Back to Journals » Therapeutics and Clinical Risk Management » Volume 16
A Dangerous Consequence of the Recent Pandemic: Early Lung Fibrosis Following COVID-19 Pneumonia – Case Reports
Authors Scelfo C , Fontana M, Casalini E, Menzella F , Piro R , Zerbini A, Spaggiari L, Ghidorsi L, Ghidoni G, Facciolongo NC
Received 7 August 2020
Accepted for publication 28 September 2020
Published 29 October 2020 Volume 2020:16 Pages 1039—1046
DOI https://doi.org/10.2147/TCRM.S275779
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Garry Walsh
Chiara Scelfo,1 Matteo Fontana,1 Eleonora Casalini,1 Francesco Menzella,1 Roberto Piro,1 Alessandro Zerbini,2 Lucia Spaggiari,3 Luca Ghidorsi,1 Giulia Ghidoni,1 Nicola C Facciolongo1
1Department of Medical Specialties, Pneumology Unit, Azienda Unità Sanitaria Locale-IRCCS di Reggio Emilia, Reggio Emilia, Italy; 2Unit of Clinical Immunology, Allergy and Advanced Biotechnologies, Azienda Unità Sanitaria Locale-IRCCS di Reggio Emilia, Reggio Emilia, Italy; 3Department of Radiology, Azienda Unità Sanitaria Locale-IRCCS di Reggio Emilia, Reggio Emilia, Italy
Correspondence: Chiara Scelfo Email [email protected]
Abstract: The outbreak of the novel severe acute respiratory syndrome coronavirus (SARS-CoV-2) started in China in December 2019. COVID-19 patients at presentation show a wide spectrum of clinical and pathological involvement. We report two cases of respiratory insufficiency due to COVID-19 pneumonia that occurred in adults without a history of respiratory diseases. Although these patients improved and were discharged from the acute ward, during the hospitalization they both progressed with a subsequent clinical and radiological worsening, pointing out one of the main concerns for these patients at discharge: the possibility of developing persistent lung abnormalities also in healthy people not having other risk factors. In conclusion, these cases represent two examples of early lung fibrosis in patients with COVID-19 pneumonia with different severity disease evolution and highlight the need for long-term follow-up strategies. The etiology of this fibrosis is under discussion: we suppose that it could be due to either a possible outcome of natural history of lung damage produced by ARDS, or to the lung injury related to high oxygen level or to the lung damage directly induced by viral infection or finally to the autoimmune response. At this moment, it is not possible to predict how many people will have consequences due to COVID-19 pneumonia, and therefore we believe that careful follow-up should be mandatory.
Keywords: COVID-19, pulmonary fibrosis, critical care, viral disease, computed tomography, follow-up
Introduction
The outbreak of the novel severe acute respiratory syndrome coronavirus (SARS-CoV-2) started in the Hubei Province in China in December 2019, and spread through the European Countries and soon all around the world.
Italy has been one of the worst-hit countries.1 The World Health Organization (WHO) declared that the coronavirus disease of 2019 (COVID-19) can be characterized as a pandemic in March 2020.2 COVID-19 patients show a wide spectrum of clinical and pathological involvement, from asymptomatic infections or mild upper respiratory tract symptoms, to pneumonia, severe acute respiratory infection (SARI), acute respiratory distress syndrome and septic shock.3 At the very early presentation, the more frequent symptoms are fever (at least 37.3°C), fatigue, dry cough, myalgias, dyspnea and other less common symptoms are headache, sore throat, rhinorrhea, conjunctivitis and gastrointestinal symptoms. The severity of presentation appeared strictly related to age and the presence of coexisting illness (among the overall population, 24% have at least one comorbidity, e.g. hypertension, diabetes mellitus and obesity).3 The spectrum of clinical conditions is varied and can evolve from a mild or moderate clinical picture to a severe acute respiratory syndrome (SARS). In March 2020, WHO published a new definition of SARI, defining it as an acute respiratory infection with history of fever or measured fever of ≥38°C, cough, with onset within the last 10 days and requiring hospitalization.
