Back to Journals » Journal of Blood Medicine » Volume 14
A Critical Review of Sickle Cell Disease Burden and Challenges in Sub-Saharan Africa
Authors Adigwe OP , Onoja SO , Onavbavba G
Received 28 January 2023
Accepted for publication 21 April 2023
Published 31 May 2023 Volume 2023:14 Pages 367—376
DOI https://doi.org/10.2147/JBM.S406196
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Martin H Bluth
Obi Peter Adigwe,1 Solomon Oloche Onoja,2 Godspower Onavbavba1
1Office of the Director General, National Institute for Pharmaceutical Research and Development, Abuja, Federal Capital Territory, Nigeria; 2Department of Medical Laboratory Sciences, University of Nigeria, Enugu, Nigeria
Correspondence: Obi Peter Adigwe, Office of the Director General, National Institute for Pharmaceutical Research and Development, Abuja, Federal Capital Territory, Nigeria, Email [email protected]
Abstract: Sickle cell disease is caused by an abnormality of the β-globin gene and is characterised by sickling of the red blood cells. Globally, sub-Saharan African countries share the highest burden of the disease. This study aimed at critically reviewing studies focusing on challenges of sickle cell anaemia in sub-Saharan Africa. A literature search was carried out in five major databases. Articles that met the inclusion criteria were included in the bibliometric review and critical analysis. A majority of the studies were undertaken in the West African region (85.5%), followed by Central Africa (9.1%). Very few studies had been undertaken in East Africa (3.6%), whilst the Southern African region had the fewest studies (1.8%). Distribution in relation to country revealed that three quarters of the studies were carried out in Nigeria (74.5%), followed by the Democratic Republic of the Congo (9.1%). According to healthcare settings, a strong majority of the studies were undertaken in tertiary health care facilities (92.7%). Major themes that emerged from the review include interventions, cost of treatment, and knowledge about sickle cell disease. Public health awareness and promotion as well as improving the quality of sickle cell centers for prompt management of patients with sickle cell disorder was identified as a critical strategy towards reducing the burden of the disease in sub-Saharan Africa. To achieve this, governments in countries located in this region need to adopt a proactive strategy in addressing gaps that have been identified in this study, as well as instituting other relevant measures, such as continuous media engagement and public health interventions relating to genetic counselling. Reforms in other areas that can help reduce the disease burden, include training of practitioners and equipping sickle cell disease treatment centers according to World Health Organization specifications.
Keywords: sickle cell anaemia, interventions, haemoglobin, genotype, genetics
Introduction
Sickle cell disease is an inherited autosomal recessive disorder of the β-globin gene characterised by clinical manifestations such as haemolytic anaemia and recurrent episodes of vascular occlusion.1 Sickle cell disease refers to a group of inherited red blood cell disorders, with sickle cell anaemia indicated as one of the disorders. Disease presentation and progression in patients with sickle cell anaemia is highly variable. Individual differences in clinical presentations are also believed to be dependent on environment, the extent of sickling, vascular endothelium, platelets, leucocytes, and plasma proteins.2
Although sickle cell disease occurs worldwide, sub-Saharan Africa is the region with the highest prevalence.3 The disease is due to a mutation in the β-globin gene at chromosome 11 within the 17th nucleotide, where adenine replaces thymine. Consequently, glutamic acid is replaced by valine during translation, leading to the formation of abnormal haemoglobin. The abnormal haemoglobin formed is insoluble and polymerises with reduced oxygen tension, trauma, stress, dehydration, acidosis, or cold environments.4 Polymerised haemoglobin produces rigid, less flexible, and fragile erythrocytes with a reduced life span. These changes lead to various acute and chronic complications.5 Clinical presentations vary among sickle cell patients. Some include leg ulcers, priapism, fatigue, dizziness, osteonecrosis, bacteraemia, and dactylitis. Others are renal disease, pulmonary hypertension, acute chest syndrome, and end-organ damage.2
Sickle cell disorder has been acknowledged to have a global impact by the World Health Organization (WHO), with remarkable public health implications for Africa.6 Available evidence suggests 4.4 million people have sickle cell disease worldwide, whilst about 43 million are living with sickle cell trait.7 About 80% of sickle cell disease cases occur in sub-Saharan Africa,8 and the mortality rate for children <5 years of age ranges from 50% to 80%.3 The high burden of the disease in this setting is further exacerbated due to lack of access to comprehensive healthcare in the region.9
Africa has been associated with the highest prevalence of the sickle cell trait, with figures suggesting that between 10% and 40% of the entire population may be affected.10 The incidence of sickle cell trait ranges from 20% to 30% in Cameroon, the Democratic Republic of the Congo, Gabon, Ghana, and Nigeria. A high prevalence of 45% has been reported in some parts of Uganda.11 Available evidence suggests that about 90% of the world’s sickle cell disease population lives in Nigeria, India, and the Democratic Republic of the Congo, where the disease affects up to 2% of the population.12 Evidence suggests Nigeria has the largest population of persons affected with sickle cell disease globally.13,14
Given the serious burden of sickle cell disease in sub-Saharan Africa, alongside inadequate measures to manage related crises arising from the condition, there is surprisingly little evidence in the extant literature that comprehensively critiques emergent issues associated with the condition. This study therefore aimed at critically reviewing studies that focused on challenges of sickle cell disease in sub-Saharan Africa.
