocytes (“white blood cells”, eg different types of monocytes/macrophages, granulocytes, T cells, B cells, and NK cells). Each has a specific repertoire of receptors not only for recognizing foreign materials (pattern recognition receptors, such as Toll-like receptors, C-type lectin receptors, NOD-like receptors or RIG-I-like receptors), but also for responding to such challenges, like phagocytosis, release of oxygen radicals, secretion of cytokines and chemokines, small molecular weight mediators, etc.7
Macrophages are present in all organs and tissues and play a central role in the organism’s response to implanted materials.8 Alternatively, they may be attracted and activated indirectly by non-cellular mechanisms such as activation of the complement system9 or the coagulation system triggered by the implantation process.10 The same is true for neutrophil granulocytes, the predominant type of immune cell in blood, which can express a wide spectrum of activities when exposed to foreign materials.11 Since implants usually come into contact with blood during surgery, a functional whole blood-based cell culture assay can provide further insights and minimize the gap between traditional biocompatibility testing and the in vivo situation.
To date, mandatory biocompatibility testing for the evaluation and approval of medical devices includes topics such as genotoxicity, carcinogenicity, blood interactions or cytotoxicity (ISO 10993), but does not take into account the human immune system, especially in view of its inherent complexity. As a result, a plethora of in vitro tests exist and are commercially offered according to ISO 10993.12
However, current immunocompatibility testing of solid materials is mostly based on the use of single types of immune cells (eg macrophages),13–15 or – when investigations aim for more complexity – mixed populations of limited diversity like peripheral blood mononuclear cells (PBMC).16 It would therefore be a major improvement in materials testing to have an assay system available that allows a more comprehensive, physiologic characterization of the response patterns of immune cells upon contact with the candidate materials. Moreover, such a system should ideally also include other physiologically relevant components. This is particularly true for blood plasma proteins, platelets17 and the complement system.18
A static cell culture model that meets all of these criteria was developed more than two decades ago to test drug activity and is widely used in clinical studies (TruCulture®).19,20 It is generally used as a static cell culture system and was primarily developed for ex vivo testing of the effects of pharmaceutical drugs on the immune system. As a closed and CO2-independent system, TruCulture provided an ideal basis for the development of a new cell culture system for testing the immunocompatibility of solid materials. However, the setup had to be changed as a static whole blood culture is sub-optimal for materials testing due to the inherent sedimentation of cellular components over time. Therefore, a rotational method had to be implemented.
The main objective in development of this innovative cell culture model was to define the optimal incubation conditions for such material testing in whole blood cultures. One of the main characteristics of whole blood, when incubated for more than a few minutes, is an inevitable, moderately rapid sedimentation of its cellular elements (erythrocytes, leukocytes and platelets).21,22 These have different densities and sedimentation rates, resulting in the formation of two layers: a thick bed of red blood cells at the bottom of the culture vessel, on top of which the white blood cells (ie the immune cells) settle much more slowly, forming a second, very thin layer called the “buffy coat”.23 Hence, the use of whole blood cultures generates a peculiar problem when testing solid or semi-solid materials: Static cultures, such as those normally used for functional immune cell assays, would require very precise positioning of the specimen in relation to the buffy coat to ensure that the material is in sufficient contact with the thin layer of white blood cells. Moreover, the position of the buffy coat is different for each blood donor because the amount of red blood cells (ie their hematocrit) varies. Alternatively, vertically inserted test bodies in such whole blood cultures, would pierce the buffy coat resulting in a very reduced contact area between the surface of the material and the immune cells forming a thin line that would minimize the sensitivity of such an assay. One possible remedy would be to rotate the cultures, preventing cell sedimentation, and redistributing immune cells from adhesion to suspension (and back again). This also increases the number of cells that get into contact with the test material during incubation. On the other hand, rotation introduces shear forces, which may lead to a premature detachment of immune cells, which in turn affects the translational value of such test results. In terms of hemocompatibility testing, Chandler loops and their modifications, are the most prominent in vitro systems for testing solid materials with whole blood.24,25 The main disadvantages are the large volumes of blood required, the relatively large contact area of the loop compared to the small samples, and incubation times of only a few hours. Therefore, there is a strong demand for alternative in vitro whole blood assays to investigate the immunocompatibility of implant materials. The experiments presented here were aimed at establishing such an assay system and optimizing basic assay parameters. In addition, three surgically used barrier membranes of different materials were tested for their immunobiological activity.
