Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 16
A Giant Mixed Infiltrative Angiolipoma of the Back with Venous Malformation- A Case Report and Related Gene Mutation Detection
Authors Zhao F , Yang Z , Yang X
Received 19 September 2022
Accepted for publication 14 December 2022
Published 6 January 2023 Volume 2023:16 Pages 53—58
DOI https://doi.org/10.2147/CCID.S389178
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jeffrey Weinberg
Fangning Zhao,* Zhenyu Yang,* Xiaonan Yang
The Department of Hemangioma and Vascular Malformation, Plastic Surgery Hospital, Chinese Academy of Medical Sciences (CAMS) and Peking Union Medical College (PUMC), Beijing, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Xiaonan Yang, Email [email protected]
Abstract: We report a case of a middle-aged male patient with a diffuse large mixed infiltrating angiolipoma on the back with venous malformation that grew from the age of 3 years. We discussed the design of the surgical flap for this large lipoma, observed its blood supply using SPY, and screened for possible causative genes, FCGR2A, BMP5, MUC2, and KRTAP4-9. To date, no invasive lipomas of this size and duration have been reported.
Keywords: case report, lipoma, infiltrating lipoma, SPY, gene detection, whole-exome sequencing
Introduction
Lipomas are one of the most common benign tumors and occur on any part of the body surface. Most lipomas develop in situ and describe small size, but a few grow into large lipomas with a diameter of more than 10 cm or overweight 1000 g.1 Infiltrating lipoma is a rare tumor that was first reported by Howard and Helwig2 in 1960. Infiltrating lipomas generally account for 5–17% of lipoma with fat and vascular components, and are capable of capsule or local infiltration of muscular tissue. Histologically, infiltrative lesions are nonencapsulated, consisting of mature adipocytes and vascular networks that intersperse with tumors and infiltrate local soft tissues.
In this study, a male patient who had undergone multiple operations and relapsed due to a back tumor was relieved after receiving our personalized surgery. The pathological results suggested that the internal components of the tumor were mainly composed of fat and blood vessels, and the tumor infiltrated to the muscle layer. Further whole-exome sequencing (WES) demonstrated potential single nucleus variants, which may contribute to possible somatic mutations.
Case Report & Operation
We report the case of a 35-year-old male patient in good physical condition with no definite family history or onset history, accompanying no systemic rheumatic immune diseases or metabolic diseases. The patient had accepted surgery when exhibited tumor growth at 3 years old. Lipoma has been demonstrated pathologically. The patient showed an arc scar under the left scapula of the back and a transverse scar at the lower waist (Figure 1). The tumor was gradually diffused with age increasing rather than eliminated. The patient complained of occasional backache and insomnia-early on the back, although there were no spots or ulcers on the tumor surface. Palpation can detect a massive lump on the back from the second thoracic vertebra to the fifth lumbar vertebra. The whole mass was an irregular quadrangle, with a width of 38 cm at the bottom, a length of 43 cm midaxillary line on the right side, a length of 26 cm on the posterior axillary line on the left side, and a height of 8.0 cm protruding from the back. It has good mobility with a soft touch and is scattered in several solid mass-like nodules.
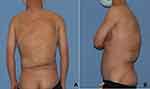 |
Figure 1 (A) back view before the operation (B) left view before the operation. |
Computed tomography (CT) also showed the main range of lesions; the tumor invaded the deep fascia (Figure 2). Magnetic resonance imaging (MRI) showed diffuse thickening of the soft tissues in the waist with a fat signal, but no abnormality was found on dynamic contrast-enhanced MRI. Before the operation, color Doppler ultrasound showed abnormal echoes in the subcutaneous tissue of the right lower back and deep fascia, suggesting abnormal blood supply through the branches, and many vascular malformations could be seen. Fibro-adipose vascular anomaly was not excluded, uneven thickness and echo of the superficial fascia and deep fascia layer of the back, and obvious fibrous tissue separation.
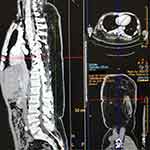 |
Figure 2 CT imaging. |
To reduce the influence of the flap on the blood supply, an incision was made at the waist scar of the last operation, the other incision was located on the right side of the back, and an S-shaped incision was made along the lateral edge of the hard nodule that could be touched under the skin of the patient. The shortest boundary distance between the two incisions was approximately 8.0cm.