Methods
We adopted a bibliometric review approach, with a critical analysis of the selected articles. This was done to enable a better understanding of the study area, which can then underpin further research, as well as guide policymakers in sub-Saharan Africa in developing new strategies to improve responses to sickle cell disease in the region. The review of the literature covered all full publications that appeared in English-language biomedical journals between 2003 and 2021. The search strategy included a combination of keywords: ‘sickle cell disease’, ‘burden, ‘interventions’, ‘challenges’, and ‘sub-Saharan Africa’. The literature search was carried out systematically using the PubMed, Scopus, Science Direct, and Google Scholar databases. Keywords and phrases were used to focus the search and to identify relevant articles. Following the search, titles and abstracts were reviewed and those articles that met the inclusion criteria were included in the review. The retrieved documents were examined to identify relevant articles. Articles considered were those that focused on sickle cell disease challenges, management, and interventions in sub-Saharan Africa. Only articles reporting primary and original research were included. Duplicates from various databases were eliminated. Data extracted from the selected articles were entered in Microsoft Excel 2016 for statistical analysis. The variables registered for each article were author, year of publication, journal title, design, and area of study. Others were healthcare setting, geographical region, sample and population of study, as well as outcomes. Descriptive statistical analysis was then undertaken, and results were summarised in figures.
Results
This bibliometric analysis and critical review of selected articles was targeted at better understanding relevant interventions, challenges of various intervention protocols, and identifying prospects for overcoming the burden of sickle cell disease in sub-Saharan Africa. The initial database search identified 1751 articles, published from 2003 to 2021. Following the elimination of duplicates as well as the application of the relevant screening and eligibility processes, a total of 55 articles were selected for critical review. Relevant details relating to the search, screening, and selection processes are presented in Figure 1.
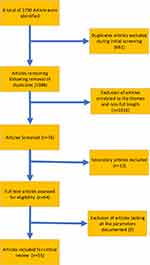 |
Figure 1 Review flow diagram. |
Bibliometry
Findings from this study revealed that investigations relating to sickle cell disease challenges and interventions had been undertaken in different healthcare settings. Various settings that emerged are presented in Figure 2. A majority of the studies (92.6%) were undertaken in tertiary healthcare facilities. Figure 2 reveals that there was a significant disparity between studies undertaken in tertiary facilities and other healthcare settings. Figure 3 gives an overview of the geographical spread of studies on sickle cell disease. Findings showed that studies undertaken in the West African region (85.5%) dominated this category. This was followed by the Central African region (9.1%). It emerged that a majority of the studies were undertaken in West Africa, and few studies had been undertaken in other parts of sub-Saharan Africa.
 |
Figure 2 Distribution of studies based on healthcare settings. |
 |
Figure 3 Geographic spread of studies. |
Figure 4 provides details of the various articles included in the review according to country. It was observed that three quarters of the studies were undertaken in Nigeria (74.5%). The studies were further categorised according to year of publication. Those that were included in the review were published between 2003 and 2021. Findings in Figure 5 shows that more studies were undertaken in 2020 (18.2%), and this was closely followed by 2019 (16.4%). Collectively, more than two thirds of the studies (69.1%) were carried out between 2017 and 2021, whilst less than a third were undertaken in 2003–2016. This shows an increase in research activity in this area in recent times.
 |
Figure 4 Publications by countries. |
 |
Figure 5 Distribution of studies according to year of publication. |
Major Themes That Emerged
Therapeutic Interventions
Various articles explored therapeutic interventions for sickle cell disease in sub-Saharan Africa. A number of the articles centred on the potency and efficacy of hydroxyurea in the management of the condition in both children and adults. For instance, Ofakunrin et al15 studied the effectiveness and safety of hydroxyurea in the treatment of sickle cell anaemia among 54 children aged 4–17 years in Nigeria for 12 months, and they noted from their findings that the number of patients who had had more than two episodes of painful crises reduced drastically, whilst those with acute chest syndrome reduced to 0. Another study on hydroxyurea among 60 adults over 3 months also showed that the medicine has significant effect in ameliorating sickle cell disease.16 Hydroxyurea is currently approved for the management of sickle cell anaemia and it has contributed towards reducing the burden of the disease globally.17 A recent study demonstrated its safety and efficacy in children of 6–9 months of age.18 Another study also demonstrated the effectiveness of moderate fixed-dose hydroxyurea in primary stroke prevention, whilst another reported a decrease in the incidence of malaria episodes following hydroxyurea therapy.19 Furthermore, it was observed that the drug has robust efficacy and safety in the treatment of children with sickle cell anemia.20 However, gaps identified in this area included short-term follow-up, inappropriate use of the intervention, and small samples.