Basic respiratory treatment is based on oxygen supplementation or mechanical ventilation in more severe clinical condition. There are many treatments for COVID-19 currently being evaluated and tested worldwide, from antivirals to anticoagulant therapies, hydroxychloroquine and corticosteroids, humanized monoclonal antibody and others, but unfortunately, the management of COVID-19 has currently more doubts than certainties.
Due to the lack of an effective therapy, the management is focused on supportive therapies.3 Focusing on radiological features, at the chest computed tomography (CT) the disease is mainly characterized by ground glass opacities (GGO), “paving stone-like”, thickening of interlobular septa with small honeycomb-like with fuzzy edge, multiple or patchy opacities in both lungs and consolidations. The findings obtained on the chest tomography have a good correlation with the natural history of the disease.4
Thanks to the experience gained from previous severe acute respiratory syndrome coronavirus (SARS) epidemic,5,6 one of the main concerns at the discharge of these patients is the possibility of developing persistent lung abnormalities such as different degrees of architectural distortions (e.g. bronchiectasis).7 The development of early lung fibrosis, characterized by the excessive and abnormal deposition of extracellular matrix in order to reconstruct the damaged alveolar epithelium, is a major concern in this disease. The pathogenesis of this fibrotic damage is not known, although it is known that SARS-CoV-2 infection involves the activation of a complex cytokine pathway that could cause a pro-fibrotic pathway. Other mechanisms involved in promoting fibrosis due to the epithelial damage and fibroblast activation are chronic inflammation and the oxidative stress produced by excessive oxygen supply that can lead to apoptosis of follicular cells. These effects add to the already known causes of pulmonary fibrosis development, such as age, smoking, viral infection, drug exposure, genetic predisposition and environmental exposures.8
The aim of this article is to describe two cases of early lung fibrosis in patients with COVID-19 pneumonia and different severities of the disease, then discuss the possible strategies for a long-term follow-up.
Case Description
Case 1
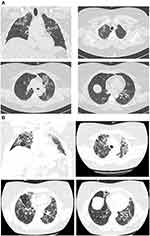
A 59-year-old woman, with a past medical history consistent with autoimmune thyroiditis, major depressive disorder, obesity with a Body Mass index (BMI) of 37.78 kg/m2 and penicillin allergy, was admitted on March 12th, 5 days after the onset of fever and cough. The swab for the detection of SARS-CoV-2 RNA, collected at nasopharyngeal and oropharyngeal levels in accordance with Centers for Disease Control (CDC) guidelines,9 resulted positive the same day and the CT scan showed bilateral GGO with poorly defined margins and multiple patchy opacity with slight thickening of pulmonary interstitial structures involving the 50% of the parenchyma (Figure 1A). Physical examination revealed fever (37.6°C), normal value of blood pressure, mild tachycardia and normal respiratory rate. Respiratory failure was detected, with an initial oxygen partial pressure (PO2) of 68 and a carbon dioxide partial pressure (PCO2) of 41 with pH normal value (7.38), at an arterial blood gas analysis (ABG) done with a fraction of inspired oxygen (FIO2) of 28%. The fraction of inspired oxygen ratio (PaO2/FiO2) was 243. Laboratory results showed normal value of procalcitonin, while C-reactive protein (CRP) was mighty elevated (Table 1). She underwent conventional oxygen therapy, hydroxychloroquine and lopinavir/ritonavir. After an initial improvement, clinical conditions rapidly worsened and a noninvasive ventilation was performed, with scarce patient adherence and no clinical improvement. As a consequence, treatment with high-flow nasal oxygen (HFNO) was started and subcutaneous Tocilizumab (324 mg), with two consecutive injections, was administered. After 20 days, the patient improved and was discharged at home with an acceptable room air saturation of 94% at room air. After a week, she was newly admitted at the Emergency Room (ER) department because of shortness of breath and fatigue: the new contrast-enhanced CT scan, performed on April 10th, about one month later the onset of initial symptoms, showed a larger extension of the opacities, with parenchymal changes including consolidations and signs of reticular interstitial thickening, linear scarring and volume loss at the upper lobes (Figure 1B). Again, the respiratory failure was present (ABG at room air: pO2 56, pCO2 52, pH 7.28), needing oxygen support with a maximum FIO2 of 60%, but not requiring ventilation. During the second hospitalization, the patient was asthenic, she was weak and unable to support effort and it was not possible to withheld oxygen therapy, so the patient was discharged to a rehabilitation ward to continue pulmonary and motor rehabilitation. The laboratory findings were normal, in particular leukocyte (5.58 x1000/μL); haemoglobin: 13.5 g/dl; liver function glutamic-oxalacetic transaminase (GOT): 34 U/l; glutamic-pyruvic transaminase (GPT): 49 U/l; renal function (1.02 mg/dl); electrolytes; Lactate de-hydrogenase (LDH): 438 U/l and C protein (0.1 mg/dL). Two swab specimens were performed, resulting both negative.