As MRI showed, there were many different levels of blood supply through branches of arteries and veins, and many vascular malformations were distributed in the adipose tissue. The superficial fascia under the dermis was then carefully peeled off. The deep side was peeled off along the superficial side of the myofascial, peeled off from the upper back to the right paravertebral line, completely penetrated the lower middle part of the two incisions, and removed the upper right and lower masses within the range that could be determined. An obvious venous mass deformity can be observed under the right latissimus dorsi muscle, embolization was performed using polycarboxylates diluted in saline. We observed the blood supply of the edge of the flap by the SPY detector. It can be seen that the blood supply at the lower left side of the back first appeared, followed by the blood supply at the lower right area, and finally, the whole middle area showed a slow blood supply. To prevent necrosis of the skin flap after the operation, resection of the upper left tumor was stopped, hemostasis was achieved by bipolar electrocoagulation, the skin flap was trimmed, the wound was washed with gentamicin diluted with normal saline, and subcutaneous and skin intermittent sutures were performed. The total blood loss of the patient was approximately 1000 mL, 4 U of suspended red blood cells and 200 mL of plasma were infused during the operation.
After the surgery, the patient was bandaged under pressure. Two weeks after the operation, the patient showed extensive exudation, and the skin and subcutaneous tissue had poor adhesion, which led to unsatisfactory wound healing. The tumor was removed again after two weeks. The purpose of this operation is mainly to remove the upper left tumor on the back. The patient recovered well postoperatively and was discharged from the hospital 10 days later with high satisfaction. Follow-up for half a year showed that the patient was in a stable condition and the wound healed well (Figure 3).
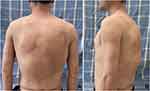 |
Figure 3 (A) back view after the operation (B) left view after the operation. |
Pathological Report and Gene Detection
We took several pieces of the tumor for pathological examination, and the return was lipomatosis, which means that lipoma shows multisite and multilevel growth, including invasive lipoma and simple lipoma. Because of the extensive vascular malformation, we considered it an infiltrative angiolipoma showing a lobular or flaky distribution of mature adipocytes, mostly accompanied by vessels with abundant blood supply (Figure 4).
 |
Figure 4 (A) a large number of vascular malformations around adipose tissue. (B) vacuolar adipose tissue. |
Considering that the tumor of the patient is a lipoma containing vascular components infiltrating the deep muscle layer, we collected peripheral blood samples and diseased tissues of the patient and conducted WES at a depth of 100X (Supplementary Materials). The results of the somatic mutation suggest that there are 111 somatic single nucleotide variants in the tumor genome, among which the splitting mutations in FCGR2A and BMP5, the synonymous mutation in MUC2, and the deletion mutation in KRTAP4-9 may contribute to the onset of tumor. No insertion and deletion mutation and meaningful copy number variation were found.
Discussion
The incidence of invasive lipoma is equal in men and women, and cases have been reported at different ages. Infiltrating lipomas occur in striated muscles, with an inconspicuous boundary, slow growth, poor mobility, and firmer texture than an ordinary lipoma.
In this case, some tumors were soft but several were hard and nodular, and there was no obvious adhesion to the skin. Pinched masses have a sense of demarcation of the dermal layer, showing a mixed situation of invasive angiolipoma and intermuscular lipoma. An infiltrative angiolipoma contains prominent blood vessels, a large amount of connective tissue and mature adipose tissue, and often degenerates muscle fibers. The patient began to develop the disease at the age of three, and the course of the disease was more than 30 years, and it was no longer a simple manifestation of a certain disease.
Controlling bleeding is the most important aspect of this procedure. In our two surgeries, the patient’s total blood loss was approximately 1500mL. The back of the lesion is thick, rich in blood vessels, and abundant in blood supply, therefore, we consider cutting off the deep surface of the lesion first, and then moving into the superficial surface of the lesion to reduce blood loss. After the operation, the deep peeling surface of the skin flap healed slowly with a large amount of exudation, mainly tissue fluid. This may also be a sign of fat liquefaction. This may also be related to incomplete or inaccurate oppression. To completely compress the wound, we used the bellyband, elastic bandage and tight vest to control the exudation, bellyband can provide continuous tension, thus having a better effect.