Materials and Methods
Blood Donors
All healthy blood donors provided written informed consent before phlebotomy, as approved by the Ethics Committee of the University of Tübingen (Project No. 457/2021BO2). Heparinized blood (50 IU/mL) of a total of 24 donors was obtained and used in the cultures not later than 60 minutes after drawing in order to avoid storage-related changes in viability and the activation state of the leukocytes. Exclusion criteria for blood donation were as follows: Symptoms of systemic or local inflammatory reactions (except for single small and superficial skin lesions), last symptoms of systemic or local inflammatory reactions of an inflammatory disease (or first symptoms of a new episode) within the last 14 days before blood donation, vaccination within the last six weeks, surgery within the last three months, chronic diseases with inflammatory components (even during symptom-free intervals), drug intake within the last 14 days (except for contraceptives) or consumption of alcohol (eg >0.5 L of wine or 1 L of beer on the evening prior to blood donation), or strenuous exercise performed within three hours before blood donation.
Test Materials
Three different types of commercially available barrier membranes made of polytetrafluoroethylene (PTFE; Cytoplast TXT, Osteogenics Biomedical), natural porcine collagen membrane (collagen; Geistlich Bio-Gide, Geistlich Pharma AG), or polycaprolactone (PCL; Osteoguide, Genoss Co.) were tested in these whole blood cultures. Nelfilcon A (NelA) contact lenses (Dailies AquaComfort Plus, Alcon), consisting of polyvinyl alcohol, hydroxypropylmethyl-cellulose, polyethylene glycol and N-formylmethyl acrylamide were used as mediate positive control and High-density polyethylene (HDPE; Food and Drug Safety Center, Hatano Research Institute) served as biological inert materials. All test materials were obtained and used sterile. Macroscopic images of the barrier membranes were taken with a Sony α58 camera.
Cell Culture System
All specimens were trimmed to fit into the 3 mL tubes (used for TruCulture) and two milliliter CO2-independent proprietary medium (TruCulture) was added. One milliliter of freshly drawn, heparinized human whole blood was transferred into these tubes and incubated at 37°C for 48h. Tubes were cultured either in a block thermostat (VLM block thermostat, Köhler Automobiltechnik GmbH) for static cultures or periodically resuspended in a sample mixer (HulaMixer, Thermo Fisher Scientific) placed in a common cell culture incubator without CO2 supply to prevent sedimentation of cellular components. Unstimulated cultures were used as negative control, while lipopolysaccharide (LPS) in combination with Staphylococcal Enterotoxin B (SEB) were used at suboptimal concentrations in order to induce a pronounced, but still not maximal cytokine response. After incubation cell cultures were centrifuged (500g; 10 minutes) and supernatants were stored (< −20°C) until cytokine detection.
Acridine Orange Staining
After incubation and supernatant collection, membranes were carefully collected, washed two times in Hanks’ Balanced Salt Solution with Ca++ and Mg++ (HBSS+) and stored in cell culture medium, as used for the incubation. Subsequently Membranes were put on a slide and stained with 50 µL of Acridine Orange working solution (0.01mg/mL in HBSS without Ca++ and Mg++) for 5 minutes until microscopic images were taken with a Nikon D7000 on a Leitz Aristoplan fluorescence microscope.
Cytokine Detection
The release of cytokines characteristic for leukocyte activation were chosen to evaluate functional immune cell responses to the different materials tested. Mediator release was measured using bead-based multiplexed sandwich immunoassays (Luminex™ technology) on a Luminex 200™ analyser system. Data was interpreted using proprietary analysis software developed by Myriad RBM (Austin, USA). The following endpoints were measured: IFNγ, IL-1β, IL-1RA, IL-6, IL-8, IL-10, IL-12p40, GM-CSF, TNFα, MCP-1, and MIP-1β.
Statistical Analysis
Statistical analyses were performed using GraphPad Prism version 9.3.1 for Windows. The comparison of the means was performed by one-way analysis of variance (ANOVA) for repeated measurements followed by Tukey’s multiple comparison test or two-way ANOVA followed by Šídák’s multiple comparison test. Differences of p < 0.05 were considered significant.
Results
Establishing the Rotated Human Whole Blood Assay to Test Solid Materials
In accordance with static whole blood cultures, rotated cultures showed similar kinetics for cytokine release (data not shown). A few mediators (eg TNFα or IL-1β) are released within the first 24 hours, whereas many other cytokines require more time to be synthesized and released (eg: IFNγ, IL-2, or IL-10). Therefore, the number of measurable endpoints in the cultures presented below was optimized by choosing an incubation time of 48 hours. Incubation times of 72 hours or more are not recommended as nutrients are gradually depleted, culture conditions deteriorate, and cell viability decreases significantly.
Various types of materials were considered appropriate controls, during the development process. As with static cultures (TruCulture), non-stimulated cultures and LPS/SEB-activated cultures could be used as negative and stimulation controls, respectively. HDPE, classified as negative control for cytotoxicity testing according to DIN EN ISO 10993–5, showed very low activation of immune cells and was used as a negative material control. NelA showed moderate, inter-individual variation in response patterns and was used as positive material control.
