Back to Journals » Research and Reports in Urology » Volume 14
A Drug-Coated Balloon Treatment for Urethral Stricture Disease: Three-Year Results from the ROBUST I Study
Authors Virasoro R , DeLong JM, Estrella RE, Pichardo M, Rodriguez Lay R, Espino G, Elliott SP
Received 2 February 2022
Accepted for publication 3 May 2022
Published 6 May 2022 Volume 2022:14 Pages 177—183
DOI https://doi.org/10.2147/RRU.S359872
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Guglielmo Mantica
Ramón Virasoro,1 Jessica M DeLong,1 Rafael E Estrella,2 Merycarla Pichardo,3 Ramón Rodriguez Lay,4 Gustavo Espino,5 Sean P Elliott6
1Urology of Virginia PLLC, Virginia Beach, VA, USA; 2Clinica Unión Medica, Santiago de los Caballeros, Dominican Republic; 3URUS, Santo Domingo, Dominican Republic; 4Cirujano Urologo Royal Center Panama City, Panama City, Panama; 5Centro Especializado San Fernando, Panama City, Panama; 6Department of Urology, University of Minnesota, Minneapolis, MN, USA
Correspondence: Ramón Virasoro, Urology of Virginia PLLC, 225 Clearfield Ave, Virginia Beach, VA, 23462, USA, Tel +1 757-457-5100, Email [email protected]
Introduction: Endoscopic management of male anterior urethral stricture disease is common; however, repeat treatment is associated with high recurrence rates. Here, we report the 3-year results of the ROBUST I trial, which evaluated the safety and efficacy of the Optilume® drug coated balloon (DCB) in men with recurrent urethral strictures.
Methods: Adult men with recurrent bulbar urethral strictures ≤ 2 cm in length and 1– 4 prior endoscopic interventions were treated with the Optilume DCB. Functional success was defined as ≥ 50% reduction in International Prostate Symptom Score (IPSS) without need for retreatment. Other outcomes included quality of life, maximum flow rate, post-void residual urine volume, erectile function, and freedom from repeat intervention.
Results: Of the 53 enrolled and treated men, 33 completed the 3-year visit, with 10 patients experiencing clinical failures at previous visits, giving a total of 43 subjects evaluable for the functional success endpoint. Functional success was achieved in 67% (29/43) and freedom from retreatment in 77% (33/43). Average IPSS improved from 25.2 at baseline to 5.5 at 3 years (p< 0.0001). Significant improvements were observed in quality of life, flow rate, and post-void residual urine volume. Erectile function was not affected by treatment. Device-related adverse events were mild or moderate in nature and resolved quickly after onset. There were no serious treatment-related adverse events.
Conclusion: Symptomatic improvement after treatment with the Optilume DCB was maintained through 3 years in a population highly susceptible to recurrent urethral stricture disease. This minimally invasive therapy is safe with no negative impact on sexual function.
Keywords: lower urinary tract symptoms, paclitaxel, urethral dilation, medical device, clinical trial
Introduction
The treatment of recurrent male anterior urethral stricture disease remains a common and challenging problem for many urologists across the globe. Current available options for recurrent urethral strictures include endoscopic management and urethral reconstruction. While open repair is considered the gold standard, with success rates of 80–95%, minimally invasive therapies are still more frequently used.1 Of the primary endoscopic procedures, urethral dilation and Direct Vision Internal Urethrotomy (DVIU) have similar efficacy, with progressively lower probability of long-term success in repeat treatments.2,3 More recently, small studies have evaluated targeted injections of antifibrotic agents as an adjunctive therapy to endoscopic procedures in an attempt to prevent or attenuate scar tissue formation.4 The Optilume® Drug Coated Balloon (DCB) (Urotronic, Inc., Plymouth, MN, USA) is the first DCB intended for the treatment of male anterior urethral strictures. This technology aims to provide immediate symptomatic relief by widening the urethral lumen using balloon dilation, while maintaining long-term urethral patency via the circumferential and local application of paclitaxel. Paclitaxel is an antimitotic agent that inhibits cell proliferation and migration and has been used extensively in cardiovascular interventions to prevent restenosis after angioplasty.5,6
We previously reported the 1- and 2-year ROBUST I trial results, which evaluated the Optilume® DCB in men with recurrent urethral strictures up to 2 cm in length.7,8 Anatomic success in ROBUST I was achieved in 70% at 1 year based on the ability to pass a 16F flexible cystoscope or 14F Foley catheter.7 Based on the study design, cystoscopy was not performed after 1 year, however functional success occurred in 70% (32/46) at 2 years, defined as International Prostate Symptom Score (IPSS) improvement ≥50% in the absence of retreatment need.8 We now report the 3-year safety and efficacy outcomes of the ROBUST I trial.