Niprisan, which is now marketed as Niclovix, is a phytomedicine that has also been proven in recent times as a therapeutic intervention for sickle cell disease.21,22 It is an anti-sickling medicine extracted from local herbs in Nigeria. Niprisan was developed by the National Institute for Pharmaceutical Research and Development for the management of patients with sickle cell disease.23 George et al evaluated the role of Niprisan among 115 children with sickle cell disease in Nigeria,22 and outcomes indicated a considerable reduction in hospital admissions from 47 (40.2%) to 13 (11.3%). It was observed that Niprisan was effective in the management of sickle cell disease. Oniyangi and Cohall,24 also reported safety and efficacy of the phytomedicinal intervention in their study.
A survey on the effect of a comprehensive clinical care programme on severely ill patients suggested marked reduction in the frequency and severity of sickle cell-related acute events, with improvement in general status and physical growth among 184 patients (78%).25 Taking into account the social, cultural, and economic backgrounds of sub-Saharan Africa, provision of information and education about the disease to affected persons has proven to be effective in reducing challenges associated with the condition.26
Challenges associated with suboptimal management had been reported. In their study on acute pain management in children with sickle cell anaemia during emergency admission, Oshikoya et al27 highlighted inadequate management of pain with analgesics. The study also identified that pain management among children was not in conformity with the guidelines of the World Health Organization.
A study undertaken in the Democratic Republic of the Congo on the management of sickle cell disease also highlighted insufficient standard-care practices for patients in Kisangani.28 Findings from this study indicated that comprehensive management of sickle cell disease, including specific interventions, such as neonatal screening, early diagnosis, preventive penicillin therapy, pneumococcal and haemophiliac influenza vaccination, and malaria prophylaxis, were all lacking. Mukinayi et al29 highlighted poor diagnostic and treatment options as factors contributing to increased morbidity and mortality for sickle cell anaemia patients in the Democratic Republic of the Congo. Galadanci et al14 reported similar findings in their study on current sickle cell disease management practices in Nigeria.
Knowledge About Sickle Cell Disease
Various studies in this review identified knowledge deficits about the haemoglobin genotype, premarital genetic counseling, and screening in sub-Saharan Africa. These gaps seemed to have contributed to the prevalence of the condition. A cross-sectional study by Obed et al30 revealed knowledge deficits about sickle cell disease among Ghanaian pregnant women, and a limited understanding of sickle cell disease among university students, particularly on the pattern of inheritance, was reported by Boadu and Addoah.31 Similar findings were reported from studies among Kisangani University students,32 families directly affected by sickle cell disease in Mbujimayi,33 and among healthcare providers in the city of Kindu, all in the Democratic Republic of the Congo.28,34 Poor knowledge about sickle cell disease was identified amongst unmarried youths in Cameroon.35 In Nigeria, poor knowledge about sickle cell disease has also been reported among secondary school students,36,37 undergraduates,1,38,39 local government workers,40 and childbearing-age adult women.41–43 Level of education has been reported as a determinant of good knowledge about sickle cell disease.44,45
A cross-sectional survey by Rasheed et al46 on married and unmarried youths in Nigeria revealed that the level of knowledge of participants was good (70.06%); however, participants had negative attitudes towards the practice of premarital sickle cell genetic screening. The findings from that study were attributed to inadequate public health awareness and lack of basic knowledge on the haemoglobin genotype and sickle cell disease. Similar findings were reported among some secondary school students in Nigeria,47–49 as well as tertiary institutions.50–52
On the contrary, several studies revealed adequate knowledge of sickle cell disease, genetic counselling, and haemoglobin genotyping with positive preventive practices and attitudes towards the condition. Significant knowledge of the causes, signs, and symptoms of sickle cell disease with positive preventive practices towards the condition was reported among students of tertiary institutions53–55 and youths in Yaba, Nigeria.56,57 Oluwole and Adeyemo58 reported high levels of awareness, good knowledge, and positive attitudes towards sickle cell disease. A majority of respondents in that study (84.9%) were also willing to screen their newborns for sickle cell disease. Good knowledge and positive attitudes were observed among religious and traditional leaders in Nigeria.59 In Ghana, studies among women60 and young unmarried adults revealed positive attitudes towards genetic testing and counselling and practices towards sickle cell disease.61 Similar findings were reported in Zambia.62
Cost of Intervention
Public perceptions relating to the cost of management of sickle cell disease have also been explored in the extant literature. In a study that explored factors relating to the financial burden of sickle cell disease in Nigeria, the findings showed that few participants (7.2%) were enrolled in the National Health Insurance Scheme, with out-of-pocket payments on the increase.63 Monthly household income ranged from ₦12,500 (US$27.15) to ₦330,000 ($716.64), whilst health expenditure was between ₦2500 ($5.43) and ₦215,000 ($466.90). The high financial burden exposed participants to financial hardship due to expenses and loans incurred in order to offset hospital bills and other relevant healthcare costs. Health insurance was however reported as an invaluable tool that can help ameliorate financial burden associated with the management of sickle cell anaemia.63 Another study explored the cost of treatment in Kenya, and the high cost of treatment was also reported.64 High cost of diagnosis was similarly identified as a factor limiting prenatal screening for sickle cell disease.65,66
Discussion
Various studies relating to the challenges and burden of sickle cell disease have been undertaken in sub-Saharan Africa. A majority of the studies carried out were in tertiary health care centres. Whilst it is understandable that tertiary health care facilities seem to be a familiar setting for such studies, this finding reveals a paucity of information from other settings. Ordinarily, primary healthcare centres should serve as the first point of contact when seeking medical intervention.66 It appears, however, that primary and secondary health facilities have not received the needed attention as it relates to challenges of sickle cell disease. One reason for this disparity could be attributed to the nature of tertiary health facilities, which combine both healthcare delivery and academic activities.