The final design of this assay, as used to generate the results presented in this study, was as follows: Test materials were placed in the TruCulture tubes and two milliliters of medium were added. Control tubes were either supplemented with LPS (bacterial lipo-polysaccharide, HyCult) plus SEB (staphylococcal enterotoxin B, Bernhard Nocht Institute, Germany; stimulation control), or left without stimulation (negative control). One milliliter of freshly drawn blood was then transferred into these tubes and incubated for a total of 48 h at 37°C in the static model or with intermittent rotation (Figure 1). At the end of incubation, the culture vessels were centrifuged (500 x g; 10 minutes) and cell culture supernatants were stored (< −20°C) until assayed for cytokine release.
The positive and negative controls were used to compare the rotated test system side-by-side with the static assay. In addition, two different types of materials were analyzed: 1) HDPE, positioned in the tubes at approximately the level where the buffy coat was expected to form during the static culture, 2) NelA, a daily disposable hydrogel contact lens that is not intended to get into direct contact with whole blood and is generally classified as a Class II medical device.
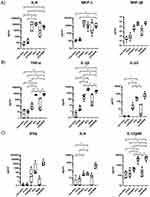
In general, there were only minor differences between the results of the static and the rotated cell culture models. Negative controls (non-stimulated and HDPE-containing cultures) were very low for both systems, with stronger effects for the positive control with known inter-individual differences in immune cell responses (NelA) and very strong effects for the stimulation control (LPS/SEB; see Figures 2 and 3). These results were promising as the static whole blood culture served as a benchmark for the rotating system. A closer look at the cytokine levels revealed that both negative as well as positive controls behaved as expected in the rotated system.
 |
Figure 3 Comparison of static versus rotated whole blood cultures. Shown are the immunoregulatory cytokines MCP-1, MIP-1β, IL-10 and IL-1RA. In accordance with Figure 2 boxplots show single values of eight independent runs, using the blood of three varying healthy donors for each run (N = 24). Statistical differences were determined using two-way ANOVA followed by Šídák’s multiple comparison test (***p < 0.001, ****p < 0.0001). Significant differences for the static model are not shown. |
In addition, the rotated model showed lower background levels for most cytokines for the unstimulated negative control and HDPE (IL-1β, IL-6, MCP-1, and MIP-1β), while mediating significantly higher MCP-1 levels for NelA with the same tendency for MIP-1β, IL-10, IL-1β and IFNγ.
Proof of Concept Experiments with Dental Barrier Membranes
Upon completion of the development of this rotating human whole blood assay, three different types of commercially available barrier membranes for dental, trauma, and maxillofacial surgery, were tested. These differed mainly in their basic composition (PTFE, collagen, and PCL) and macroscopic surface texture, reflecting their natural or synthetic origin (Figure 4A). Barrier membranes were ideal specimens for this type of test system as they are in direct contact with whole blood in vivo, as will be the case for most other implantable materials. These experiments were carried out on the blood of six different healthy volunteers in order to identify inter-donor variations.
After incubation in human whole blood cultures, the supernatants were collected and mediator secretion was quantified by multiplex immunoassays. In addition, the membranes were removed and macroscopic images were taken. Subsequently, the membranes were stained with Acridine Orange for microscopy (Figure 4C).
Compared to the untreated PTFE membranes, incubation in human whole blood cultures resulted in virtually no optical or mechanical changes in the synthetic PTFE membranes. In contrast, the collagen and PCL membranes became soft and elastic (collagen) or very fragile (PCL), resulting in rupture of the PCL membranes. In addition, faint (PCL) or obvious red deposits (collagen) were seen on the membranes (Figure 4B). This finding was also confirmed by microscopic images, showing darker areas on the collagen membranes (Figure 4C).
Acridine Orange-staining also revealed adherent cells in a material-dependent manner (PTFE: low; collagen: medium; PCL: high). The visibility and morphological structure of the nuclei (polymorphic or horseshoe-shaped) indicated immune cells (eg neutrophiles or monocytes/macrophages) (Figure 4D). Simultaneously, cytokines were quantified in the culture supernatants. Figure 5 shows nine representative analytes, that can be grouped into chemokines (Figure 5A), cytokines mainly produced by myeloid cells (Figure 5B) and/or by lymphocytes (Figure 5C). Negative controls consistently showed low to undetectable levels, whereas stimulation controls (LPS/SEB) induced high levels of these analytes in the whole blood cultures from all six donors.