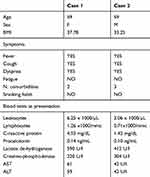 |
Table 1 Demographic, Clinical and Laboratory Features |
Serum autoantibody panel was negative and the detection of serum immunoglobulin G and M against SARS-COV2 was not yet available at the time of admission.
Case 2
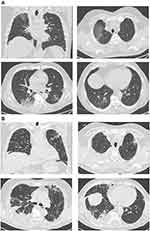
A 59-year-old man, with a history of hypertension, obesity with a BMI of 33.25 kg/m2 and benign prostate hyperplasia started complaining cough, asthenia and fever on March 10th and was first admitted in the emergency department ten days after. He was not taking any chronic therapy except angiotensin binding receptor antagonist for the control of arterial hypertension. The swab was soon performed because he had a history of exposure to an infected person, but the mild clinical presentation led to discharge him. In this occasion, the CT scan showed multiple patchy, nodular opacity and GGO under the pleura, with poorly defined margins and few consolidations at the right lower lobe with a cumulative extent of 20% (Figure 2A). He had fever (37.8°C), normal value of blood pressure and pulsation, tachypnea (respiratory rate of 24 breaths per minute). At the first presentation, he already had characteristics of a more severe disease, such as leukopenia with lymphopenia (Table 1). He came back at the emergency department after a week with the evidence of respiratory failure (ABG at room air: pO2 54, pCO2 30, pH 7.50 and the PO2/FiO2 ratio was 257) and was admitted in a Medicine ward. Because of the lack of intravenous doses, a subcutaneous Tocilizumab (324 mg) with two consecutive injections was performed, together with hydroxychloroquine and lopinavir/ritonavir, but the situation suddenly worsened (the PaO2/FiO2ratio fell to 100 on day 2 with a FIO2 of 60%) and, after a brief trial of non-invasive ventilation in the Respiratory ward, he was admitted to the Intensive Care Unit (ICU) and underwent endotracheal intubation (EOT) the day after. The endotracheal tube was removed after a week and noninvasive ventilation was continued for a few days, then stopped and supplemental oxygen gradually tapered. He was discharged to a medical rehabilitation unit (MRU) still requiring low flow oxygen therapy which he continued even in the following days. Because of the delay in recovery and weaning from oxygen support (ABG at room air: pO2 57, pCO2 39, pH 7.48), a CT scan was therefore repeated, showing a right sided pleural effusion and the presence of sub-pleural consolidations and reticular thickening involving the upper lobes (Figure 2B). Again, autoantibody screening was negative and the detection of serum immunoglobulin G and M against SARS-COV2 was not performed because not available yet.