Findings from this study also revealed disparity between the West African region, where a significant proportion of the studies were carried out, compared to the much smaller proportions from other regions in sub-Saharan Africa. The high proportion of studies from the West African region could be associated with the high burden of sickle cell disease in the region.3 In the Central African region, only the Democratic Republic of the Congo had considerable research activity relating to this subject area, whilst only Kenya and Sudan had undertaken such studies in the East African region. Furthermore, the classification of the articles according to country revealed that three-quarters of the studies were undertaken in Nigeria, and this may be perhaps due to the high prevalence of sickle cell disease in the country compared to other nations.13,14 The findings of this study not only provide a basis for further studies that can address the knowledge gaps but they also clearly reveal the urgent need for a review of research and development strategies that can better contribute to the control and eradication of the disease.
Sickle cell disease remains a public health challenge in sub-Saharan Africa.67 Findings from this study suggest that various intervention efforts such as the use of hydroxyurea in managing the condition as well as the development of Niprisan have yielded significant result in reducing the burden of the disease. Hydroxyurea changes the complex pathophysiology of sickle cell anaemia through such mechanisms as improvement of cellular hydration, induction of fetal haemoglobin production, reduction of leucocyte and platelet counts, and production of nitric oxide. Clinically, this translates to reduced frequency and intensity of sickle cell crisis, hospitalisations, and blood transfusions, with holistic improvement in the quality of life of sickle cell patients.15,16,68 However, inappropriate use of hydroxyurea was observed in this review. This can consequently affect clinical outcomes despite its robust benefits and proven efficacy in the management of sickle cell disease. There is a need for training and retraining of healthcare professionals on the use and safety of hydroxyurea, Niprisan and other relevant products for the management of sickle cell disease so as to improve clinical outcomes. Findings from this study also highlight the need for the prioritisation of phytomedicine development in sub-Saharan Africa, as Niprisan, which was developed from four plants, was found to be efficacious in the management of the disease.21–24,69
This study further revealed that interventions with respect to timely diagnosis were inadequate. This suggests the need to scale up point-of-care testing as well as infant screening for early identification and management of individuals affected with the disorder so as to prevent unnecessary loss of lives.70 Other factors that contribute to the high prevalence of sickle cell anaemia in sub-Saharan Africa include poor knowledge about the condition. Therefore, developing capacity in public health awareness, genetic counselling, and proper premarital education can serve as vital means of reducing the prevalence of the disease.71 Keeping citizens adequately informed about the condition is key to reducing the burden of the disease in sub-Saharan Africa. In addition, the inclusion of topics relating to sickle cell disease in educational curricula, especially at an early stage, can help improve awareness about the condition at a young age. Improved strategic collaboration between the ministries of health and education can play a critical role in addressing some of these concerns. Improving awareness at the grassroots level can be amplified and expedited through community-based structures such as cultural and religious groups.
Findings from this study suggest the management of sickle cell disease is associated with high financial burden and the payment for healthcare services were mainly out-of-pocket spending. Based on these findings, there is a need for governments in countries with high prevalence of the disease to come up with a robust and comprehensive policy that can alleviate the high cost of care associated with managing the condition. Such an approach can help improve the quality of health for patients with sickle cell disorder.64,65
Conclusion
This study adopted a novel critical review strategy to appraise the body of work undertaken in sickle cell disease over approximately two decades. Findings highlighted various challenges in the current management of the disease, as well as the importance of increasing efforts towards public enlightenment about the disease so as to increase awareness.
The gaps from the holistic review also underscore the need for countries across sub-Saharan Africa to equip treatment centers with adequate facilities as well as to regularly train healthcare personnel with respect to the latest techniques for prevention, management and treatment of the disease. The need for adherence to standard sickle cell disease management protocol at all levels also emerged as an important strategy to reduce the burden associated with the disease. Despite the criticality of primary health centres in healthcare provision, several of these facilities across sub-Saharan African countries seemed underutilised, suggesting necessary reforms, revitalisation, and adequate equipping to enhance their utility in sickle cell prevention and care.
