Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 15
A Cross-Sectional Study of Atherosclerosis in Newly Diagnosed Patients with Ketosis-Prone Type 2 Diabetes
Authors Wang Y, Lu C, Augusto Monteiro Cardoso Lopes M, Chen L, Luo Y , Wu W, Gu X
Received 1 December 2021
Accepted for publication 7 March 2022
Published 25 March 2022 Volume 2022:15 Pages 933—941
DOI https://doi.org/10.2147/DMSO.S349467
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Juei-Tang Cheng
Yuxia Wang,1,2 Chaoyin Lu,2,3 Mauro Augusto Monteiro Cardoso Lopes,2 Lingqiao Chen,1,2 Yan Luo,1,2 Wenjun Wu,1,2 Xuemei Gu1,2
1Department of Endocrinology, The First Affiliated Hospital of Wenzhou Medical University, Wenzhou, Zhejiang, People’s Republic of China; 2The First Clinical School of Wenzhou Medical University, Wenzhou, Zhejiang, People’s Republic of China; 3Department of Endocrinology, Taizhou Central Hospital (Taizhou University Hospital), Taizhou, Zhejiang, People’s Republic of China
Correspondence: Xuemei Gu, Department of Endocrinology, The First Affiliated Hospital of Wenzhou Medical University, Nan Bai Xiang Road, Wenzhou, 325006, People’s Republic of China, Tel +86-577-55579385, Fax +86-577-88069555, Email [email protected]
Purpose: To investigate the prevalence, clinical and metabolic characteristics of atherosclerosis (AS) in newly diagnosed patients with ketosis-prone type 2 diabetes (KPT2D) or non-ketotic type 2 diabetes (NKPT2D).
Patients and Methods: About 1072 subjects with non-autoimmune new-onset diabetes were included in the cross-sectional study. Patients were classified as non-ketotic type 2 diabetes (NKPT2D, n = 662) or ketosis-prone type 2 diabetes (KPT2D, n = 410). Blood samples were collected to determine the levels of glucose, HbA1c, insulin and C-peptide. Routine liver and kidney function tests were also performed. AS was determined by vascular ultrasonography.
Results: The levels of fasting blood glucose and HbA1c were significant higher in the KPT2D group when compared to the NKPT2D group (P< 0.001). The levels of fasting C-peptide, 2 h C-peptide and HOMA-β were lower in the KPT2D group than those in NKPT2D group (P< 0.001). However, no significant difference was observed for HOMA-IR between the two groups. The onset age of the patients with KPT2D was significantly lower compared to NKPT2D patients (38± 13 vs 49± 14, P< 0.001). After adjusting age of the two groups, the KPT2D patients had a higher prevalence of AS compared to the NKPT2D patients (31.4% vs 21.1%, P=0.005). In both groups, age and gender were independent risk factors for AS, whereas estimated glomerular filtration rate (eGFR) was an independent risk factor in the NKPT2D patients and 2-h postprandial plasma glucose (2h-PPG) was an independent risk factor in the KPT2D patients.
Conclusion: AS was more prevalent in KPT2D patients compared to the NKPT2D cohort, which was independent of age and gender. These data suggest that KPT2D patients may have a higher risk of macrovascular complications compared to NKPT2D of the same age.
Keywords: ketosis, diabetes, atherosclerosis
Introduction
Ketosis-prone type 2 diabetes (KPT2D) is a term that has been previously used to describe a range of forms of diabetes (FlatBush diabetes,1 idiopathic type 1 diabetes,2 atypical diabetes,3 and ketosis-prone diabetes4), which was originally reported in young African-Americans.5 Ketosis-prone type 2 diabetes (KPT2D) is manifested as unprovoked ketosis or ketoacidosis and is characterized by impairment of both insulin secretion and action with the absence of islet autoantibodies.
Patients with KPT2D present with mixed features of Type 1 and Type 2 diabetes, which makes clinical classification challenging. Recent studies have increasingly reported the differences between KPT2D and typical type 1 diabetes. However, it is difficult to distinguish KPT2D from typical type 2 diabetes. Lu et al6 found that KPT2D had improved islet β-cell function and higher insulin resistance compared to patients with NKPT2D. Also, the latest WHO guidelines7 classified KPT2D as a hybrid type of diabetes meaning that it is not a subset of type 1 or type 2 diabetes. The clinical and metabolic differences between KPT2D and the typical type 2 diabetes remain unclear particularly concerning differences in macrovascular comorbidities and complications between the diseases.
