Back to Journals » Cancer Management and Research » Volume 13
A Cytoplasm-Enriched circRNA circ-MYBL2 is Downregulated in Non-Small Cell Lung Cancer and Sponges Oncogenic miR-28 to Regulate Cancer Cell Proliferation and Apoptosis
Received 9 March 2021
Accepted for publication 20 July 2021
Published 16 August 2021 Volume 2021:13 Pages 6499—6506
DOI https://doi.org/10.2147/CMAR.S309924
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Seema Singh
This paper has been retracted.
Yanqing Mao, 1, 2 Chunjie Wang 1
1Department of General Practice, Dushu Lake Hospital Affiliated to Soochow University, Suzhou, 215000, People’s Republic of China; 2Department of General Practice, The First Affiliated Hospital of Soochow University, Suzhou, 215000, People’s Republic of China
Correspondence: Chunjie Wang
Department of General Practice, Dushu Lake Hospital Affiliated to Soochow University, No. 8, Chongwen Road, Suzhou Industrial Park, Suzhou City, Jiangsu Province, 215000, People’s Republic of China
Tel +86 0512-65955019
Email [email protected]
Background: Recent studies have reported different roles of circRNA circ-MYBL2 in different cancers. However, the involvement of circ-MYBL2 in non-small cell lung cancer (NSCLC) is unknown. This study was carried out to explore the role of circ-MYBL2 in NSCLC.
Methods: The expression of circ-MYBL2 and miR-28 was detected by RT-qPCR. A 5-year follow-up study was performed for survival analysis. Nuclear fractionation assay was used for subcellular localization analysis. RNA pull-down assay was performed to detect the interaction between circ-MYBL2 and miR-28. The role of circ-MYBL2 and miR-28 in regulating the expression of each other was evaluated by overexpression assay. BrdU incorporation assay and cell apoptosis assay were performed to investigate the role of circ-MYBL2 and miR-28 in cell proliferation and apoptosis.
Results: NSCLC tissues exhibited significantly higher expression levels of miR-28 and lower expression levels of circ-MYBL2. Close correlations between circ-MYBL2 and miR-28 and patients’ survival were observed. Circ-MYBL2, which was found to be mainly enriched in cytoplasm, directly interacted with miR-28. Although circ-MYBL2 and miR-28 showed no regulatory role in the expression of each other, circ-MYBL2 suppressed the effects of miR-28 on cell proliferation and apoptosis.
Conclusion: Circ-MYBL2 is enriched in cytoplasm, and it sponges oncogenic miR-28 to suppress cancer cell proliferation in NSCLC and promote cell apoptosis.
Keywords: circ-MYBL2, miR-28, non-small cell lung cancer, proliferation
Introduction
Lung cancer is the most commonly diagnosed cancer and the leading cause of cancer deaths.1,2 Lung cancers can be divided into two subgroups including small and non-small cell lung cancer (SCLC and NSCLC).3 NSCLC accounts for more than 85% of all lung cancer cases.1,2 NSCLC is more often in smokers than in non-smokers, while SCLC is rarely diagnosed in non-smokers.3 Therefore, the pathogenesis of NSCLC is more complex than that of SCLC4. Although the long-term (5-year) survival rate of NSCLC (24%) is much higher than that of SCLC (6%), the high incidence rate of NSCLC makes it a major cause of cancer-related deaths.5,6 Therefore, new treatment approaches are needed for NSCLC.
Since NSCLC is often diagnosed in never-smokers, tumorigenesis of NSCLC requires the participation of other factors, such as molecular alterations.7,8 To boost the advances in NSCLC treatments, some molecular factors, such as epidermal growth factor receptor tyrosine kinase inhibitors (EGFR TKIs), have been demonstrated as candidates for targeted therapy of NSCLC.9–11 However, more specific and efficient targets remain lack. Recent studies have shown that, circRNAs, as self-closed RNA transcripts, do not have protein-coding capacity but affect protein synthesis to participate in cancer biology,12 suggesting the potential clinical application of circRNAs in the treatment of cancers. However, functional characterization of circRNAs is required prior to clinical applications.13 Recent studies have reported different roles of circ-MYBL2 in different cancers. However, the involvement of circ-MYBL2 in NSCLC is unknown. This study was carried out to explore the role of circ-MYBL2 in NSCLC.14,15 We predicted that circ-MYBL2 could interact with miR-28, which promotes lung cancer.16 We then studied the crosstalk between circ-MYBL2 and miR-28 in NSCLC.