Genetic variants that may contribute to the disease as suggested by our exon sequencing results include FCGR2A, BMP5, MUC2, and KRTAP4-9. BMPs belongs to the TGFβ family and is involved in adipose tissue development and adipogenesis and other processes through intracellular signaling pathways such as SMAD and MAPK.3,4 In porcine model, BMP5 may regulate lipid metabolism,5 and in vitro experiments using stromal cells suggest that BMP5 may play an imperative role in the transformation between white and brown adipose tissue.6 As a subtype of FcR receptor, FCGR2A is highly expressed in both human adipose tissue and cells, suggesting that the mutation of FCGR2A may further regulate the metabolic activity and cell function of fat by affecting the function of immunoglobulin.7 MUC2 mainly exists in mucosa-containing tissue epithelium, such as the intestinal tract. It is thought that it can provide a protective lubricating barrier for particles and infectious pathogens on the mucosal surface,8 while KRTAP4-9 is a keratin-related protein that is essential for the formation of hard hair.To date, we have not found any reports of lipoma related to the genes. A larger group of patients is required for further analysis.
Conclusion
In summary, the main treatment for infiltrating lipoma with vascular malformation is still surgical resection. Because of its diffuse invasive growth, the boundary is unclear, it is difficult to completely remove, and there is a certain probability of recurrence. However, even if it recurs, it is mostly local and can be treated by surgically again. FCGR2A, BMP5, MUC2, and KRTAP4-9 may contribute to the infiltrating lipoma with vascular malformation, this also brings us a new follow-up research direction. After two surgeries, the patient achieved satisfactory results. We will closely follow up with this patient for a long time to judge the prognosis of the disease, and we also expect more peers to discuss and communicate with us about this case.
Ethical Statement
All the case details have been permitted to be published in this study under the authority of the Ethics Committee of Plastic Surgery Hospital, CAMS. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Consent Statement
Written informed consent was provided by the patient to have the case details and accompanying images published.
Funding
This study was financially supported by the Multidisciplinary diagnosis and treatment cooperation project (No.1112320139).
Disclosure
Fangning Zhao and Zhenyu Yang are co-first authors for this study. All authors declare no conflicts of interest for this work.
References
1. Szewc M, Gawlik P, Zebrowski R, et al. Giant lipoma in the fronto-temporo-parietal region in an adult man: case report and literature review. Clin Cosmet Investig Dermatol. 2020;13:1015–1020. doi:10.2147/CCID.S273189
2. Lin JJ, Lin F. Two entities in angiolipoma. A study of 459 cases of lipoma with review of literature on infiltrating angiolipoma. Cancer. 1974;34(3):720–727. doi:10.1002/1097-0142(197409)34:3<720::AID-CNCR2820340331>3.0.CO;2-K
3. Buffolo M, Pires KM, Ferhat M, et al. Identification of a Paracrine Signaling Mechanism Linking Cd34(High) Progenitors to the Regulation of Visceral Fat Expansion and Remodeling. Cell Rep. 2019;29(2):270–282.e5. doi:10.1016/j.celrep.2019.08.092
4. Jia S, Meng A. Tgfβ family signaling and development. Development. 2021;148(5). doi:10.1242/dev.188490
5. Shao GC, Luo LF, jiang SW, et al. A C/T mutation in microRNA target sites in bmp5 gene is potentially associated with fatness in pigs. Meat Sci. 2011;87(3):299–303. doi:10.1016/j.meatsci.2010.09.013
6. Mae J, Nagaya K, Okamatsu-Ogura Y, et al. Adipocytes and stromal cells regulate brown adipogenesis through secretory factors during the postnatal white-to-brown conversion of adipose tissue in Syrian hamsters. Front Cell Dev Biol. 2021;9:698692. doi:10.3389/fcell.2021.698692
7. Palming J, Gabrielsson BG, Jennische E, et al. Plasma cells and fc receptors in human adipose tissue-lipogenic and anti-inflammatory effects of immunoglobulins on adipocytes. Biochem Biophys Res Commun. 2006;343(1):43–48. doi:10.1016/j.bbrc.2006.02.114
8. Steininger H, Pfofe DA, Muller H, et al. Expression of Cdx2 and Muc2 in Barrett’s Mucosa. Pathol Res Pract. 2005;201(8–9):573–577. doi:10.1016/j.prp.2005.03.010
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