In diabetes, atherosclerosis (AS) is accelerated by the unique diabetic milieu and has been a focus of major research and clinical discussions given the high prevalence of the condition in prediabetic and diabetic patients.8 The prevalence of AS has been reported to be more than 70% in T2DM.9 Also, AS can significantly increase the risk of cardiovascular events and deaths in patients with type 2 diabetes. Previous studies have been reported using ultrasound examination of the carotid or lower extremities arteries.10,11 Li et al9 found that the combination of carotid and lower extremity ultrasonography can improve the detection of AS in type 2 diabetes patients. To characterize AS in KPT2D, we used a combined approach in a larger cohort of new-onset diabetic patients and compared the differences between KPT2D and NKPT2D patients.
Patients and Methods
Subjects
In the present study, data were analyzed from patients treated at the Department of Endocrinology of the first affiliated hospital of Wenzhou Medical University from January 2013 to December 2019. Diabetes was diagnosed according to the diagnostic criteria of the American Diabetes Association (2010).12 The inclusion criteria for the study were: 1. Newly diagnosed diabetes that had not been treated before, 2. Typical symptoms of diabetes for ≤ 6 months, 3. Positive for urine ketones at the time of diagnosis and blood ketones >0.5 mmol/L, 4. Negative for islet cell antibodies (ICAs), glutamate decarboxylase autoantibodies (GADs), and insulin autoantibodies (IAAs). The exclusion criteria were: 1. Patients with serious dysfunction of lung, liver, heart, or kidneys (AST or ALT≧3 times of the upper limit of normal; eGFR< 45 mL/min/1.73 m2), 2. Gestational and secondary diabetes attributed to taking agents known to affect carbohydrate metabolism, 3. Pancreatitis, pancreatic cancer or other endocrine diseases, 4. Obvious precipitating causes for the development of ketosis (such as stress, infection, prolonged fasting, alcohol ingestion, trauma or use of glucocorticoids), and 5. Type 1 diabetes, latent autoimmune diabetes in adults, maturity onset diabetes in the young, mitochondrial diabetes and monogenic diabetes.
A total of 1072 hospitalized patients were enrolled in the study. Diabetic ketosis was defined as the presence of moderate to heavy urine ketones (urine ketone body was positive) and blood ketones >0.5 mmol/L, as well as hyperglycemia.6,10 1072 cases were classified into two categories based on the presence or absence of diabetic ketosis. Amongst these cases, 410 patients were diagnosed with KPT2D and 662 patients were diagnosed with NKPT2D.The study was approved by the Ethics Committee of the first affiliated hospital of Wenzhou Medical University. And all the procedures performed in the present study involving human participants were accordance with the ethical standards of the institutional research committee. A waiver of informed consent was approved because of the retrospective nature of our study. All of the patients’ data are covered confidentiality in our hospital. This study was conducted in compliance with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Clinical Data Collection
All subjects received physical and biochemical examinations after admission to the hospital. Weight and height were measured using established protocols.13 The body mass index (BMI) was calculated as the weight in kilograms divided by the square of height in meters and divided into three categories: normal, <24 kg/m2, overweight, ≥ 24 to < 28 kg/m2; obesity, ≥ 28 kg/m2.14 Blood pressure was measured with an OMRON electronic sphygmomanometer. Hypertension was diagnosed by current treatment for hypertension, systolic blood pressure (SBP)≧140 mmHg or diastolic blood pressure (DBP)≧90 mmHg. All patients were interviewed to obtain their family history, alcohol consumption and smoking habits. Random blood glucose (RBG) was measured on the first hospital visit. Other venous blood samples were taken on the following morning after overnight fasting for at least 8 hours during hospitalization. Glycosylated hemoglobin A1C (HbA1c), creatinine (Cr), uric acid (UA), albumin, aspartate aminotransferase (AST), alanine aminotransferase (ALT), total triglycerides (TG), total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), and low-density lipoprotein cholesterol (LDL-C) levels were determined by standard laboratory methods. When the blood glucose levels of the patients had stabilized, fasting plasma glucose (FPG), fasting C-peptide (FCP), 2-h postprandial plasma glucose (2h-PPG) and 2-h post-prandial C-peptide (2h-C-P) levels were collected.