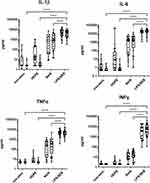
Among the materials tested, NelA mediated the greatest variability between donors. In general, immune cells from two donors were highly activated for several cytokines (see Figure 4: IL-8, MCP-1, TNFα, IL-1β, and IL-10), whereas those from the other donors secreted comparatively low concentrations of these mediators. However, when looking at the results of the barrier membranes, PCL showed significantly higher levels of most mediators compared to the other membranes, NelA and the negative control. In contrast, PTFE showed no significant immune cell activation and cytokine and chemokine levels similar to the negative control (see Figure 5). The collagen membrane, on the other hand, showed moderate induced cytokine release (see Figure 5). Some cytokine levels induced by the barrier membranes even exceeded the levels of the stimulation control, such as IL-8, TNFα, and IL-1β for PCL and MCP-1 for collagen.
Characterization of Immune Cell Responses Measured by Means of This Whole Blood System
Based on the results shown in Figure 5, an additional method was used to deconvolve the data. For better visualization, the data has been transformed, normalized and displayed as a heatmap. The heatmap underlined barrier membrane-specific clusters that could also be seen in each graph of Figure 5 (see Figure 6). The very low values induced by PTFE were quite similar to the heatmap pattern observed for the unstimulated control, whereas this type of evaluation also clearly differentiated the responses to collagen, on the one hand, and PCL on the other. For some mediators, PCL even looked quite similar to LPS/SEB. Based on the subject specific response patterns, NelA seemed to confirm its intermediate position in this ranking. The negative, positive and stimulation controls, as well as the three different materials tested in this initial series of experiments, showed clearly distinct activity profiles when interacting with immune cells in these rotated whole blood cultures.
Discussion
The whole blood-based test system described above was specifically designed to provide a more comprehensive and in vivo-like characterization of potential immune cell interactions with solid and semi-solid materials. The overall goal of this project was to use untouched immune cells to avoid any artificial loss or gain of activity due to handling. Such undesirable activities can easily be caused by storage, transport or manipulation during immune cell preparation. Therefore, cultures should be started no later than 1 h after blood collection.26,27 In addition to this, shear forces and temperature shifts, often being the result of blood sample shipping, means additional stress to immune cells.28,29 However, the most crucial point is the preparation of the immune cells prior to cultivation, including centrifugation and resuspension of the cells, or exposure to buffers, different from their natural matrix, etc.27,30,31
Moreover, whole blood cultures mimic excellently the complexity of the human immune system in vivo.32–34 All types of immune cells, such as monocytes, macrophages, T cells, B cells, NK cells or granulocytes, as well as platelets, soluble factors such as complement proteins, antibodies, etc., are still present in their native composition. Each of these elements has the ability to either trigger or at least modulate the response of immune cells to materials.35 Thus, maintaining an in vivo-like complexity greatly enhances the translational value of results obtained with these models considerably.30,33,36 Macroscopic and microscopic images showing adherent erythrocytes and immune cells such as neutrohiles and monocytes after incubation support our hypothesis that the absence of only a single immune cell type (eg granulocytes) or even erythrocytes in in vitro Test systems (eg based on PBMCs) can alter the interaction between the cells and the material, but also the cell-to-cell communication after getting in contact with the test material, and hence distort the overall findings in the end. Therefore, the most decisive component in these cultures is human whole blood. In order to obtain reliable and reproducible results, strict inclusion and exclusion criteria must be met when selecting the donors (see Material and Methods). These must ensure that immune cell activity has not already been triggered (or suppressed) in vivo, as a consequence of immunological illnesses, medication, surgery, vaccination or similar.37–41
The easiest and also most sensitive endpoints to measure in whole blood culture models are cytokines secreted into the culture fluid by the different types of immune cells.42 This allows even larger sample series to be processed easily and quickly using standard immunoassays, such as single ELISAs, or the more powerful multiplexed assays such as Luminex.43 It is self-evident that multiplexed assays, which provide a far more comprehensive overview of the activities of a wide range of cell types, are best suited to the complexity represented by such organotypic cell culture systems. Besides, other parameters, such as mRNA expression, surface activation-markers, cell viability, morphology, intra-cellular cytokine levels, etc. can be determined by recovery of the cellular components from these cultures as well.33,44,45
A very important aspect when developing new test models is the definition of controls and reference samples, as already investigated and published in our recent preprint.46 In this culture system, the unsimulated negative control defined the basal level of immune cell activity. This was of great importance for further data analysis and interpretation. The stimulation control illustrated, whether immune cells from each donor could be properly activated. Both, the negative control and the stimulation control, were well established for static whole blood culture model (TruCulture).33,47 HDPE was found the most suitable negative control material for this test model with very low, almost basal cytokine levels. HDPE was also selected as negative control for cytotoxicity testing according to DIN EN ISO 10993–5. More difficult was the search for an appropriate positive control since it was meant to activate immune cells without causing hemolysis or cytotoxicity. NelA fulfilled these criteria, although its immune cells activation was donor dependent with inter-individual variations. Only by considering the results of negative and stimulation control cultures, as well as those containing negative and positive material controls, will allow a reliable processing of such data.