Given the high prevalence of sickle cell disease in sub-Saharan Africa, there is an urgent need for governments and relevant stakeholders in this region to prioritise strategies that can underpin effective management of the disease. In addition to managing the disease, novel findings that emerged due to the unique methodology adopted can enable public health synergies that will reduce the burden whilst targeting total elimination. Genetic counselling, premarital genotype screening, and comprehensive health insurance coverage for all sickle cell disease patients are some key elements in strategic reforms that can improve access to care whilst also contributing to an overarching reduction of the disease burden.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Ugwu NI. Sickle cell disease: awareness, knowledge and attitude among undergraduate students of a Nigerian tertiary educational institution. Asian J Med Sci. 2016;7(5):87–92. doi:10.3126/ajms.v7i5.15044
2. Onoja SO, Eluke BC, Dangana A, Musa S, Abdullah IN. Evaluation of von Willebrand factor and other coagulation homeostasis profile of patients with sickle cell anemia attending a tertiary hospital at Enugu, Nigeria. Med J Zambia. 2020;47(4):269–275. doi:10.55320/mjz.47.4.715
3. Aliyu ZY, Kato GJ, Taylor J, et al. Sickle cell disease and pulmonary hypertension in Africa: a global perspective and review of epidemiology, pathophysiology, and management. Am J Hematol. 2008;83(1):63–70. doi:10.1002/ajh.21057
4. De Franceschi L, Cappellini MD, Olivieri O. Thrombosis and sickle cell disease. InSeminars in thrombosis and hemostasis. Thieme Med Pub. 2011;37(3):226–236. doi:10.1055/s-0031-1273087
5. Malowany JI, Butany J. Pathology of sickle cell disease. Semin Diagn Pathol. 2012;29(1):49–55. doi:10.1053/j.semdp.2011.07.005
6. Hsu L, Nnodu OE, Brown BJ, et al. White paper: pathways to progress in newborn screening for sickle cell disease in Sub-Saharan Africa. J Trop Dis Public Health. 2018;6(2):260. doi:10.4172/2329-891X.1000260
7. Global Burden of Disease Study. Collaborators (2015). Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013”. Lancet. 2013;386(9995):743–800. doi:10.1016/s0140-6736(15)60692-4
8. Rees DC, Williams TN, Gladwin MT. Sickle-cell disease. Lancet. 2010;376(9757):2018–2031. doi:10.1016/s0140-6736(10)61029-x
9. Piel FB, Patil AP, Howes RE, et al. Global epidemiology of sickle haemoglobin in neonates: a contemporary geostatistical model-based map and population estimates. Lancet. 2013;381(9861):142–151. doi:10.1016/S0140-6736(12)61229-X
10. Agasa B, Bosunga K, Opara A, et al. Prevalence of sickle cell disease in a northeastern region of the Democratic Republic of Congo: what impact on transfusion policy? Transfus Med. 2010;20(1):62–65. doi:10.1111/j.1365-3148.2009.00943.x
11. Afolayan JA, Jolayemi FT. Parental attitude to children with sickle cell disease in selected health facilities in Irepodun Local Government, Kwara State, Nigeria. Stud Ethno Med. 2011;5(1):33–40. doi:10.1080/09735070.2011.11886389
12. Kadima BT, Gini Ehungu JL, Ngiyulu RM, Ekulu PM, Aloni MN. High rate of sickle cell anaemia in S ub‐S aharan A frica underlines the need to screen all children with severe anaemia for the disease. Acta Paediatr. 2015;104(12):1269–1273. doi:10.1111/apa.13040
13. Fleming AF, Storey J, Molineaux L, Iroko EA, Attai ED. Abnormal haemoglobins in the Sudan savanna of Nigeria: i. Prevalence of haemoglobins and relationships between sickle cell trait, malaria and survival. Ann Trop Med Parasitol. 1979;73(2):161–172. doi:10.1080/00034983.1979.11687243
14. Galadanci N, Wudil BJ, Balogun TM, et al. Current sickle cell disease management practices in Nigeria. Int Health. 2014;6(1):23–28. doi:10.1093/inthealth/iht022
15. Ofakunrin AO, Oguche S, Adekola K, et al. Effectiveness and safety of hydroxyurea in the treatment of sickle cell anaemia children in Jos, North Central Nigeria. J Trop Pediatr. 2020;66(3):290–298. doi:10.1093/tropej/fmz070
16. Adewoyin AS, Oghuvwu OS, Awodu OA. Hydroxyurea therapy in adult Nigerian sickle cell disease: a monocentric survey on pattern of use, clinical effects and patient’s compliance. Afr Health Sci. 2017;17(1):255–261. doi:10.4314/ahs.v17i1.31
17. Halsey C, Roberts IA. The role of hydroxyurea in sickle cell disease. Br J Haematol. 2003;120(2):177–186. doi:10.1046/j.1365-2141.2003.03849.x
18. Thornburg CD, Files BA, Luo Z, et al. Impact of hydroxyurea on clinical events in the BABY HUG trial. J Am Soc Hematol. 2012;120(22):4304–4310. doi:10.1182/blood-2012-03-419879
19. Galadanci NA, Umar Abdullahi S, Vance LD, et al. Feasibility trial for primary stroke prevention in children with sickle cell anemia in Nigeria (SPIN trial). Am J Hematol. 2017;92(8):780–788. doi:10.1002/ajh.24770
20. Tshilolo L, Tomlinson G, Williams TN, et al. Hydroxyurea for children with sickle cell anemia in sub-Saharan Africa. N Engl J Med. 2019;380(2):121–131. doi:10.1056/NEJMoa1813598
21. Wambebe CO, Bamgboye EA, Badru BO, et al. Efficacy of niprisan in the prophylactic management of patients with sickle cell disease. Curr Ther Res. 2001;62(1):26–34. doi:10.1016/S0011-393X(01)80039-4
22. George IO, Frank-Briggs A, Odigie JO. Nicosan therapy: any role in children with sickle cell anemia in Nigeria. Int J Trop Med. 2011;6(6):121–123.