The estimated glomerular filtration rate (eGFR) was calculated using the CKD-EPI formula: a× (creatinine/b)c × (0.993)age in which the values of a, b and c are dependent on gender, race and creatinine.15 Homoeostasis model assessment was used to estimate basal β-cell function (HOMA-β) and insulin resistance (HOMA-IR).16 HOMA-β and HOMA-IR were calculated using the following formulas: HOMA-β= Fasting C-Peptide [pmol/L] × 0.27/(Fasting Plasma Glucose [mmol/L] −3.5) and HOMA-IR = 1.5 + Fasting Plasma Glucose [mmol/L]× Fasting C-Peptide [pmol/L] /2800.
The levels of islet-related autoantibodies including glutamic acid decarboxylase antibody (GADA), IA-2 (protein tyrosine phosphatase antibody) and islet cell antibody (ICA) were determined by ELISA. The levels of urine ketones were measured using the Rothera test and blood ketones were detected using a fast blood ketone meter (Abbott).
Ultrasonography Measurements
Ultrasound measurements were conducted by two experienced ultra-sonographers under a standardized protocol and the cases were reassessed by an independent senior expert. Color Doppler sonography was performed using a Hitachi Ultrasound Machine (MODEL:EZU-MT28-S1). All of the ultrasound assessors were blinded to the diabetes phenotype. The blood vessels measured included the bilateral carotid, vertebral, femoral, popliteal, anterior tibial, posterior tibial, and peroneal arteries. According to the Mannheim consensus,17 an atherosclerotic plaque was defined as a focal structure encroaching into the arterial lumen of 0.5 mm or 50% of the surrounding IMT value or demonstrating a thickness of≧ 1.5 mm as measured from the media–adventitia interface to the intimal interface. AS was defined as the presence of carotid or lower extremity arterial atherosclerotic plaques in any of the above-mentioned artery segments.
Statistical Analyses
The data were analyzed using SPSS 25.0 software. Normally distributed and continuous variables were presented as mean ± SD and were analyzed using a t-test. Data which were not normally distributed were presented as the median with an interquartile range and were analyzed using a Mann–Whitney test. Categorical variables were expressed as percentages and the frequencies of categorical variables were determined using a Chi-square test. A paired Chi-square test and conditional logistic regression were used to compare the rates and risks of atherosclerosis between the two subgroups after age matching of the subjects. Binary multivariate logistic regression was conducted to determine the risk factors for atherosclerotic plaque. Statistical significance was defined as a two-tailed P-value of <0.05.
Results
Characteristics and Anthropometric Features of the Subjects
1072 patients who were initially diagnosed with type 2 diabetes were enrolled in the study. The clinical characteristics of the subjects are summarized in Table 1. The mean age of the patients with KPT2D was significantly lower than the NKPT2D patients (38±13 vs 49±14, P<0.001), which was consistent with findings reported in a previous study.18 There was a strong male predominance in both groups but this phenomenon was more evident in the KPT2D group (82.4% vs 70.1%, P<0.001) in which the ratio of men to women was close to 5:1. KPT2D was found to be more closely associated with a family history of diabetes compared with NKPT2D (22.2% vs 17.4%, P=0.049). The heights and weights of patients with KPT2D were higher than patients with NKPT2D (168.8 ± 8.3cm vs 165.3 ± 9.4cm, 71.9 ± 15.2 kg vs 68.7 ± 13 kg, P<0.001). No significant difference in BMI was found between the two groups (25.0 ± 4.2 vs 24.9 ± 3.6 kg/m2, P=0.567).