As proof of concept, three different types of commercially available barrier membranes, all of them being used for maxillofacial surgery, were examined for their potential interactions with immune cells in this new whole blood test system. The materials, PTFE, collagen, and PCL, can be regarded as excellent representative examples to benchmark such test systems, given their wide range of physical, chemical and biological properties.48 They not only revealed different strengths of responses (low – medium – high), but also reaction patterns of different, yet reproducible complexity. Interestingly, the cytokine release triggered by the membranes correlated with the number of immune cells adhered to the membranes. Thus, images may provide a preliminary indication of immune cell responses. It is particularly the availability of all relevant immune cell types and sub-types in whole blood cultures that enables the detection of a wide spectrum of primary as well as secondary effects of materials, as shown in this paper.
On the other hand, some results obtained with collagen indicated another interesting feature, which will surely not be limited to this material: While inducing moderate to high concentrations of several mediators, unexpectedly low values occurred for others, like MIP-1β (see Figure 3) or MMP3 (data not shown). This was likely caused by an adsorption of these proteins to the collagens, which has been shown for MIP-1β,49 as well as for MMP3.50 Besides the fact that such properties will interfere with a reliable quantitation of these mediators, properties like these will also have the potential to contribute to the integration process of implants such as collagen membranes into the surrounding tissues. The whole blood test system presented in this paper is also able to detect such additional features of implant materials (eg barrier membranes) not only of natural origin, but also of synthetic composition. Further investigations will be needed to characterize the influence of the adhesion of specific mediators.
Follow-up experiments will also focus on immune activating properties of materials used frequently for medicinal purposes and try to establish correlations between reaction patterns observed in this novel whole blood culture system and clinical outcome. Integration of this information into a database will ease the characterization of the analyzed materials. In addition, future tests will address additional culture conditions, such as co-activation of immune cells by bacterial stimuli and other inflammatory signals, but also use of a wider spectrum of endpoints in order to characterize material properties more comprehensively. However, since this test system is focusing on the immune cell – implant material interaction, it is worth mentioning that there are some limitations using this setup.
First, when selecting anticoagulants for the test system, Ca++ chelating agents (eg citrate or EDTA) must be excluded, as they prevent the essential Ca++ influx during immune cell activation and thus artificially inhibit or alter their responses, leading to false negative results. Another limitation is the analysis of typical hemocompatibility parameters from such supernatants (eg D-Dimers, fibrinopeptide A (FPA), thrombin-antithrombin III complex (TAT), platelet factor 4 (PF4), β-Thromboglobulin (β-TG), soluble complement complex C5b9 (sC5b9), complement factor C3a).51 This is mainly caused by the extended incubation time of up to 48h compared to hemocompatibility tests, which usually do not exceed an incubation time of 4h.52,53 Also, uncontrolled activation of the coagulation system during incubation would lead to an altered immune cell response (eg, after platelet and complement activation)51,53 and must be prevented by using higher doses of anticoagulants. As a consequence, the complex network between coagulation, complement and platelet activation is altered and supernatants obtained in the described setup do not represent reliable hemocompatibility parameter levels. If these parameters are still needed, the setup can be modified with little effort to meet the requirements for reliable hemocompatibility testing.
Conclusion
Overall, the presented results show that this innovative whole blood assay for testing immunocompatibility of implantable materials is able to safely differentiate between a) materials that do not elicit much activity in immune cells, b) others triggering weaker (and/or less variable) responses, c) those generating strong and more extensive responses, as well as d) characteristic, but subject-specific differences in reaction patterns.
The new in vitro model employed in these experiments provided a reliable means to sensitively detect complex reaction profiles of native human immune cells while avoiding cell culture artifacts, such as stress-induced false positive or false negative results when using immune cells that need to be isolated from whole blood before testing.
Abbreviations
C3a, complement factor 3a; FPA, fibrinopeptide A; GM-CSF, granulocyte/monocyte colony-stimulating factor; IL-X, interleukin X; LPS, lipopolysaccharide; IFNγ, interferon gamma; MIP-1β, macrophage inflammatory protein 1 beta; NelA, Nelfilcon A; PBMC, peripheral blood mononuclear cells; PCA, principal component analysis; PCL, polycaprolactone; PF4, platelet factor 4; PTFE, polytetrafluoroethylene; sC5b9, soluble complement complex C5b9; SEB, staphylococcal enterotoxin B; TAT, thrombin-antithrombin III complex; β-TG, β-thromboglobulin; TNFα, tumor necrosis factor alpha.