23. Iyamu EW, Turner EA, Asakura T. In vitro effects of NIPRISAN (Nix‐0699): a naturally occurring, potent antisickling agent. Br J Haematol. 2002;118(1):337–343. doi:10.1046/j.1365-2141.2002.03593.x
24. Oniyangi O, Cohall DH. Phytomedicines (medicines derived from plants) for sickle cell disease. Cochrane Database Syst Rev. 2020;9. doi:10.1002/14651858.CD004448.pub7
25. Rahimy MC, Gangbo A, Ahouignan G, Adjou R, Deguenon C, Goussanou S. Effect of a comprehensive clinical care program on disease course in severely ill children with sickle cell anemia in a sub-Saharan African setting. Blood. 2003;102:834–838. doi:10.1182/blood-2002-05-1453
26. Ohaeri JU, Shokunbi WA. Psychosocial burden of sickle cell disease on caregivers in a Nigerian setting. J Natl Med Assoc. 2002;94(12):1058–1070.
27. Oshikoya KA, Edun B, Oreagba IA. Acute pain management in children with sickle cell anaemia during emergency admission to a teaching hospital in Lagos, Nigeria. S Afr J Child Health. 2015;9(4):119–123. doi:10.7196/SAJCH.2015.v9i4.968
28. Kambale-Kombi P, Marini Djang’eing’a R, Alworong’a Opara JP, Tonen-Wolyec S, Kayembe Tshilumba C, Batina-Agasa S. Students’ knowledge on sickle cell disease in Kisangani, Democratic Republic of the Congo. Hematology. 2020;25(1):91–94. doi:10.1080/16078454.2020.1727174
29. Mukinayi MB, Tumba DG, Gulbis B. Sickle cell disease in the democratic republic of Congo: assessing physicians’ knowledge and practices. Trop Med Infect Dis. 2020;5(3):127. doi:10.3390/tropicalmed5030127
30. Obed SA, Asah-Opoku K, Aboagye S, Torto M, Oppong SA, Nuamah MA. Awareness of sickle cell trait status: a cross-sectional survey of antenatal women in Ghana. Am J Trop Med Hyg. 2017;96(3):735–740. doi:10.4269/ajtmh.16-0396
31. Boadu I, Addoah T. Knowledge, beliefs and attitude towards sickle cell disease among university students. J Community Med Health Educ. 2018;8(1):1000593. doi:10.4172/2161-0711.1000593
32. Kambale-Kombi P, Marini Djang’eing’a R, Alworong’a Opara JP, et al. Management of sickle cell disease: current practices and challenges in a northeastern region of the Democratic Republic of the Congo. Hematology. 2021;26(1):199–205. doi:10.1080/16078454.2021.1880752
33. Mukinayi BM, Kalenda DK, Mbelu S, Gulbis B. Awareness and attitudes of 50 Congolese families affected by sickle cell disease: a local survey. Pan Afr Med J. 2018;29:24. doi:10.11604/pamj.2018.29.24.12276
34. Katawandja AL. Knowledge and practices of health providers on the diagnosis and biological monitoring of sickle cell disease in the City of Kindu, in the East of the Democratic Republic of Congo. Open Access Libr J. 2020;7:e6757. doi:10.4236/oalib.1106757
35. Ngwengi NY, Fon PN, Mbanya D. Distribution of haemoglobin genotypes, knowledge, attitude and practices towards sickle cell disease among unmarried youths in the Buea Health District, Cameroon. Pan Afr Med J. 2020;37:109. doi:10.11604/pamj.2020.37.109.17864
36. Bazuaye GN, Olayemi EE. Knowledge and attitude of senior secondary school students in Benin City Nigeria to sickle cell disease. World J Medical Sci. 2009;4(1):46–49.