Discussion
These two cases describe different clinical presentations and severity of the disease caused by COVID-19, both started with radiological characteristics consistent with the recent scientific literature10 and ended in the development of early fibrotic damage. Results like these are not usual for respiratory viral infections, in fact, it is well known that subacute viral infections are associated with the development of late fibrosis in the long term.11 There is still debate on what interpretation give to fibrosis, but in these cases, the presence of fibrosis in the early stage of the disease was not related to a worse prognosis.12
Moreover, it is possible that the older the lung, the greater the risk of virus susceptibility.13 The pathogenesis of this fibrotic damage is not known: we are aware that the disease caused by SARS-CoV-2 involves the activation of a complex cytokine pathway that could cause the various manifestations of the disease, including a pro-fibrotic pathway.14–16 For this reason, Tocilizumab (a humanized monoclonal antibody directed against interleukin-6 receptor) has been used in clinical practice for COVID-19 patients within clinical trials or compassionate use in order to limit the cytokine storm; in these cases, due to the lack of the drug, we were not able to administer it intravenously or (in the first case) at the full dosage.17,18 These two cases were not treated with corticosteroids because the Tocilizumab protocol excluded the possibility of concomitant use. However, this does not rule out the possibility of using steroids for the treatment in the acute disease phase, as recently published.19
A similar pro-fibrotic pathway is involved also in the development of some types of lung fibrosis, such as idiopathic pulmonary fibrosis (IPF).20,21 On the other hand, in many cases, the clinical syndrome associated with this pathogen is comparable with the acute respiratory distress syndrome (ARDS), in which the fibrotic damage is a possible and well-known result.22 The mechanical ventilation may also have a role in worsening the lung damage and causing the development of fibrosis23,24 and these patients both demonstrated the presence of fibrosis, even after different types of respiratory support and severity of the disease. Furthermore, the potential pro-fibrotic role represented by oxygen free radicals (ROS) produced by the prolonged use of high oxygen fraction must also be considered.25,26
There is also the possibility that the microclots in the pulmonary vessels together with the fibrin deposition in the air spaces and lung parenchyma may have a role in developing fibrosis and worsening respiratory failure.8,27
Finally, the negativity of the search for autoantibodies allows us to exclude the possible concomitant development of an autoimmune disease, which could be associated with interstitial lung diseases with autoimmune features.28
The research aimed at identifying one or more drugs that are truly effective in treating COVID-19 is massive and supported by huge investments. Numerous studies are underway on more or less promising drugs with different molecular targets. It would be impossible to report all the studies in progress, so we will refer to one study that summarizes most of the treatments to be considered. Lechowicz et al review analyze possible treatments currently to be considered to prevent or limit post-COVID-19 fibrosis. Considering inflammation, steroids are used to decrease the host inflammatory response meanwhile Tocilizumab is used to block the cytokine cascade. Among humanized antibody, other studies are ongoing with Canakinumab (ClinicalTrials.gov Identifier: NCT04362813) and with Sarilumab (ClinicalTrials.gov Identifier: NCT04315298, NCT04327388, NCT04324073, NCT04322773, NCT04321993). Spironolactone seems to have an antioxidant action in animal models. For years there has been interest in the potential use of anticoagulant drugs in the treatment of ARDS. Recently these drugs are hypothesized to been used in patients with very severe COVID-19-induced ARDS, in particular tissue plasminogen activator (tPA). The use of antivirals in this field is intended to reduce the duration of lung infection and the possible consequent damage. There are many trials ongoing on potential novel strategies,29 most of them consider antifibrotic agents (Nintedanib and Pirfenidone), vaccine, hydroxycorochine combined with antivirals, vitamins (vitamin C and D), corticosteroids, plasma therapy, humanized monoclonal antibody with anti-interleukin effect (tocilizumab, siltuximab), mesenchymal stromal stem cells, individualized-Chinese herbal medicine and others. The real solution is the identification of a safe vaccine, and in this regard, preliminary report seems reassuring about efficacy and safety.30
Conclusion
In conclusion, we suppose that the mechanism underlying the fibrotic process could be due to an outcome of natural history of lung damage produced by ARDS or his treatment (high oxygen level, ventilator support), or the lung damage directly induced by viral infection or finally the autoimmune response, triggered by viral infection.
To our knowledge, these are the first case reports showing the development of rapid onset lung fibrosis after SARS-CoV-2 infection in previously healthy respiratory patients, resulting in clinical worsening. We expect the number of patients who develop such lung damages will be high and therefore, we believe that these patients will need to be taken care of with a structured follow-up program in order to understand in which ones the fibrotic evolution will take place, when it will appear and in which case it will cause restrictive pattern and eventually chronic respiratory failure.31
Finally, these cases should alert lung specialists to consider COVID-19 as a possible differential diagnosis for causes of pulmonary fibrosis.