Ethics Approval and Consent to Participate
The study complies with the Declaration of Helsinki. All healthy donors provided written informed consent before phlebotomy, as approved by the Ethics Committee of the University of Tübingen (Project No. 457/2021BO2). No clinical data were obtained.
Acknowledgments
We would like to thank Ms. Tine Abel, who conducted all cytokine assays, for her outstanding performance.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
No funding was received for conducting this study.
Disclosure
HOT Screen GmbH is a private contract research organization and Geistlich Pharma AG is a commercial manufacturer of regenerative medical devices.
- During the development of this assay procedure, SK was employed as scientist at HOT Screen GmbH.
- MW is scientist at HOT Screen GmbH.
- JC-S has no competing interests to declare that are relevant to the content of this article.
- TK is chief executive officer and head of multi-analyte profiling at HOT Screen GmbH.
- AM-F has no competing interests to declare that are relevant to the content of this article.
- BS is Senior Executive Scientific Manager at Geistlich Pharma AG.
- MS is chief executive officer and chief scientific officer at HOT Screen GmbH.
References
1. Pałka K, Pokrowiecki R. Porous titanium implants: a review. Adv Eng Mater. 2018;20(5):1700648. doi:10.1002/adem.201700648
2. Lowe B, Ottensmeyer MP, Xu C, He Y, Ye Q, Troulis MJ. The regenerative applicability of bioactive glass and beta-tricalcium phosphate in bone tissue engineering: a transformation perspective. J Funct Biomater. 2019;10(1):16. doi:10.3390/jfb10010016
3. Elgali I, Omar O, Dahlin C, Thomsen P. Guided bone regeneration: materials and biological mechanisms revisited. Eur J Oral Sci. 2017;125(5):315–337. doi:10.1111/eos.12364
4. Bernard M, Jubeli E, Pungente MD, Yagoubi N. Biocompatibility of polymer-based biomaterials and medical devices - regulations, in vitro screening and risk-management. Biomater Sci. 2018;6(8):2025–2053. doi:10.1039/c8bm00518d
5. Swetha B, Mathew S, Murthy S, Nagaraja S, Bhandi S. Determination of biocompatibility: a review. Int Dent Med J Adv Res. 2015;1:1–6. doi:10.15713/ins.idmjar.2
6. Mariani E, Lisignoli G, Borzì RM, Pulsatelli L. Biomaterials: foreign bodies or tuners for the immune response? Int J Mol Sci. 2019;20(3):636. doi:10.3390/ijms20030636
7. Fang P, Li X, Dai J, et al. Immune cell subset differentiation and tissue inflammation. J Hematol Oncol. 2018;11(1):97. doi:10.1186/s13045-018-0637-x
8. Kzhyshkowska J, Gudima A, Riabov V, Dollinger C, Lavalle P, Vrana NE. Macrophage responses to implants: prospects for personalized medicine. J Leukoc Biol. 2015;98(6):953–962. doi:10.1189/jlb.5VMR0415-166R
9. Bohlson SS, O’Conner SD, Hulsebus HJ, Ho -M-M, Fraser DA. Complement, C1q, and C1q-related molecules regulate macrophage polarization. Front Immunol. 2014;5. doi:10.3389/fimmu.2014.00402
10. Hsieh JY, Smith TD, Meli VS, Tran TN, Botvinick EL, Liu WF. Differential regulation of macrophage inflammatory activation by fibrin and fibrinogen. Acta Biomater. 2017;47:14–24. doi:10.1016/j.actbio.2016.09.024
11. Rosales C. Neutrophil: a cell with many roles in inflammation or several cell types? Front Physiol. 2018;9:113. doi:10.3389/fphys.2018.00113
12. Lock A, Cornish J, Musson DS. The role of in vitro immune response assessment for biomaterials. J Funct Biomater. 2019;10(3):31. doi:10.3390/jfb10030031
13. Chu C, Liu L, Rung S, et al. Modulation of foreign body reaction and macrophage phenotypes concerning microenvironment. J Biomed Mater Res Part A. 2020;108(1):127–135. doi:10.1002/jbm.a.36798
14. Hachim D, LoPresti ST, Yates CC, Brown BN. Shifts in macrophage phenotype at the biomaterial interface via IL-4 eluting coatings are associated with improved implant integration. Biomaterials. 2017;112:95–107. doi:10.1016/j.biomaterials.2016.10.019
15. Zipursky A, Bow E, Seshadri RS, Brown EJ. Leukocyte density and volume in normal subjects and in patients with acute lymphoblastic leukemia. Blood. 1976;48(3):361–371. doi:10.1182/blood.V48.3.361.361
16. Schildhauer TA, Peter E, Muhr G, Köller M. Activation of human leukocytes on tantalum trabecular metal in comparison to commonly used orthopedic metal implant materials. J Biomed Mater Res Part A. 2009;88A(2):332–341. doi:10.1002/jbm.a.31850
17. Takahashi A, Takahashi S, Tsujino T, et al. Platelet adhesion on commercially pure titanium plates in vitro I: effects of plasma components and involvement of the von Willebrand factor and fibronectin. Int J Implant Dentist. 2019;5(1):5. doi:10.1186/s40729-019-0160-z
18. Mödinger Y, Teixeira GQ, Neidlinger-Wilke C, Ignatius A. Role of the complement system in the response to orthopedic biomaterials. Int J Mol Sci. 2018;19(11):3367. doi:10.3390/ijms19113367
19. Bindja J, Weiss ME, Schmolz M, et al. Synthetic ligands against TLR2-9 in TruCultureTM - whole blood assays distinguish clinical stages of SIRS (trauma) and sepsis. Trauma Shock Inflamm Sepsis. 2010;393:55–63.