37. Olakunle OS, Kenneth E, Olakekan AW, Adenike OB. Knowledge and attitude of secondary school students in Jos, Nigeria on sickle cell disease. Pan Afr Med J. 2013;15(1). doi:10.11604/pamj.2013.15.127.2712
38. Hussaini MA, Durbunde AA, Jobbi YD, et al. Assessment of experience, perception and attitude towards premarital sickle cell disease screening among students attending federal college of education, Kano, Nigeria. Int J Res Rep Hematol. 2019;4:1–2. doi:10.11604/pamj.2021.38.350.20894
39. Alao OO, Araoye M, Ojabo C. Knowledge of sickle cell disease and haemoglobin electrophoresis: a survey of students of a tertiary institution. Niger J Med. 2009;18(3):326–329. doi:10.4314/njm.v18i3.51208
40. Abioye-Kuteyi EA, Osakwe C, Oyegbade O, Bello I. Sickle cell knowledge, premarital screening and marital decisions among local government workers in Ile-Ife, Nigeria. Afr J Prim Health Care Fam Med. 2009;1(1):1–5. doi:10.4102/phcfm.v1i1.22
41. Otovwe A, Sunday UI, Oghenenioborue Rume OB, Awulo DM. Knowledge and attitude of premarital genotype screening among women of child-bearing age in kumo-akko local government area of Gombe State Nigeria. Open J Public Health. 2019;1(2):1006.
42. Babalola OA, Chen CS, Brown BJ, Cursio JF, Falusi AG, Olopade OI. Knowledge and health beliefs assessment of sickle cell disease as a prelude to neonatal screening in Ibadan, Nigeria. J Glob Health Rep. 2019;3:e2019062. doi:10.29392/joghr.3.e2019062
43. Agofure O, Danzaria MA. Knowledge and attitude towards premarital genotype screening among women of child-bearing age in kumo akko local government area of Gombe State. Open J Med Res. 2020;1(1):10–19. doi:10.52417/ojmr.v1i1.73
44. Owolabi RS, Alabi P, Olusoji D, Ajayi S, Otu T, Ogundiran A. Knowledge and attitudes of secondary school students in Federal Capital Territory (FCT), Abuja, Nigeria towards sickle cell disease. Niger J Med. 2011;20(4):479–485.
45. Daak AA, Elsamani E, Ali EH, et al. Sickle cell disease in western Sudan: genetic epidemiology and predictors of knowledge attitude and practices. Trop Med Int Health. 2016;21(5):642–653. doi:10.1111/tmi.12689
46. Rasheed TO, Afolabi WA, Abdul Rasheed RO, Ajala RA. Premarital sickle cell genetic screening knowledge, attitude and practice compared among married and unmarried youths in Nigeria. World J Public Health. 2018;3(3):76–82. doi:10.11648/j.wjph.20180303.12
47. Oluwadamilola AD, Akinreni TI, Adefisan MA, Olayiwola SD. Knowledge, attitude and control practices of sickle cell diseases among senior secondary students in Osun State, Nigeria. Pan Afr Med J. 2021;38:350. doi:10.11604/pamj.2021.38.350.20894
48. Abdulhameed A, Yalma RM. Knowledge, attitude, and practice of genotype screening among junior and senior secondary school students in gwagwalada area council, FCT Abuja. Int J Prog Sci Technol. 2021;26(2):272–280.
49. Ezenwosu OU, Chukwu BF, Ezenwosu IL, Ikefuna AN, Emodi IJ, Ezeanolue EE. Knowledge and awareness of individual sickle cell genotype among adolescents in a unity school in Southeast, Nigeria: a cross-sectional study. Int J Adolesc Med Health. 2020;20190149. doi:10.1515/ijamh-2019-0149
50. Faremi AF, Olatubi IM, Lawal YR. Knowledge of sickle cell disease and pre marital genotype screening among students of a tertiary educational institution in south western Nigeria. Int J Caring Sci. 2018;11(1):285–295.
51. Uche E, Olowoselu O, Augustine B, et al. An assessment of knowledge, awareness, and attitude of undergraduates toward sickle cell disease in Lagos, Nigeria. Niger J Med. 2017;58(6):167. doi:10.4103/nmj.NMJ_111_18
52. Bosah CN, Alagbu CE. Knowledge and attitude of Nnamdi Azikiwe University undergraduate students towards pre-marital sickle cell screening. J Early Child Prim Educ. 2020;2(1):87–99.
53. Chukwurah EF, Oduma FC, Madubuattah CG, Chukwurah FC. Assessment of knowledge and attitude of sickle cell genetic screening among fresh undergraduate students of Ebonyi State University, NigeriaAbakaliki. J Med Lab Sci. 2019;29(3):8–20.