Abbreviations
ARDS, acute respiratory distress syndrome; BMI, body mass index; CDC, Centers for Disease Control; COVID-19, coronavirus disease; CRP, C-reactive protein CT, computed tomography; EOT, endotracheal intubation; ER, emergency room; FIO2, fraction of inspired oxygen; GGO, ground glass opacities; GOT, glutamic-oxalacetic transaminase; GPT, glutamic-pyruvic transaminase; HFNO, high-flow nasal oxygen; ICU, intensive care unit; IPF, idiopathic pulmonary fibrosis; MRU, medical rehabilitation unit; PO2, partial oxygen pressure; PCO2, partial dioxide partial pressure; PO2/FIO2, arterial oxygen pressure/fraction of inspired oxygen; ROS, oxygen free radicals; SARI, severe acute respiratory infection; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; TpA, tissue plasminogen activator; WHO, World Health Organization.
Ethics Approval and Consent to Participate
N/A.
Consent for Publication
Written informed consent was obtained from the patients for publication of this Case report and any and all accompanying images. A copy of the written consent is available for review upon request.
Acknowledgments
N/A.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest for this work.
References
1. Remuzzi A, Remuzzi G. COVID-19 and Italy: what next? The Lancet. 2020;395:1225–1228. doi:10.1016/S0140-6736(20)30627-9
2. World Health Organization. WHO Clinical management of severe acute respiratory infection (SARI) when COVID-19 disease is suspected. Interim Guidance. 2020.
3. Guan W, Ni Z, Hu Y, et al. Clinical characteristics of Coronavirus Disease 2019 in China. N Engl J Med. 2020;382(18):1708–1720. doi:10.1056/NEJMoa2002032
4. Wang Y, Dong C, Hu Y. Temporal changes of CT findings in 90 patients with COVID-19 pneumonia: a longitudinal study. Radiology. 2020;19:200843. doi:10.1148/radiol.2020200843.
5. Hui DS, Wong KT, Ko FW, et al. The 1-year impact of severe acute respiratory syndrome on pulmonary function, exercise capacity, and quality of life in a cohort of survivors. Chest. 2005;128:2247–2261. doi:10.1378/chest.128.4.2247
6. Chang Y-C, Chong-Jen Y, Chang S-C, et al. Pulmonary sequelae in convalescent patients after severe acute respiratory syndrome: evaluation with thin-section CT. Radiology. 2005;236(3):1067–1075. doi:10.1148/radiol.2363040958
7. Zornetzer GA, Frieman MB, Rosenzweig E, et al. Transcriptomic analysis reveals a mechanism for a prefibrotic phenotype in STAT1 knockout mice during severe acute respiratory syndrome coronavirus infection. J Virol. 2010;84(21):11297–11309. doi:10.1128/JVI.01130-10
8. Lechowicz K, Drożdżal S, Machaj F, et al. COVID-19: the potential treatment of pulmonary fibrosis associated with SARS-CoV-2 infection. J Clin Med. 2020;9(6):E1917. doi:10.3390/jcm9061917
9. Centers for Disease Control and Prevention. Interim guidelines for collecting, handling, and testing clinical specimens from patients under investigation (PUIs) for 2019 novel coronavirus (2019-nCoV). 2020. Available from: https://www.cdc.gov/coronavirus/2019-nCoV/guidelines-clinical-specimens.html.opens in new tab.