20. Nalos M, Huang S, Sluyter R, et al. ‘Host tissue damage’ signal ATP impairs IL-12 and IFNγ secretion in LPS stimulated whole human blood. Intensive Care Med. 2008;34(10):1891. doi:10.1007/s00134-008-1156-y
21. Hung WT, Collings AF, Low J. Erythrocyte sedimentation rate studies in whole human blood. Phys Med Biol. 1994;39(11):1855–1873. doi:10.1088/0031-9155/39/11/005
22. Yin W, Xu Z, Sheng J, Xie X, Zhang C. Erythrocyte sedimentation rate and fibrinogen concentration of whole blood influences the cellular composition of platelet-rich plasma obtained from centrifugation methods. Exp Ther Med. 2017;14(3):1909–1918. doi:10.3892/etm.2017.4724
23. Taylor JR. On the nature and cause of the buffy coat of the blood. Lond Med Phys J. 1831;11(63):187–192.
24. Chandler AB. In vitro thrombotic coagulation of the blood; a method for producing a thrombus. Lab Invest. 1958;7(2):110–114.
25. Slee JB, Alferiev IS, Levy RJ, Stachelek SJ. The use of the ex vivo Chandler Loop Apparatus to assess the biocompatibility of modified polymeric blood conduits. J Vis Exp. 2014;(90). doi:10.3791/51871
26. Jerram A, Guy TV, Beutler L, et al. Effects of storage time and temperature on highly multiparametric flow analysis of peripheral blood samples; implications for clinical trial samples. Biosci Rep. 2021;41(2):BSR20203827. doi:10.1042/BSR20203827
27. Goods BA, Vahey JM, Steinschneider AF, Askenase MH, Sansing L, Christopher Love J. Blood handling and leukocyte isolation methods impact the global transcriptome of immune cells. BMC Immunol. 2018;19(1):30. doi:10.1186/s12865-018-0268-6
28. Posevitz-Fejfár A, Posevitz V, Gross CC, et al. Effects of blood transportation on human peripheral mononuclear cell yield, phenotype and function: implications for immune cell biobanking. PLoS One. 2014;9(12):e115920. doi:10.1371/journal.pone.0115920
29. Diks AM, Bonroy C, Teodosio C, et al. Impact of blood storage and sample handling on quality of high dimensional flow cytometric data in multicenter clinical research. J Immunol Methods. 2019;475:112616. doi:10.1016/j.jim.2019.06.007
30. He D, Yang CX, Sahin B, et al. Whole blood vs PBMC: compartmental differences in gene expression profiling exemplified in asthma. Allergy Asthma Clin Immunol. 2019;15(1):67. doi:10.1186/s13223-019-0382-x
31. Gottfried-Blackmore A, Rubin SJS, Bai L, et al. Effects of processing conditions on stability of immune analytes in human blood. Sci Rep. 2020;10(1, Art. no. 1). doi:10.1038/s41598-020-74274-8
32. Duffy D, Rouilly V, Libri V, et al. Functional Analysis via standardized whole-blood stimulation systems defines the boundaries of a healthy immune response to complex stimuli. Immunity. 2014;40(3):436–450. doi:10.1016/j.immuni.2014.03.002
33. Duffy D, Rouilly V, Braudeau C, et al. Standardized whole blood stimulation improves immunomonitoring of induced immune responses in multi-center study. Clin Immunol. 2017;183:325–335. doi:10.1016/j.clim.2017.09.019
34. Appay V, Reynard S, Voelter V, Romero P, Speiser D, Leyvraz S. Immuno-monitoring of CD8+ T cells in whole blood versus PBMC samples. J Immunol Methods. 2006;309(1–2):192–199. doi:10.1016/j.jim.2005.11.007
35. Rus H, Cudrici C, Niculescu F. The role of the complement system in innate immunity. Immunol Res. 2005;33(2):103–112. doi:10.1385/IR:33:2:103
36. Brooks P, Emery P, Evans JF, et al. Interpreting the clinical significance of the differential inhibition of cyclooxygenase-1 and cyclooxygenase-2. Rheumatology. 1999;38(8):779–788. doi:10.1093/rheumatology/38.8.779
