Back to Journals » Drug, Healthcare and Patient Safety » Volume 8
A fatal adverse effect of cefazolin administration: severe brain edema in a patient with multiple meningiomas
Authors Tribuddharat S, Sathitkarnmanee T , Kitkhuandee A, Theerapongpakdee S, Ngamsangsirisup K, Chantawong S
Received 1 July 2015
Accepted for publication 18 December 2015
Published 9 February 2016 Volume 2016:8 Pages 9—12
DOI https://doi.org/10.2147/DHPS.S91514
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Chul Ahn
Sirirat Tribuddharat,1 Thepakorn Sathitkarnmanee,1 Amnat Kitkhuandee,2 Sunchai Theerapongpakdee,1 Kriangsak Ngamsaengsirisup,1 Sarinya Chanthawong,1
1Department of Anesthesiology, 2Department of Surgery, Faculty of Medicine, Khon Kaen University, Khon Kaen, Thailand
Abstract: Cefazolin is commonly administered before surgery as a prophylactic antibiotic. Hypersensitivity to cefazolin is not uncommon, and the symptoms mostly include urticaria, skin reaction, diarrhea, vomiting, and transient neutropenia, which are rarely life threatening. We present a rare case of fatal cefazolin hypersensitivity in a female who was diagnosed with multiple meningiomas and scheduled for craniotomy and tumor removal. Immediately after cefazolin IV administration, the patient developed acute hypertensive crisis, which resolved within 10 minutes after the treatment. This was followed by unexplained metabolic acidosis. The patient then developed severe brain edema 100 minutes later. The patient had facial edema when her face was exposed for the next 30 minutes. A computed tomography scan revealed global brain edema with herniation. She was admitted to the intensive care unit for symptomatic treatment and died 10 days after surgery from multiorgan failure. The serum IgE level was very high (734 IU/mL). Single-dose administration of cefazolin for surgical prophylaxis may lead to rare, fatal adverse reaction. The warning signs are sudden, unexplained metabolic acidosis, hypertensive crisis, tachycardia, and facial angioedema predominating with or without cutaneous symptoms like urticaria.
Keywords: cefazolin, adverse effect, drug hypersensitivity, brain edema, hypertension
Introduction
Cefazolin is a first-generation cephalosporin broad-spectrum antibiotic. It has been used for the treatment of serious gram-positive and gram-negative infections and has been extensively used as a prophylaxis antibiotic before performing a wide range of surgical operations.1–4 This drug is known to be associated with rare and mild adverse effects such as urticaria, skin reaction, diarrhea, vomiting, and transient neutropenia. In addition, there are some serious adverse reactions such as difficulty in breathing, headache, dizziness, overactive reflexes, Stevens–Johnson syndrome, and pain or swelling of face, lips, tongue, or throat. However, these symptoms are rarely life threatening.5,6 We report a case of cefazolin-induced fatal hypersensitivity presenting with acute hypertensive crisis, followed by severe brain edema and multiorgan failure.
Case report
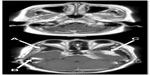
This report was approved by the institutional review board of Khon Kaen University (HE561235). Written informed consent was given by the patient’s husband. A 50-year-old female presented with chronic headache without associated symptoms for 3 years before this admission. Medical history revealed no known drug allergies or any medical problems. This patient’s vital signs were normal, and general physical examination was unremarkable. Routine preoperative laboratory tests on admission, including complete blood count, blood glucose level, blood chemistry, and chest X-ray were within normal limits. Brain magnetic resonance imaging showed multiple enhancing lesion at right and left sphenoid wing and right temporal area, with hyperostosis of bone in the right sphenoid wing (Figure 1). The diagnosis made was multiple meningiomas. Multisite craniotomy to remove the tumors was planned.