Methods
ROBUST I is a prospective, multicenter, single arm, open-label study evaluating the safety and efficacy of the Optilume® DCB for the treatment of male recurrent anterior urethral strictures. Adult men with a single bulbar stricture <12F and ≤2 cm long on urethrogram were eligible to participate. Patients had to have 1–4 prior endoscopic treatments (none within 3 months of enrollment), IPSS ≥13, and peak urinary flow rate (Qmax) <10 mL/sec. Protocol exclusions included prior urethroplasty, radical prostatectomy, lichen sclerosus, penile prosthesis or artificial urinary sphincter, and history of pelvic radiation.
Strictures were pre-dilated using an uncoated balloon and/or DVIU, followed by treatment with the Optilume® DCB inflated to rated burst pressure for a minimum of 5 minutes. Post-procedural follow-up occurred at 5 days (Foley removal), 14 days, 1 month, 3 months, 6 months, and annually through 3 years post-procedure. Outcomes include IPSS, Urethral Stricture Surgery Patient-Reported Outcome Measure (USS-PROM), Qmax, post-void residual (PVR) urine volume, and freedom from repeat intervention. Functional success is reported as the percentage of subjects with IPSS improvement ≥50% without need for retreatment. Urethral Lumen Test (ULT) results performed at 6 and 12 months were previously reported.7 Safety was addressed by adverse event collection and the “overall satisfaction” question of the International Index of Erectile Function (IIEF).
The study was performed at 4 sites in Latin America and was approved by all study center ethics committees. All Procedures performed in the study involving human participants were in accordance with the ethical standards of the institutional research committees and with the 1964 Helsinki declaration and its later amendments for compatible ethical standards. Panamá sites were approved by Comité de Bioética de la Investigación, Instituto Conmemorativo Gorgas de Estudios de la Salud (CBI-ICGES). Dominican Republic sites where approved by Consejo Nacional de Bioética en Salud (CONABIOS). Subjects provided written informed consent prior to participation. The study is registered on clinicaltrials.gov as NCT03014726.
The required sample size for the study was based on the primary efficacy endpoint evaluated at 90 days; no additional sample size requirements were associated with long-term follow-up.7 Data were summarized using appropriate descriptive statistics. Subjects who required retreatment with the Optilume® DCB or exited the study due to treatment failure were considered failures for the functional success endpoint. Subgroup analyses for functional success rates were performed by balloon diameter, stricture length, and number of prior endoscopic treatments. Changes from baseline were tested using the unpaired t-test and subgroup results using the chi-squared test. Statistical tests were evaluated at a 0.05 significance level.
Results
Study Population
Fifty-three men with bulbar strictures were enrolled and treated with the Optilume® DCB between November 2016 and September 2017. The mean age was 50.7 years (range 22.0 to 81.0) and the average number of prior dilations was 1.7 (range 1 to 4). Strictures averaged 0.9 cm in length and 2.3 mm in diameter. IPSS at baseline was 25.2 for the cohort. A total of 33 subjects completed the 3-year follow-up visit, while 3 subjects missed their 3-year visit but were not considered lost to follow-up. Ten subjects discontinued standard follow-up prior to the 3-year visit due to receiving repeat treatment, 4 were lost to follow-up, 3 were withdrawn due to confounding adverse events (BPH), and one subject withdrew consent. Subjects receiving repeat treatment were considered failures for the functional success and freedom from repeat intervention endpoints, giving a total of 43 evaluable subjects for these endpoints. Detailed demographics were reported in previous publications.7,8
Efficacy
At 3 years, 67% (29/43) of subjects achieved functional success based on an improvement in IPSS ≥50% without retreatment. Of the 29 subjects with functional success, 3 had previously failed the ULT (1 subject at 12 months and 2 subjects at 6 months). Four subjects had IPSS improvement from baseline of 50–75% and 25 improved by >75%. The 14 failures included 4 subjects with improvement in IPSS <50%, 5 subjects who were retreated with the Optilume® DCB, and 5 subjects who exited due to treatment failure (2 underwent urethroplasty, 1 received an additional dilation, and 2 exited before the repeat intervention type was known). Subjects who were lost to follow-up, withdrawn due to confounding BPH, or missed their 3-year visit were censored from the analysis. Subjects with BPH had cystoscopic confirmation of no urethral stricture recurrence prior to study exit. These subjects exhibited <50% improvement in IPSS, but had a patent urethra at the time of study exit and therefore were censored and not considered treatment failures for future visits as symptoms were attributed to prostatic enlargement and not stricture recurrence. Six of the 10 censored subjects had ≥50% reduction in IPSS at their last available visit, resulting in a functional success rate of 66% (35/53) using a last observation carried forward approach, which is consistent with the observed results.