Materials and Methods
Patient Information
This study enrolled 64 NSCLC patients who were admitted at Dushu Lake Hospital Affiliated to Soochow University. NSCLC tumor tissues and adjacent non-tumor tissues (within 3 cm around tumors) were collected from these patients. This study was approved by the Ethics Committee of aforementioned hospital. Due to the fact that not all patients were suitable for surgical resection, tissues were either collected during surgical resection (n = 46) or through fine-needle mediated biopsies (n = 18). All tissue samples were analyzed by more than 3 pathologists, and uncertain cases were excluded. Tissue samples were stored at −80 °C prior to the isolation of total RNAs. Informed consent was signed by all patients. General clinical data for all patients are listed in Table 1.
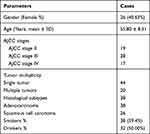 |
Table 1 Clinical Data for Patients |
Survival Analysis
After admission, a 5-year follow-up study was carried out to record their survival conditions. All patients were contacted by telephone on a monthly manner. In some cases, patients were checked during outpatient visit. Survival condition of each patient at each month was recorded and saved for subsequent survival analysis.
Cell Culture
The well-characterized human NSCLC cell lines H1299 (squamous cell carcinoma) and A549 (adenocarcinoma) (ATCC, USA) were used in this study. RPMI 1640 medium and DMEM supplemented with 10% fetal bovine serum (FBS, Invitrogen, China) were used to cultivate A549 and H1299 cells, respectively. Cells were cultivated at 37 °C in a 5% CO2 incubator.
Transient Cell Transfection
H1299 and A549 cells were transfected with either pcDNA3.1- circ-MYBL2 vector or miR-28 mimic (Invitrogen, China). Briefly, a 6-well plate was seeded with these cells at a density of 106 cells per well, and cell culture was performed overnight. After that, Neon Electroporation Transfection (Thermo Fisher Scientific) was used to transiently transfect vector or miRNA into cells. Cells were washed with fresh medium at 6 h after transfection, followed by cell culture in fresh medium for another 48 h. The same system was also applied to transfect negative control (NC) miRNA and empty vector as the control.
RNA Preparations
Total RNAs were isolated using Direct-zol RNA Kit (ZYMO RESEARCH). DNA removal was performed using DNase I. Agilent 2100 Bioanalyzer (Agilent Technologies, USA) was used to analyze RNA quality.
RT-qPCRs
M-MLV reverse transcriptase (Promega) was used to reverse transcribe total RNAs (1000 ng) into cDNA samples, which was used as the template (1 µL per 20 µL system) to perform qPCRs. 18S rRNA was used as the internal control to determine the expression levels of circ-MYBL2. The expression of miR-28 was determined using the All-in-One™ miRNA qRT-PCR Reagent Kit (Genecopoeia) with U6 as the endogenous control. The 2−ΔΔCt method was used to process data as amplification rate close to 100% was achieved in all cases.
Nuclear Fractionation Assay
H1299 (squamous cell carcinoma) and A549 (adenocarcinoma) cells were subjected to the preparation of cytosolic and nuclear samples using the Nuclear/Cytosol Fractionation Kit (BioVision, # K266). All operations were performed following the manufacturer’s instructions. Cytosolic and nuclear fractions were prepared by centrifugation at 2500g at 4 °C for 10 min. The supernatant (cytosolic fraction) and pellet (nuclear fraction) samples were collected, followed by RNA isolation and RTs through the same methods mentioned above. Routine Taq (Invitrogen) was used to perform PCR reactions to measure the expression of circ-MYBL2 and GAPDH. After electrophoresis to separate PCR products, EB staining was performed, and MyECL imager (Bio-Rad) was used to take images.