She underwent standard general anesthesia. The standard monitoring included intra-arterial and central venous blood pressure. Anesthesia was induced by administration of lidocaine, fentanyl, propofol, cisatracurium, and desflurane. She was uneventfully intubated. Three minutes after intubation, cefazolin 1 g intravenous (IV) was given as prophylaxis antibiotic. Within 1 minute, she developed unexplained acute hypertensive crisis with systolic blood pressure ranging between 200 and 230 mmHg and diastolic blood pressure between 110 and 120 mmHg. Tachycardia ensued (90–110 beats/min). She had neither generalized erythema nor urticaria. The anesthesiologist suspected that this patient was under light anesthesia, and more fentanyl, propofol, and desflurane were given. Her vital signs gradually returned to the normal range, and the neurosurgeon pinned her head and then started the operation. Arterial blood gas analysis revealed moderate metabolic acidosis (base excess –7 mEq/L), while other values were within normal limits. The patient was managed to maintain the vital signs within normal range. Operations at the first two sites were uneventful. One hundred minutes from the start of surgery while performing craniotomy for the third site, the surgeon reported that her brain developed acute severe edema with protrusion through craniotomy site. The anesthesiologist rapidly infused mannitol 60 g and applied hyperventilation to control the PaCO2 to 25–30 mmHg, but the edema worsened. The operation was stopped, and the incision wound was closed. After the patient’s face was exposed, a marked facial edema was observed. She was immediately moved to the Department of Radiology for a computed tomography brain scan. The computed tomography brain scan showed global brain swelling causing midline shift to the left, with transcalvarial and descending transtentorial brain herniation (Figure 2). Subsequently, this patient was admitted to the intensive care unit (ICU).
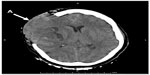  | Figure 2 The CT brain scan showed global brain swelling causing transcalvarial brain herniation (A). |
On ICU admission, the patient had a blood pressure of 110/60 mmHg with severe metabolic acidosis (base excess –11 mEq/L), a Glasgow Coma Scale Score of 2T (E1VTM1), and pupil 4–5 mm fixed (both eyes). Within 2 hours after the ICU admission, the patient developed neurogenic shock and received high doses of inotropics, including dopamine, dobutamine, norepinephrine, and epinephrine to support her hemodynamics. The patient received two more administrations of 1 g cefazolin IV within 24 hours after surgery. She had progressive edema in the face and the neck, but was easy to ventilate. The possibility of anaphylaxis to cefazolin was then suspected. Blood sample was sent for Immunoglobulin E (IgE) immunoassays.
Two days later, she had progressive generalized edema, Glasgow Coma Scale Score of 2T, pupil 5–6 mm fixed (both eyes), multiorgan impairment (renal, respiratory, hematologic, and metabolic systems), and hemodynamic instability. Arterial blood gas analysis showed severe metabolic acidosis (base excess –20 mEq/L) with low PaO2/FiO2 ratio of 150 mmHg. Her hemoglobin was 7.6–11.2 g/dL, total white blood cells count 17,100–31,250/mm3, normal differential count (neutrophils 60%–70% and lymphocytes 20%–30%), and platelet count 38,000–119,000/mm3. She had high serum creatinine (from 0.8 to 3.9 mg/dL) and a refractory high blood glucose level (up to 593 mg/dL). She experienced no seizures or skin rash. Serum IgE level was 734 IU/mL (normal <100 IU/mL). The outcomes never improved, and she died on the tenth day after surgery from multiorgan failure.