The average IPSS improved from 25.2 at baseline to 5.5 at 3 years (p<0.0001; Table 1). Stricture length and number of prior endoscopic procedures were not predictors of functional success at 3 years (Table 2). However, treatment success was significantly dependent on balloon size. Functional success was 50% (10/20) with the 24F balloon and 83% (19/23) with the 30F balloon (p=0.02).
 |
Table 1 Summary of Outcome Measures by Visit |
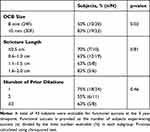 |
Table 2 Functional Success at 3 Years by Subgroup |
Freedom from repeat intervention was 77% (33/43) at 3 years. Most failures for this endpoint occurred within 12 months of the index procedure. Of the 10 subjects who were retreated with the Optilume® DCB or pursued alternative therapy, 5 occurred after the 3-month visit, 3 after the 6-month visit, and one each after the 1-year and 2-year visits. In addition to the 29 functional successes, we included 4 subjects with IPSS<50% and no need for additional intervention on the original urethral stricture.
Voiding function and patient-reported outcome measures were positively impacted after treatment. There were significant improvements from baseline in quality of life (IPSS QOL), Qmax, PVR, and USS-PROM at 3 years (p<0.0001 for all; Table 1).
Safety
A total of 73 adverse events in 35 subjects were reported through 3 years, with only 3 being reported between 2 and 3 years. Five subjects experienced 6 serious adverse events; none were related to the study device or procedure. These events included myocardial infarction, two events of abdominal pain (appendicitis, kidney stones), a fall necessitating leg surgery, bladder infection, and prostate cancer. The most common adverse event was urinary tract infection (20.8%), followed by dysuria, fever, acute urinary retention, and urethral stricture (9.4% for each event). The majority (89%) of events were classified as mild or moderate using the Common Terminology Criteria for Adverse Events (CTCAE).
There were 13 treatment-related adverse events, most (12/13) occurring within the first year post-procedure (Table 3). Four events were classified as probably or possibly related to the study device, including one event each of hematuria, urinary tract infection, dysuria, and acute urinary retention (1.9% for each). All device-related events occurred within 6 months of the index procedure and resolved within 3 weeks with either antibiotics or no treatment. The most common procedure-related events included dysuria, fever, and hematuria (3.8% for each). The one event deemed possibly related to treatment after the first year was a stricture recurrence.
 |
Table 3 Number of Adverse Events by Time Period |
Interestingly, sexual function improved from baseline. The average IIEF Overall Satisfaction (OS) sub-score increased from 6.5 at baseline to 8.2 at 3 years (p=0.003; Table 1), while the average Erectile Function (EF) sub-score increased from 16.0 at baseline to 20.6 at 3 years (p=0.015).
Discussion
The ROBUST I trial evaluated the safety and efficacy of the Optilume DCB for the treatment of male anterior bulbar urethral strictures. At 3 years, functional success occurred in 67% with no treatment-related serious adverse events.