 |
Table 1 The Characteristics and Anthropometric Features of the KPT2D and NKPT2D Patients in This Study |
Biochemical Analysis of the Subjects
The biochemical results of the patients in the two groups were summarized in Table 2. The levels of random blood glucose and HbA1c were higher in the KPT2D group compared to the NKPT2D group (P<0.001). The indices reflecting the function of islets including the levels of fasting C-peptide, 2 h C-peptide and HOMA-β were lower in the KPT2D group compared to the NKPT2D group (P<0.001). No significant difference was observed in the HOMA-IR between the two groups indicating that they had comparable insulin sensitivity. Blood pressure in the KPT2D group was lower compared to that in the NKPT2D group (P<0.05) and the prevalence of hypertension was also lower in the KPT2D group compared to the NKPT2D group (42.4% vs 57.3%, P<0.001). There were significant group differences in creatinine and eGFR (P<0.001). Also, the NKPT2D patients had higher levels of HDL- cholesterol than KPT2D patients (P<0.001) yet the levels of triglyceride, total cholesterol and LDL-cholesterol levels were not significantly different between the two groups. Liver function measured by the levels of AST and ALT were similar between the two groups.
 |
Table 2 Biochemical Parameters of the Subjects |
Comparison of Atherosclerosis Between the KPT2D and NKPT2D Groups
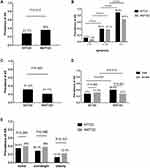
According to the diagnostic criteria, the prevalence of atherosclerosis detected by ultrasound examination was 27.7% in the KPT2D group and 36% in the NKPT2D group (P=0.012; Figure 1A). However, the onset age of the patients with KPT2D was significantly lower than the NKPT2D patients in the study. Bembi et al discovered that the prevalence of atherosclerosis was directly proportional to the age of the patients.18 Thus, we divided the patients into three age groups and it was shown that the prevalence of AS significantly increased with increasing age (P<0.05; Figure 1B). We speculated that age was a confounding factor due to the obvious differences in the age of onset between the KPT2D and NKPT2D groups. Multivariate logistic regression analysis of the variables in all subjects showed that the odds ratio of AS was higher in the KPT2D group compared to the NKPT2D group (OR: 1.789, 95% CI: 1.218–2.628; P=0.003) after adjusting for gender and age.
Furthermore, case control matching was performed to pair the patients of two groups according to their ages through SPSS. After matching the age and pairing, we finally got 261 age-paired patients in the study. It was interesting that the prevalence of AS in the KPT2D group was now significantly higher than that in the NKPT2D group amongst the age-paired subjects (31.4% vs 21.1%, P=0.005; Figure 1C). Conditional logistic regression showed that the odds ratio of AS was higher in the KPT2D group compared to the NKPT2D group (OR: 1.491, 95% CI: 1.059–2.098; P=0.022).
The rates of AS stratified by gender and BMI in each group are shown in Figure 1D~E. No difference in the prevalence of AS in the patients with KPT2D was observed between genders. However, in the NKPT2D group, the prevalence of AS was higher in males compared to females (39.9% vs 27%, P=0.003) (Figure 1D). All subjects were divided into three groups based on BMI. Among the three BMI groups, there was no difference in the prevalence of AS between KPT2D group and NKPT2D group whilst obese patients had a lower prevalence of AS compared to patients with a normal weight and overweight patients in the two groups (Figure 1E). We further compared the age of onset between the three groups and the data showed that the obesity group was younger than the overweight or normal weight groups (42 ± 14 vs 48 ± 13, 47 ± 14; P<0.001).
Analyses of Atherosclerosis Risk Factors in Each Diabetic Group
Binary logistic regression was performed to identify the risk factors independently associated with atherosclerosis after adjusting for variables (Table 3). The independent variables were significantly different between the groups or accepted to influence the occurrence of atherosclerosis. In the KPT2D group, the age of onset (OR:1.146, 95% CI:1.101–1.193; P<0.001), gender (OR:0.209, 95% CI:0.072 −0.608; P=0.004), and 2-h postprandial plasma glucose (OR:1.09, 95% CI:1.003–1.185; P=0.043) were significantly associated with AS. The age of onset (OR:1.146, 95% CI:1.11–1.184; P<0.001), gender (OR:0.208, 95% CI:0.116–0.373; P<0.001), and eGFR (OR:1.02, 95% CI:1.002–1.039; P=0.032) were found to be independent risk factors for atherosclerosis in NKPT2D group. Meanwhile, binary logistic regression analysis in all subjects showed that the age of onset (OR:1.141, 95% CI:1.114–1.168; P<0.001), gender (OR:0.228, 95% CI:0.147 −0.353; P<0.001) and the KPT2D group (OR:1.685, 95% CI:1.131–2.509; P=0.01) were significantly associated with AS.