37. Aasvang EK, Pitter S, Hansen CP, et al. Preoperative TruCulture® whole blood cytokine response predicts post-operative inflammation in pancreaticoduodenectomy patients-A pilot cohort study. Scand J Immunol. 2020;92(3):e12930. doi:10.1111/sji.12930
38. Del Valle DM, Kim-Schulze S, Huang -H-H, et al. An inflammatory cytokine signature predicts COVID-19 severity and survival. Nature Med. 2020;26(10, Art. no. 10):1636–1643. doi:10.1038/s41591-020-1051-9
39. Niu X, Chen G. Clinical biomarkers and pathogenic-related cytokines in rheumatoid arthritis. J Immunol Res. 2014;2014:1–7. doi:10.1155/2014/698192
40. Monastero RN, Pentyala S. Cytokines as biomarkers and their respective clinical cutoff levels. Int J Inflam. 2017;2017. doi:10.1155/2017/4309485
41. Divekar AA, Zaiss DMW, Lee FE-H, et al. Protein vaccines induce uncommitted IL-2-secreting human and mouse CD4 T cells, whereas infections induce more IFN-gamma-secreting cells. J Immunol. 2006;176(3):1465–1473. doi:10.4049/jimmunol.176.3.1465
42. Damsgaard CT, Lauritzen L, Calder PC, Kjær TMR, Frøkiær H. Whole-blood culture is a valid low-cost method to measure monocytic cytokines — a comparison of cytokine production in cultures of human whole-blood, mononuclear cells and monocytes. J Immunol Methods. 2009;340(2):95–101. doi:10.1016/j.jim.2008.10.005
43. Vignali DA. Multiplexed particle-based flow cytometric assays. J Immunol Methods. 2000;243(1–2):243–255. doi:10.1016/s0022-1759(00)00238-6
44. Rodrigues KB, Dufort MJ, Llibre A, et al. Innate immune stimulation of whole blood reveals IFN-1 hyper-responsiveness in type 1 diabetes. Diabetologia. 2020;63(8):1576–1587. doi:10.1007/s00125-020-05179-4
45. Drabe CH, Sørensen SS, Rasmussen A, et al. Immune function as predictor of infectious complications and clinical outcome in patients undergoing solid organ transplantation (the ImmuneMo:SOT study): a prospective non-interventional observational trial. BMC Infect Dis. 2019;19(1):573. doi:10.1186/s12879-019-4207-9
46. Klimosch S, Caballé-Serrano J, Knorpp T, Munar-Frau A, Schaefer B, Schmolz M. Surgically used barrier membranes show distinct reaction profiles in an innovative human whole blood culture system. Res Square. 2022. doi:10.21203/rs.3.rs-1243026/v1
47. Urrutia A, Duffy D, Rouilly V, et al. Standardized whole-blood transcriptional profiling enables the deconvolution of complex induced immune responses. Cell Rep. 2016;16(10):2777–2791. doi:10.1016/j.celrep.2016.08.011
48. Yang Z, Wu C, Shi H, et al. Advances in barrier membranes for guided bone regeneration techniques. Front Bioeng Biotechnol. 2022;10:921576. doi:10.3389/fbioe.2022.921576
49. Proudfoot AEI, Handel TM, Johnson Z, et al. Glycosaminoglycan binding and oligomerization are essential for the in vivo activity of certain chemokines. Proc Natl Acad Sci U S A. 2003;100(4):1885–1890. doi:10.1073/pnas.0334864100
50. Manka SW, Bihan D, Farndale RW. Structural studies of the MMP-3 interaction with triple-helical collagen introduce new roles for the enzyme in tissue remodelling. Sci Rep. 2019;9(1):18785. doi:10.1038/s41598-019-55266-9
51. Weber M, Steinle H, Golombek S, et al. Blood-contacting biomaterials: in vitro evaluation of the hemocompatibility. Front Bioeng Biotechnol. 2018;6:99. doi:10.3389/fbioe.2018.00099
52. Braune S, Latour RA, Reinthaler M, Landmesser U, Lendlein A, Jung F. In vitro thrombogenicity testing of biomaterials. Adv Healthcare Mater. 2019;8(21):1900527. doi:10.1002/adhm.201900527
53. van Oeveren W. Obstacles in haemocompatibility testing. Scientifica. 2013;2013:392584. doi:10.1155/2013/392584
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.