RNA Pull-Down Assay
Biotinylated-labeled miR-28 (Bio-miR-28) and NC (Bio-NC) were designed and prepared by RiboBio (Guangzhou, China). Through the aforementioned methods, Bio-miR-28 and Bio-NC were transfected into H1299 and A549 cells. After cell lysis, streptavidin agarose magnetic beads (Life Technologies) were used to pull-down RNA complex. Following RNA isolation from pull-down samples, qPCRs were performed to analyze the expression of circ-MYBL2.
BrdU Incorporation Assay (Proliferation Assay)
BrdU incorporation was used to reflect the proliferation of cells after transfections. Briefly, 3000 cells were seeded into each well of a 96-well plate. At 48 h post-transfection, BrdU was added (10 µM), and cells were cultured for another 24 h. After that, cells were fixed and incubated with peroxidase-coupled anti-BrdU-antibody (Sigma-Aldrich) for 60 min. Following PBS washing, peroxidase substrate was used to incubate with the cells for 60 min. OD values (450 nm) were then measured to reflect cell proliferation.
Cell Apoptosis Assay
Annexin V-FITC/propidium iodide (PI) cell apoptosis detection kit (Sigma-Aldrich) was used to stain cells collected at 48 h post-transfection. Staining was performed at 37 °C for 1 h. After that, flow cytometry was performed to analyze cell apoptosis. Flow Jo V10 software was used for data analysis.
Statistical Analysis
Paired t-test was used to compare paired tissues. ANOVA Tukey’s test was used for comparisons among multiple independent groups. The 64 patients were divided into two groups (cutoff value = the median expression level of circ-MYBL2 or miR-28). Survival curves were plotted for each group and compared by Log rank test. p < 0.05 was statistically significant.
Results
Differential Expression of circ-MYBL2 and miR-28 in NSCLC Predicted the Survival of Patients
The expression levels of circ-MYBL2 and miR-28 in paired tissues (n = 64) were detected. The results showed that circ-MYBL2 was downregulated in NSCLC (Figure 1A, p < 0.01), while miR-28 was upregulated in NSCLC (Figure 1B, p < 0.01). Survival analysis was carried out to study the role of circ-MYBL2 and miR-218 in predicting the survival of patients. It showed that low expression levels of circ-MYBL2 (Figure 1C) and high expression levels of miR-218 (Figure 1D) were closely correlated with the poor survival of NSCLC patients.
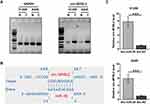
Subcellular Location Analysis of circ-MYBL2 and Its Direct Interaction with miR-218
Nuclear fractionation assay was performed to analyze the subcellular location of circ-MYBL2 in both H1299 and A549 cells. The results showed that circ-MYBL2 could be detected in both cytosolic (C) and nuclear (N) samples, while the expression levels of circ-MYBL in N were much higher than that in C samples (Figure 2A). Therefore, circ-MYBL2 was likely enriched in cytoplasm. The potential base pairs that could be formed between circ-MYBL2 and miR-28 were predicted by IntaRNA2.0. The prediction revealed that circ-MYBL2 could form multiple base pairs with miR-28 (Figure 2B). RNA pull-down assay analysis illustrated that circ-MYBL2 was significantly enriched in Bio-miR-28 pull-down samples than that in Bio-NC pull-down samples (Figure 2C, p < 0.001), suggesting the direct interaction between circ-MYBL2 and miR-28.
The Role of circ-MYBL2 and miR-28 in Regulating the Expression of Each Other
Pearson’s correlation coefficient was used to analyze the correlations between circ-MYBL2 and miR-28 across both NSCLC (Figure 3A) and non-tumor (Figure 3B) tissues. It was observed that circ-MYBL2 and miR-28 were not correlated with each other. H1299 and A549 cells were overexpressed with circ-MYBL2 or miR-28, and the overexpression was confirmed every 24 h until 96 h (Figure 3C, p < 0.05). Surprisingly, circ-MYBL2 showed no role in regulating the expression of miR-28 (Figure 3D). Similarly, miR-28 also did not affect the expression of circ-MYBL2 (Figure 3E).