 |
Table 3 Results of Binary Logistic Regression Analysis of the Risk Factors for Atherosclerosis |
Discussion
Recently, an increasing number of studies have reported a special type of diabetes that is diagnosed with ketosis or ketoacidosis and reversible β-cell impairment but is not associated with islet autoimmune markers. In the current study, we compared the clinical characteristics and prevalence of atherosclerosis between the patients with KPT2D and NKPT2D. KPT2D was shown to have a lower age of onset compared to NKPT2D, which was consistent with data reported in a previous study.19 Also, we found a higher predominance of male patients in the KPT2D group that was close to the rates reported by Ye et al.20 However, the underlying pathogenesis of this phenomenon is not yet clear. Body fat distribution, insulin sensitivity and sex hormones might interact to cause the syndrome.21,22 In keeping with previous reports, our results showed that a family history of diabetes was more strongly associated with KPT2D than NKPT2D.
In this study, indicators related to elevated glucose levels including FBG, 2h-PPG and HbA1c in KPT2D were higher compared to the KPT2D suggesting that there were more serious metabolic disturbances in KPT2D. Also, the KPT2D patients had lower levels of fasting C-peptide, 2h-C-peptide and insulin evaluated by the HOMA-β compared to the NKPT2D patients. However, no significant difference was observed in HOMA-IR or lipid levels except for HDL-C between the two groups. These results are consistent with other reports.23 Although patients in both groups had similar insulin sensitivity, their insulin secretion was lower in the KPT2D group suggesting that impairment of β-cell function played an important role in the onset of KPT2D that might be caused by glucotoxicity and glucolipotoxicity.20,24
AS is one of the most common macrovascular complications of diabetes that can increase the risk of cardiovascular events such as myocardial infarction, stroke, and death. Insulin resistance and metabolic changes in diabetes accelerate the development of AS,25 highlighting the importance of the early detection of diabetes in the clinic. Previous studies have demonstrated that the prevalence of carotid atherosclerosis or lower limb atherosclerotic lesions in KPT2D are comparable to NKPT2D.10,11
In most hospitalized diabetic patients, carotid and lower extremity ultrasound examination are used to evaluate the condition of macroangiopathy. However, previous studies are mostly based on imaging of a single area that may underestimate the seriousness of AS. In this study, we combined two examinations to more accurately estimate the characteristics of atherosclerosis in each group. AS is a progressive disease that begins before or around the onset of one or more known risk factors and often precedes hyperglycemia indicating that the development of AS begins before the clinical diagnosis of diabetes. Hence, the new-onset diabetes patients whose duration of diabetes was less than half a year were selected in our study.
Baseline data showed that the prevalence of atherosclerosis in the KPT2D group was lower than in the NKPT2D group. In this study, we found that the age of onset for KPT2D was significantly lower than for NKPT2D which may contribute to this phenomenon. It is well established that AS is closely associated with age. So, we divided the patients into three different age groups and found that the prevalence of AS increased with increasing age. Further, data showed that there was a positive correlation between KPT2D and AS after adjusting for age and gender. After age matching the patients in the two groups, we found that the prevalence of atherosclerosis in the KPT2D group was higher than in the NKPT2D group. These data are inconsistent with previous cross-sectional studies10,11 and may be due to more severe metabolic disorder in the KPT2D group than the NKPT2D that could accelerate the development of AS. Also, it has been reported that diabetic ketosis is associated with an increased incidence of vascular disease.26 Another possible explanation is that ketosis plays an important role in the development of AS.
Although carotid and lower extremity ultrasonography were combined in our study, the rates of atherosclerosis in both groups were lower than carotid or lower atherosclerosis in previous studies. This phenomenon may be due to the duration of new-onset diabetes in our study being less than 6 months. In agreement with a previous study,9 the NKPT2D group had a higher prevalence of atherosclerosis in males compared to females. However, our results displayed no gender difference in the prevalence of atherosclerosis in the KPT2D group. The difference in the prevalence of AS between men and women in the two groups may be attributed to the higher predominance of males in the KPT2D group compared to the NKPT2D group. Among the three BMI groups, the rates of AS in the KPT2D group and NKPT2D group were comparable. Interestingly, obese patients had a lower prevalence of AS compared to normal weight and overweight patients in the two groups. These observations may be due to obese patients being younger than overweight or normal weight patients in our study.