This study focused on patient symptoms and the lack of need for additional intervention to define long-term treatment successful. Functional outcomes used in the literature to define patients free from clinically significant stricture recurrence include improvement in IPSS, Qmax >15 mL/sec, and patient-reported outcome measures such as the USS-PROM. Functional success in this study was based on improvement in IPSS and had similar rates at 2 and 3 years (68% and 67%, respectively).8 The reduction in IPSS by an average of 72% (19.7 points) was considered clinically meaningful through 3 years post-treatment for a population with moderate-to-severe symptoms at baseline.9 Significant improvements were also observed in voiding function, with average Qmax remaining >15 mL/sec at all follow-up visits through 3 years. Although Qmax has decreased over time, all patient-reported measures (IPSS, IPSS QOL, USS-PROM, IIEF) remained consistently and significantly improved from baseline. Freedom from repeat intervention was 77% at 3 years. This rate was higher than the rate of functional success due to the inclusion of 4 additional subjects with <50% improvement in IPSS who had not pursued additional intervention prior to their 3-year visit. Long-term anatomic data was not available for this study as the ULT was not performed after one year to avoid additional invasive diagnosis tests. While cystoscopic recurrence after urethroplasty has been found to be predictive of additional intervention, it was not correlated to patient symptoms.10 In this study, of the 29 subjects achieving functional success at 3 years, three were considered prior ULT failures. While anatomic data is important in determining stricture recurrence, the pragmatic approach of utilizing both re-intervention rate and PROMs is a common practice to evaluate long-term treatment success.
Literature reports for outcomes after endoscopic treatment of recurrent urethral strictures show success rates ranging from 50% to 0% for those with 2 and 3 prior interventions, respectively.3,11 Freedom from re-intervention and functional success outcomes after treatment with the Optilume® DCB compare favorably with other endoscopic interventions, with 77% remaining free from repeat intervention at 3 years. Subjects in ROBUST I had an average of 1.7 prior endoscopic procedures, with 43% (23/53) having at least 2 before study enrollment. Treatment with the Optilume® DCB was therefore the second or third procedure for the study population. The Optilume DCB has shown excellent results when used on recurrent bulbar urethral strictures. How these outcomes translate to use in treatment naïve strictures is not directly understood, however it can reasonably be inferred that if the Optilume DCB works in this difficult patient population it would show similarly positive results in a treatment naïve population.
Consistent with the 2-year results, balloon size was a significant predictor of success. This was largely due to the availability of only 24F balloon diameters in the initial phase of the study, which was then expanded to include 30F balloons. Device sizing is currently based on stricture length, stricture diameter, and the diameter of healthy urethra distal to the stricture. Literature suggests that stricture length and the number of prior dilations are important prognostic indicators for outcomes after routine endoscopic treatment.11,12 Long-term success in this study was not dependent on baseline characteristics such as the number of prior endoscopic procedures or stricture length. It is not clear whether this is due to the small sample sizes within each subgroup or due to the nature of the Optilume DCB treatment, however these results were confirmed in a follow-up randomized study comparing the Optilume DCB to standard of care endoscopic dilation.13
Adverse events were generally mild to moderate, and similar to those associated with urethroplasty or other lower urinary tract procedures. Only one treatment-related event was reported after 1 year (stricture recurrence), indicating that the risk of having a device-related adverse event is low in the long term. Erectile function did not appear to be impacted by the treatment.
Study limitations include the lack of a control arm, although higher rates of stricture recurrence would be expected in this population based on published data of patients with multiple repeat endoscopic treatments.3,11 Results from a single blind, randomized, controlled trial comparing the Optilume® DCB to standard of care dilation were recently published, showing a significant improvement in the rate of anatomic success and functional improvement for patients treated with the Optilume® DCB.13 Patients at increased risk of stricture recurrence (eg, prior radiation, lichen sclerosus) or other anatomic locations (bladder neck) were excluded from the study to obtain a homogeneous population, thus performance in these subgroups is unknown. Although cystoscopy was not performed after one year, both retreatment rate and patient reported questionnaires symptoms were used to determine long-term success. Subject follow-up will continue through 5 years to further evaluate durability of the treatment.
Conclusions
Symptomatic improvement after treatment with the Optilume DCB was maintained through 3 years in a population susceptible to high stricture recurrence rate. The therapy is safe with no negative impact on sexual function. Data from the ROBUST I study will be reported through five years. Additionally, a Phase III randomized clinical trial comparing the Optilume® DCB to DVIU or plain balloon dilation has recently been published.