54. Kehinde ME, Sowunmi C. Sickle cell disease knowledge, premarital genotype screening and marital decision among unmarried students of Lagos state Polytechnic Ikorodu, Lagos, Nigeria. EASIJ. 2021;3(5):53–65. doi:10.5281/zenodo.4768648
55. Yalma RM, Awodiji MM. Knowledge, attitude and practice of genotype screening among undergraduate students of the university of Abuja, Nigeria. Eur J Prev Med. 2021;9(2):63–70. doi:10.11648/j.ejpm.20210902.16
56. Oludare GO, Ogili MC. Knowledge, attitude and practice of premarital counseling for sickle cell disease among youth in Yaba, Nigeria. Afr J Reprod Health. 2013;17(4):175–182.
57. Adegbite OA. Young people’s knowledge of sickle cell disease and willingness for genotype screening in Ibadan, Nigeria. Afr J Biomed Res. 2021;24(2):211–217.
58. Oluwole EO, Adeyemo TA. Knowledge, attitude and willingness to screen younger infants for sickle cell disease among mothers attending immunization clinic in an urban community in Lagos, Nigeria. J Comm Med Prim Health Care. 2021;33(2):52–67. doi:10.4314/jcmphc.v33i2.4
59. Abubakar SB, Abdulqadir I, Magaji BA, Sanusi N, Ibrahim F, Maiturare M. Knowledge, attitude and perception of traditional and religious leaders on pre-marital screening for sickle cell disease in Sokoto. Int J Med Public Health. 2019;9(2):36–41. doi:10.5530/ijmedph.2019.2.10
60. Ross PT, Lypson ML, Ursu DC, Everett LA, Rodrigues O, Campbell AD. Attitudes of Ghanaian women toward genetic testing for sickle cell trait. Int J Gynaecol Obstet. 2011;115(3):264–268. doi:10.1016/j.ijgo.2011.08.004
61. Appiah S, Korsah KA, AmpongAdjei C, Appiah OE. Genetic counselling in sickle cell disease: views of single young adults in Ghana. J Community Genet. 2020;11(4):485–493. doi:10.1007/s12687-020-00474-4
62. Nakazwe E, Mwanakasale V, Siziya S. Knowledge attitude and practices of parents with children suffering from sickle cell disease towards factors that precipitate sickle cell crises, at arthur davidson children’s hospital in Ndola Zambia. Asian Pac J Health Sci. 2017;18:18–27. doi:10.21276/apjhs.2017.4.3.26
63. Olatunya OS, Ogundare EO, Fadare JO, et al. The financial burden of sickle cell disease on households in Ekiti, Southwest Nigeria. CEOR. 2015;7:545. doi:10.2147/CEOR.S86599
64. Amendah DD, Mukamah G, Komba A, Ndila C, Williams TN. Routine paediatric sickle cell disease (SCD) outpatient care in a rural Kenyan hospital: utilization and costs. PLoS One. 2013;8(4):e61130. doi:10.1371/journal.pone.0061130
65. Olatunya OS, Babatola AO, Ogundare EO, et al. Perceptions and practice of early diagnosis of sickle cell disease by parents and physicians in a southwestern state of Nigeria. Sci World J. 2020;2020:4801087. doi:10.1155/2020/4801087
66. Nnodu OE, Adegoke SA, Ezenwosu OU, et al. A multi-centre survey of acceptability of newborn screening for sickle cell disease in Nigeria. Cureus. 2018;10(3). doi:10.7759/cureus.2354
67. Akinbami A, Dosunmu A, Adediran A, Oshinaike O, Adebola P, Arogundade O. Haematological values in homozygous sickle cell disease in steady state and haemoglobin phenotypes AA controls in Lagos, Nigeria. BMC Res Notes. 2012;5(1):1–6. doi:10.1186/1756-0500-5-396
68. Ware RE. How I use hydroxyurea to treat young patients with sickle cell anemia. J Am Soc Hematol. 2010;115(26):5300–5311. doi:10.1182/blood-2009-04-146852
69. Adzu B, Masimirembwa C, Mustapha KB, Thelingwani R, Kirim RA, Gamaniel KS. Effect of NIPRISAN® on CYP3A4 activity in vitro. Eur J Drug Metab Pharmacokinet. 2015;40(1):115–118. doi:10.1007/s13318-014-0173-1
70. Nnodu O, Isa H, Nwegbu M, et al. HemoType SC, a low-cost point-of-care testing device for sickle cell disease: promises and challenges. Blood Cells Mol Dis. 2019;78:22–28. doi:10.1016/j.bcmd.2019.01.007
71. Olatona FA, Odeyemi KA, Onajole AT, Asuzu MC. Effects of health education on knowledge and attitude of youth corps members to sickle cell disease and its screening in Lagos State. J Community Med Health Educ. 2012;2:163. doi:10.4172/2161-0711.1000163
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