Finally, we explored the risk factors of AS in the two groups by multivariate logistic regression. Our results showed that age and gender were independent predictors of AS in KPT2D and NKPT2D patients. The 2h-PPG and eGFR were independent risk factors for KPT2D and NKPT2D, respectively. A possible explanation is that patients with chronic renal insufficiency always have dyslipidemia and develop arteriosclerosis during the early stages of NKPT2D, whilst blood glucose levels play a more important role in the development of AS due to acute metabolic disorder in KPT2D patients. These results showed that the risk factors for atherosclerosis in the two diabetic groups were not identical.
Whilst our study provides robust data, it has several limitations particularly as it was performed retrospectively. Prospective studies are needed to assess the long-term effects of diabetic ketosis on atherosclerosis. Also, our study was performed at a single-center study and so validation is required through larger multi-center studies to further clarify the atherosclerotic features of KPT2D.The subjects in our study usually had severe hyperglycemia which requires hospitalization for intensive treatment. Our conclusions may fail to apply outpatients with mild to moderate hyperglycemia.
Conclusions
In this study, we showed that KPT2D patients are younger and have a more obvious male predominance compared to NKPT2D patients. However, pancreatic islet function is reduced in KPT2D patients compared to NKPT2D patients in the acute phase. Also, AS is more prevalent in KPT2D patients than in NKPT2D patients after adjusting for age. The KPT2D group was an independent predictor of AS in non-autoimmune diabetes. The current study provides reference points to distinguish KPT2D from NKPT2D and is beneficial in clinical diagnosis and treatment.
Acknowledgments
We would like to thank all of the subjects for participating in this study. The investigators are grateful to the dedicated participants and all of the research staff involved in the study particularly Dr. Wenyue Liu for providing guidance on statistical analysis. This study was supported by projects of Wenzhou Science and Technology Bureau (grant No.Y20190129 and Y2020263).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Lebovitz HE, Banerji MA. Ketosis-prone diabetes (flatbush diabetes): an emerging worldwide clinically important entity. Curr Diab Rep. 2018;18(11):120. doi:10.1007/s11892-018-1075-4
2. Gupta RD, Ramachandran R, Gangadhara P, et al. Clinical characteristics, beta-cell dysfunction and treatment outcomes in patients with A − β + ketosis-prone diabetes (KPD): the first identified cohort amongst Asian Indians. J Diabetes Complications. 2017;31(9):1401–1407. doi:10.1016/j.jdiacomp.2017.06.008
3. Boyne MS. Arginine metabolism and A-β + ketosis-prone diabetes. J Nutr. 2018;148(2):170–171. doi:10.1093/jn/nxx066
4. Choukem SP, Sobngwi E, Boudou P, et al. β- and α-cell dysfunctions in Africans with ketosis-prone atypical diabetes during near-normoglycemic remission. Diabetes Care. 2013;36(1):118–123. doi:10.2337/dc12-0798
5. Winter WE, Maclaren NK, Riley WJ, Clarke DW, Kappy MS, Spillar RP. Maturity-onset diabetes of youth in black Americans. N Engl J Med. 1987;316(6):285–291. doi:10.1056/NEJM198702053160601
6. Lu H, Hu F, Zeng Y, et al. Ketosis onset type 2 diabetes had better Islet β -cell function and more serious insulin resistance. J Diabetes Res. 2014;2014:1–6. doi:10.1155/2014/510643
7. World Health Organization. Classification of diabetes mellitus. World Health Organization; 2019. Available from: https://apps.who.int/iris/handle/10665/325182.