Abbreviations
BPH, benign prostatic hyperplasia; CTCAE, Common Terminology Criteria for Adverse Events; DCB, drug coated balloon; DVIU, direct vision internal urethrotomy; EF, erectile function; IIEF, International Index of Erectile Function; IPSS, International Prostate Symptom Score; LUTS, lower urinary tract symptom scores; OS, overall satisfaction; PVR, post-void residual; Qmax, peak urinary flow rate; QoL, quality of life; ULT, urethral lumen test; USS-PROM, urethral stricture surgery patient reported outcome measure.
Data Sharing Statement
Further data sharing is not planned outside of the contents of this manuscript and those reported on the clinicaltrials.gov website.
Acknowledgments
The authors would like to thank Gabi Molnar for her contributions with manuscript writing and data analysis.
Funding
The study was sponsored and funded by Urotronic, Inc.
Disclosure
R Virasoro, JM DeLong and SP Elliott are paid consultants of Urotronic, Inc. The other authors report no conflicts of interest in this work.
References
1. Bullock TL, Brandes SB. Adult anterior urethral strictures: a national practice patterns survey of board certified urologists in the United States. J Urol. 2007;177(2):685–690. doi:10.1016/j.juro.2006.09.052
2. Steenkamp JW, Heyns CF, de Kock ML. Internal urethrotomy versus dilation as treatment for male urethral strictures: a prospective, randomized comparison. J Urol. 1997;157(1):98–101. doi:10.1016/S0022-5347(01)65296-0
3. Santucci R, Eisenberg L. Urethrotomy has a much lower success rate than previously reported. J Urol. 2010;183(5):1859–1862. doi:10.1016/j.juro.2010.01.020
4. Pang KH, Chapple CR, Chatters R, et al. A systematic review and meta-analysis of adjuncts to minimally invasive treatment of urethral stricture in men. Eur Urol. 2021;80(4):467–479. doi:10.1016/j.eururo.2021.06.022
5. Axel DI, Kunert W, Goggelmann C, et al. Paclitaxel inhibits arterial smooth muscle cell proliferation and migration in vitro and in vivo using local drug delivery. Circulation. 1997;96(2):636–645. doi:10.1161/01.CIR.96.2.636
6. Herten M, Torsello GB, Schonefeld E, Stahlhoff S. Critical appraisal of paclitaxel balloon angioplasty for femoral-popliteal arterial disease. Vasc Health Risk Manag. 2016;12:341–356. doi:10.2147/VHRM.S81122
7. Virasoro R, DeLong JM, Mann RA, et al. A drug-coated balloon treatment for urethral stricture disease: interim results from the ROBUST I study. Can Urol Assoc J. 2020;14(6):187–191. doi:10.5489/cuaj.6323
8. Mann RA, Virasoro R, DeLong JM, et al. A drug-coated balloon treatment for urethral stricture disease: two-year results from the ROBUST I study. Can Urol Assoc J. 2021;15(2):20–25. doi:10.5489/cuaj.6661
9. Barry MJ, Williford WO, Chang Y, et al. Benign prostatic hyperplasia specific health status measures in clinical research: how much change in the American Urological Association symptom index and the benign prostatic hyperplasia impact index is perceptible to patients? J Urol. 1995;154(5):1770–1774. doi:10.1016/S0022-5347(01)66780-6
10. Baradaran N, Fergus KB, Moses RA, et al. Clinical significance of cystoscopic urethral stricture recurrence after anterior urethroplasty: a multi-institution analysis from Trauma and Urologic Reconstructive Network of Surgeons (TURNS). World J Urol. 2019;37(12):2763–2768. doi:10.1007/s00345-019-02653-6
11. Heyns CF, Steenkamp JW, De Kock ML, Whitaker P. Treatment of male urethral strictures: is repeated dilation or internal urethrotomy useful? J Urol. 1998;160(2):356–358. doi:10.1016/S0022-5347(01)62894-5
12. Pansadora V, Emiliozzi P. Internal urethrotomy in the management of anterior urethral strictures: long-term followup. J Urol. 1996;156(1):73–75. doi:10.1016/S0022-5347(01)65942-1
13. Elliott SP, Coutinho K, Robertson KJ, et al. One-year results for the ROBUST III randomized controlled trial evaluating the optilume drug-coated balloon for anterior urethral strictures. J Urol. 2021;206(1):10–97. doi:10.1097/JU.0000000000001655
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