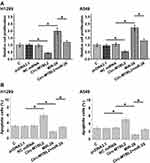
The Functions of circ-MYBL2 and miR-28 in NSCLC Cell Proliferation and Apoptosis
The roles of circ-MYBL2 and miR-28 in the proliferation and apoptosis of H1299 and A549 cells were analyzed by BrdU incorporation assay and cell apoptosis assay, respectively. The results showed that circ-MYBL2 could decrease cell proliferation and increase cell apoptosis. MiR-28 could increase cell proliferation (Figure 4A) and decrease cell apoptosis (Figure 4B). Moreover, circ-MYBL2 suppressed the role of miR-28 in cell proliferation and apoptosis (p < 0.05).
Discussion
The interaction between circ-MYBL2 and miR-28, their prognostic values for the survival of NSCLC patients, and their roles in NSCLC cell proliferation were investigated in this study. We observed that circ-MYBL2 may interact with miR-28 to suppress its role in promoting cancer cell proliferation.
The functions of circ-MYBL2 have been investigated in myeloma and cervical cancers, and opposite roles of circ-MYBL2 were observed in these two cancers.14,15 Circ-MYBL2 is downregulated in multiple myeloma and overexpression of circ-MYBL2 suppressed cancer cell mobility and cell cycle progression. In addition, circ-MYBL2 also suppresses the transcription of multiple oncogenes, suggesting its role as a tumor suppressor in multiple myeloma.14 In contrast, upregulation of circ-MYBL2 has been observed in cervical cancer, and circ-MYBL2 can sponge miR-361-3p to promote cancer cell invasion and proliferation.15 We analyzed the differential expression of circ-MYBL2 in NSCLC and observed its downregulation in cancer tissues. Moreover, overexpression of circ-MYBL2 suppressed NSCLC cell proliferation and increased cell apoptosis. Therefore, circ-MYBL2 is likely a tumor suppressor in NSCLC.
MiRNA-28 is upregulated in NSCLC and it targets PTEN, a well-known tumor suppressor and cell death inducer, to promote cancer cell proliferation.16 Our study confirmed the upregulation of miR-28 in NSCLC and its enhancing effects on cell proliferation and its inhibitory effects on cell apoptosis. However, based on our knowledge, the upstream regulator or miR-28 in cancers, such as NSCLC, is unclear. In this study, we showed that circ-MYBL2 could directly interact with miR-28, while overexpression assay indicated that circ-MYBL2 and miR-28 did not regulate the expression of each other. It has been well established that circRNAs are mostly enriched in cytoplasm.17 Consistently, our study showed that circ-MYBL2 is a cytoplasm-enriched circRNA. Mature miRNAs are mainly localized to cytoplasm. Taken together, we concluded that circ-MYBL2 in cytoplasm may sponge miR-28 to suppress its role in NSCLC cell proliferation and apoptosis.
The present study included H1299 and A549 two cell lines. H1299 is a squamous cell carcinoma cell line, and A549 is adenocarcinoma cell line. Circ-MYBL2 and miR-28 showed similar roles in regulating the proliferation and apoptosis of these two cell lines. Moreover, the present study enrolled 38 cases of patients with adenocarcinoma and 26 cases of squamous cell carcinoma. No significant difference in the expression levels of circ-MYBL2 and miR-28 was observed between these two subtypes (data not shown). Therefore, circ-MYBL2 and miR-28 may play similar roles in these two subtypes.
Our study also reported the prognostic value of circ-MYBL2 and miR-28 for the overall survival of NSCLC patients. However, their clinical applications as molecular targets to treat NSCLC and their roles in the early diagnosis of NSCLC remain to be investigated.
Conclusion
In conclusion, circ-MYBL2 is downregulated in NSCLC, and it may sponge miR-28 to suppress its role in cancer cell proliferation and apoptosis.
Availability of Supporting Data
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to their containing information that could compromise the privacy of research participants.
Ethical Approval and Consent to Participate
Informed consent was signed by all patients. All producers were approved by Dushu Lake Hospital Affiliated to Soochow University Ethics Committee. Procedures operated in this research were completed in keeping with the standards set out in the Announcement of Helsinki and laboratory guidelines of research in China.