8. Tabák AG, Herder C, Rathmann W, Brunner EJ, Kivimäki M. Prediabetes: a high-risk state for diabetes development. Lancet. 2012;379(9833):2279–2290. doi:10.1016/S0140-6736(12)60283-9
9. Li L, Yu H, Zhu J, et al. The combination of carotid and lower extremity ultrasonography increases the detection of atherosclerosis in type 2 diabetes patients. J Diabetes Complications. 2012;26(1):23–28. doi:10.1016/j.jdiacomp.2011.11.006
10. Li LX, Zhao CC, Ren Y, et al. Prevalence and clinical characteristics of carotid atherosclerosis in newly diagnosed patients with ketosis-onset diabetes: a cross-sectional study. Cardiovasc Diabetol. 2013;12(1):18. doi:10.1186/1475-2840-12-18
11. Li MF, Ren Y, Zhao CC, et al. Prevalence and clinical characteristics of lower limb atherosclerotic lesions in newly diagnosed patients with ketosis-onset diabetes: a cross-sectional study. DiabetolMetabSyndr. 2014;6:71. doi:10.1186/1758-5996-6-71
12. American Diabetes Association. 2. Classification and diagnosis of diabetes: standards of medical care in diabetes-2020. Diabetes Care. 2020;43(Suppl 1):S14–S31. doi:10.2337/dc20-S002
13. Lu B, Yang Y, Song X, et al. An evaluation of the International Diabetes Federation definition of metabolic syndrome in Chinese patients older than 30 years and diagnosed with type 2 diabetes mellitus. Metabolism. 2006;55(8):1088–1096. doi:10.1016/j.metabol.2006.04.003
14. Yin X, Yang X, Zhang T, et al. Changes of body mass index and body shape in relation to risk of gastric cancer: a population-based case-control study. J Cancer. 2021;12(10):3089–3097. doi:10.7150/jca.56149
15. Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–612. doi:10.7326/0003-4819-150-9-200905050-00006
16. Fan H, Pan Q, Zhang P, Liu J, Xu Y, Yang X. Influence of islet function on typing and prognosis of new-onset diabetes after intensive insulin therapy. Med Sci Monit. 2013;19:787–793. doi:10.12659/MSM.889099
17. Touboul PJ, Hennerici MG, Meairs S, et al. Mannheim intima-media thickness consensus. Cerebrovasc Dis. 2004;18(4):346–349. doi:10.1159/000081812
18. Bembi V, Singh S, Singh P, Aneja GK, Arya TVS, Arora R. Prevalence of peripheral arterial disease in a cohort of diabetic patients. South Med J. 2006;99(6):564–569. doi:10.1097/01.smj.0000221624.68378.5d
19. Li TT, Wang AP, Lu JX, et al. Prevalence and clinical characteristics of non-alcoholic fatty liver disease in newly diagnosed patients with ketosis-onset diabetes. Diabetes Metab. 2018;44(5):437–443. doi:10.1016/j.diabet.2018.03.002
20. Ye S, Ran H, Zhang H, et al. Elevated serum triglycerides are associated with ketosis-prone type 2 diabetes in young individuals. Diabetes Metab Syndr Obes. 2021;14:497–504. doi:10.2147/DMSO.S296085
21. Blouin K, Boivin A, Tchernof A. Androgens and body fat distribution. J Steroid Biochem Mol Biol. 2008;108(3–5):272–280. doi:10.1016/j.jsbmb.2007.09.001
22. van Nas A, Guhathakurta D, Wang SS, et al. Elucidating the role of gonadal hormones in sexually dimorphic gene coexpression networks. Endocrinology. 2009;150(3):1235–1249. doi:10.1210/en.2008-0563
23. Zhang M, Li Y, Cui W, et al. The clinical and metabolic characteristics of young-onset ketosis-prone type 2 diabetes in China. Endocr Pract. 2015;21(12):1364–1371. doi:10.4158/EP15778.OR
24. Umpierrez GE, Smiley D, Gosmanov A, Thomason D. Ketosis-prone type 2 diabetes: effect of hyperglycemia on β-cell function and skeletal muscle insulin signaling. Endocr Pract. 2007;13(3):283–290. doi:10.4158/EP.13.3.283
25. Haas AV, McDonnell ME. Pathogenesis of cardiovascular disease in diabetes. Endocrinol Metab Clin North Am. 2018;47(1):51–63. doi:10.1016/j.ecl.2017.10.010
26. Jain SK, McVie R, Jaramillo JJ, Chen Y. Hyperketonemia (acetoacetate) increases the oxidizability of LDL + VLDL in type-I diabetic patients. Free Radic Biol Med. 1998;24(1):175–181. doi:10.1016/s0891-5849(97)00213-x
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.