Discussion
The incidence of perioperative anaphylaxis in patients undergoing general anesthesia is estimated to be between one in 5,000 and one in 25,000 anesthesias, with a mortality rate of 3.4%–10.0%.1,5–9 Neuromuscular blocking agents, antibiotics, and latex represent the most common cause (top three) of anaphylactic substances.1,5–6,9–13 After reviewing the literature, we found many reports of a variety of adverse drug reactions, but no case of death, to cephalosporin antibiotics such as cefazolin, cefotaxime, ceftazidime, ceftriaxone, and cefuroxime.11,14–18
The fatal adverse reaction to cefazolin administration for surgical prophylaxis in our patient is a very rare perioperative complication. The warning signs during general anesthesia are sudden, unexplained hypertensive crisis, tachycardia, and facial angioedema predominating with or without cutaneous symptoms like urticaria.5–6 The hypertensive crisis in this patient developed after uneventful intubation, before pinning the skull, without sympathetic stimulation. The diagnosis of fatal adverse reaction to cefazolin becomes difficult without eliminating other causes such as light anesthesia and other IV anesthetic agents. We ruled out lidocaine, fentanyl, propofol, cisatracurium, and desflurane, which were also used in this patient, as the causes because these drugs do not induce histamine release and because the vital signs of the patients were uneventful and stable after these drugs were administered. Although extensive brain swelling with neurological deterioration was previously reported after intracranial meningioma surgery,19 the disaster happened postoperatively, not intraoperatively like in our case. The diagnosis should be established by various immunological tests like measuring IgE level and skin prick test.1,5–7,11,20,21 Because patients with intracranial meningioma had lower total serum IgE than normal “control” participants,22 the very high IgE level in this patient, although without antigen-specific IgE measurement, confirms our diagnosis.
Considering that cerebral edema in this patient did not respond to treatment with mannitol and hyperventilation, which made it even worse, the mechanism of edema is postulated to be due to the hypersensitivity reaction, which causes an overwhelming inflammatory response that damages endothelial membranes leading to disruption of the blood–brain barrier and microcirculation failure in other organs.23,24 This may be the explanation as to why moderate metabolic acidosis developed after the hypertensive crisis but without hypoxemia, anemia, or hypotension and the aggravation of brain edema after mannitol infusion, as well as the multiorgan failure.
Treatment of adverse drug reactions during surgery consists of stopping the administration of any suspected medication and fluid resuscitation with crystalloids, along with the administration of vasopressors/vasodilators support (depending on the patients’ hemodynamics) and corticosteroids. The use of histamine receptor blockers and corticosteroids for medical prevention remains controversial.25 In case brain edema is suspected, osmotic diuretic should be avoided and loop diuretic with moderate hyperventilation should be considered.
Limitation
Since we did not perform serum IgE antibody to cefazolin measurement to confirm the diagnosis, our conclusion depends on the process of deductive reasoning.
Conclusion
Single-dose cefazolin administration for surgical prophylaxis may lead to a rare, fatal adverse reaction. The warning signs are sudden, unexplained metabolic acidosis, hypertensive crisis, tachycardia, and facial angioedema predominating with or without cutaneous symptoms like urticaria. The diagnosis of fatal adverse reaction to cefazolin becomes difficult without eliminating other causes such as light anesthesia and other IV anesthetic agents. The diagnosis should be confirmed by serum IgE measurement.
Acknowledgments
The authors thank Mr Bryan Roderick Hamman and Mrs Janice Loewen-Hamman for their assistance with the English-language presentation of the manuscript via Publication Clinic Khon Kaen University, Thailand.