10. Revel MP, Parkar AP, Prosch H, et al. COVID-19 patients and the radiology department - advice from the European Society of Radiology (ESR) and the European Society of Thoracic Imaging (ESTI). European Society of Radiology (ESR) and the European Society of Thoracic Imaging (ESTI). Eur Radiol. 2020. doi:10.1007/s00330-020-06865-y
11. Sheng G, Chen P, Wei Y. Viral infection increases the risk of idiopathic pulmonary fibrosis: a meta-analysis. Chest. 2019. doi:10.1016/j.chest.2019.10.032
12. Zheng Y, Zhang Y, Wang Y, et al. Chest CT manifestations of new coronavirus disease 2019 (COVID-19): a pictorial review. Eur Radiol. 2020;30(8):4381–4389. doi:10.1007/s00330-020-06801-0
13. Naik PK, Moore BB. Viral infection and aging as cofactors for the development of pulmonary fibrosis. Expert Rev Respir Med. 2010;4(6):759–771. doi:10.1586/ers.10.73
14. Yuki K, Fujiogi M, Koutsogiannaki S. COVID-19 pathophysiology: a review. Clin Immunol. 2020;20:108427. doi:10.1016/j.clim.2020.108427
15. Li H, Liu L, Zhang D, et al. SARS-CoV-2 and viral sepsis: observations and hypotheses. Lancet. 2020. doi:10.1016/s0140-6736(20)30920-x
16. Ingraham NE, Lotfi-Emran S, Thielen BK, et al. Immunomodulation in COVID-19. Lancet Respir Med. 2020;S2213–2600(20):30226. doi:10.1016/S2213-2600(20)30226-5
17. Sciascia S, Aprà F, Baffa A, et al. Pilot prospective open, single-arm multicentre study on off-label use of tocilizumab in patients with severe COVID-19. Clin Exp Rheumatol. 2020.
18. Xu X, Han M, Li T, et al. Effective treatment of severe COVID-19 patients with tocilizumab. Proc Natl Acad Sci. 2020. doi:10.1073/pnas.2005615117
19. The RECOVERY Collaborative Group. Dexamethasone in Hospitalized Patients with Covid-19 — preliminary Report. NEJM. 2020. doi:10.1056/NEJMoa2021436
20. Sgalla G, Iovene B, Calvello M, et al. Idiopathic pulmonary fibrosis: pathogenesis and management. Respir Res. 2018;19(1):32. doi:10.1186/s12931-018-0730-2
21. Wang J, Wang BG, Jang YC, et al. Advances in the research of mechanism of pulmonary fibrosis induced by Corona Virus Disease 2019 and the corresponding therapeutic measures. Zhonghua Shao Shang Za Zhi. 2020. 36:E006. doi:10.3760/cma.j.cn501120-20200307-00132
22. Thompson BT, Chambers RC, Liu KD. Acute respiratory distress syndrome. N Engl J Med. 2017;377(6):562–572. doi:10.1056/NEJMra1608077
23. Cabrera-Benitez NE, Laffey JG, Parotto M, et al. Mechanical ventilation-associated lung fibrosis in acute respiratory distress syndrome: a significant contributor to poor outcome. Anesthesiology. 2014;121(1):189–198. doi:10.1097/ALN.0000000000000264
24. Yu Z, Wang T, Zhang L, et al. WISP1 and TLR4 on macrophages contribute to ventilator-induced lung injury. Inflammation. 2020;43(2):425–432. doi:10.1007/s10753-019-01103-0
25. Gonzalez-Gonzalez FJ, Chandel NS, Jain M, et al. Reactive oxygen species as signaling molecules in the development of lung fibrosis. Transl Res J Lab Clin Med. 2017;190:61–68.
26. Otoupalova E, Smith S, Cheng G, et al. Oxidative stress in pulmonary fibrosis. Compr Physiol. 2020;10:509–547.
27. Yang Y, Tang H. Aberrant coagulation causes a hyper-inflammatory response in severe influenza pneumonia. Cell Mol Immunol. 2016;13:432–442. doi:10.1038/cmi.2016.1
28. Sambataro G, Sambataro D, Torrisi SE, et al. Clinical, serological and radiological features of a prospective cohort of Interstitial Pneumonia with Autoimmune Features (IPAF) patients. Respir Med. 2019;150:154–160. doi:10.1016/j.rmed.2019.03.011
29. Available from: https://www.clinicaltrials.gov/ct2/results?cond=Covid19&term=lung+fibrosis&cntry=&state=&city=&dist=.
30. Jackson LA, Anderson EJ, Rouphael NG. et al. An mRNA vaccine against SARS-CoV-2 - preliminary report. N Engl J Med. 2020. NEJMoa2022483. doi:10.1056/NEJMoa2022483
31. Xiaoneng M, Jian W, Zhuquan S, et al. Abnormal pulmonary function in COVID-19 patients at time of hospital discharge. Eur Respir J. 2020;12;2001217. doi:10.1183/13993003.01217-2020
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.