Funding
There is no funding to report.
Disclosure
The authors report that they do not have any commercial or associative interest that represents a conflict of interest in connection with the work submitted.
References
1. Barta JA, Powell CA, Wisnivesky JP. Global epidemiology of lung cancer. Ann Glob Health. 2019;85(1). doi:10.5334/aogh.2419
2. Cao M, Chen W. Epidemiology of lung cancer in China. Thorac Cancer. 2019;10(1):3–7. doi:10.1111/1759-7714.12916
3. Busch SE, Hanke ML, Kargl J, Metz HE, MacPherson D, Houghton AM. Lung cancer subtypes generate unique immune responses. J Immunol. 2016;197(11):4493–4503. doi:10.4049/jimmunol.1600576
4. Couraud S, Zalcman G, Milleron B, Morin F, Souquet PJ. Lung cancer in never smokers–a review. Eur J Cancer. 2012;48(9):1299–1311. doi:10.1016/j.ejca.2012.03.007
5. Antonia SJ, Villegas A, Daniel D, et al. Overall survival with durvalumab after chemoradiotherapy in stage III NSCLC. N Engl J Med. 2018;379(24):2342–2350. doi:10.1056/NEJMoa1809697
6. Ramalingam SS, Vansteenkiste J, Planchard D, et al. Overall survival with osimertinib in untreated, EGFR-mutated advanced NSCLC. N Engl J Med. 2020;382(1):41–50. doi:10.1056/NEJMoa1913662
7. Tang A, Ahmad U, Toth AJ, et al. Non-small cell lung cancer in never- and ever-smokers: is it the same disease? J Thorac Cardiovasc Surg. 2020;161(6):1903–1917.
8. Santoro IL, Ramos RP, Franceschini J, Jamnik S, Fernandes AL. Non-small cell lung cancer in never smokers: a clinical entity to be identified. Clinics. 2011;66(11):1873–1877. doi:10.1590/S1807-59322011001100005
9. Yuan M, Huang LL, Chen JH, Wu J, Xu Q. The emerging treatment landscape of targeted therapy in non-small-cell lung cancer. Signal Transduct Target Ther. 2019;4:61. doi:10.1038/s41392-019-0099-9
10. Janku F, Stewart DJ, Kurzrock R. Targeted therapy in non-small-cell lung cancer–is it becoming a reality? Nat Rev Clin Oncol. 2010;7(7):401–414. doi:10.1038/nrclinonc.2010.64
11. Wu SG, Shih JY. Management of acquired resistance to EGFR TKI-targeted therapy in advanced non-small cell lung cancer. Mol Cancer. 2018;17(1):38. doi:10.1186/s12943-018-0777-1
12. Patop IL, Kadener S. circRNAs in cancer. Curr Opin Genet Dev. 2018;48:121–127. doi:10.1016/j.gde.2017.11.007
13. Wang C, Tan S, Li J, Liu WR, Peng Y, Li W. CircRNAs in lung cancer - biogenesis, function and clinical implication. Cancer Lett. 2020;492:106–115. doi:10.1016/j.canlet.2020.08.013
14. Yu S, Ai L, Wei W, Pan J. circRNA circ-MYBL2 is a novel tumor suppressor and potential biomarker in multiple myeloma. Human Cell. 2021;34(1):219–228. doi:10.1007/s13577-020-00441-8
15. Wang J, Li H, Liang Z. circ-MYBL2 serves as a sponge for miR-361-3p promoting cervical cancer cells proliferation and invasion. Onco Targets Ther. 2019;12:9957–9964. doi:10.2147/OTT.S218976
16. Cui F, Zhou Q, Xiao K, Qian H. MicroRNA‑28 promotes the proliferation of non‑small‑cell lung cancer cells by targeting PTEN. Mol Med Rep. 2020;21(6):2589–2596.
17. Li J, Yang J, Zhou P, et al. Circular RNAs in cancer: novel insights into origins, properties, functions and implications. Am J Cancer Res. 2015;5(2):472–480.
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.