Author contributions
All authors contributed toward data analysis, drafting and critically revising the paper and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
Dewachter P, Mouton-Faivre C, Emala CW. Anaphylaxis and anesthesia: controversies and new insights. Anesthesiology. 2009;111(5):1141–1150. | |
Adriani KS, van de Beek D, Brouwer MC, Spanjaard L, de Gans J. Community-acquired recurrent bacterial meningitis in adults. Clin Infect Dis. 2007;45(5):e46–e51. | |
Rockowitz J, Tunkel AR. Bacterial meningitis. Practical guidelines for management. Drugs. 1995;50(5):838–853. | |
Weisfelt M, de Gans J, van de Beek D. Bacterial meningitis: a review of effective pharmacotherapy. Expert Opin Pharmacother. 2007;8(10):1493–1504. | |
Lee CW, Castells MC. Perioperative anaphylaxis to cefazolin. Allergy Asthma Proc. 2004;25(1):23–26. | |
Mertes PM, Tajima K, Regnier-Kimmoun MA, et al. Perioperative anaphylaxis. Med Clin North Am. 2010;94(4):761–789, xi. | |
Gibbs MW, Kuczkowski KM, Benumof JL. Complete recovery from prolonged cardio-pulmonary resuscitation following anaphylactic reaction to readministered intravenous cefazolin. Acta Anaesthesiol Scand. 2003;47(2):230–232. | |
Faulk D, Rodosevich K, Katz RL, Calmes SH. Anaphylactic reaction to intravenous cefazolin. Nurse Anesth. 1993;4(4):188–192. | |
Mertes PM, Aimone-Gastin I, Gueant-Rodriguez RM, et al. Hypersensitivity reactions to neuromuscular blocking agents. Curr Pharm Des. 2008;14(27):2809–2825. | |
Mertes PM, Laxenaire MC, Lienhart A, et al. Reducing the risk of anaphylaxis during anaesthesia: guidelines for clinical practice. J Investig Allergol Clin Immunol. 2005;15(2):91–101. | |
Culp JA, Palis RI, Castells MC, Lucas SR, Borish L. Perioperative anaphylaxis in a 44-year-old man. Allergy Asthma Proc. 2007;28(5):602–605. | |
Mertes PM, Laxenaire MC. Allergy and anaphylaxis in anaesthesia. Minerva Anestesiol. 2004;70(5):285–291. | |
Mertes PM, Laxenaire MC, Alla F. Anaphylactic and anaphylactoid reactions occurring during anesthesia in France in 1999–2000. Anesthesiology. 2003;99(3):536–545. | |
Kim KB, Kim SM, Park W, Kim JS, Kwon SK, Kim HY. Ceftiaxone-induced neurotoxicity: case report, pharmacokinetic considerations, and literature review. J Korean Med Sci. 2012;27(9):1120–1123. | |
Bijie H, Kulpradist S, Manalaysay M, Soebandrio A. In vitro activity, pharmacokinetics, clinical efficacy, safety and pharmacoeconomics of ceftriaxone compared with third and fourth generation cephalosporins: review. J Chemother. 2005;17(1):3–24. | |
Klein NC, Cunha BA. Third-generation cephalosporins. Med Clin North Am. 1995;79(4):705–719. | |
Aouam K, Chaabane A, Toumi A, et al. Drug rash with eosinophilia and systemic symptoms (DRESS) probably induced by cefotaxime: a report of two cases. Clin Med Res. 2012;10(1):32–35. | |
Richards DM, Heel RC, Brogden RN, Speight TM, Avery GS. Ceftriaxone. A review of its antibacterial activity, pharmacological properties and therapeutic use. Drugs. 1984;27(6):469–527. | |
Asgari S, Bassiouni H, Hunold A, Klassen D, Stolke D, Sandalcioglu IE. Extensive brain swelling with neurological deterioration after intracranial meningioma surgery – venous complication or “unspecific” increase in tissue permeability. Zentralbl Neurochir. 2008;69(1):22–29. | |
Romano A, Mayorga C, Torres MJ, et al. Immediate allergic reactions to cephalosporins: cross-reactivity and selective responses. J Allergy Clin Immunol. 2000;106(6):1177–1183. | |
Khan RA, Kazi T, O’Donohoe B. Near fatal intra-operative anaphylaxis to chlorhexidine – is it time to change practice? BMJ Case Rep. 2011;2011:bcr0920092300. | |
Wiemels JL, Wrensch M, Sison JD, et al. Reduced allergy and immunoglobulin E among adults with intracranial meningioma compared to controls. Int J Cancer. 2011;129(8):1932–1939. | |
Danese S, Dejana E, Fiocchi C. Immune regulation by microvascular endothelial cells: directing innate and adaptive immunity, coagulation, and inflammation. J Immunol. 2007;178(10):6017–6022. | |
Muller WA. Leukocyte-endothelial cell interactions in the inflammatory response. Lab Invest. 2002;82(5):521–533. | |
Alansari M, Alsanouri I. A typical intraoperative anaphylactic shock with ECG changes secondary to non-ruptured hepatic hydatid cyst. BMJ Case Rep. 2013;2013:bcr2012008442. |
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.