Back to Journals » ClinicoEconomics and Outcomes Research » Volume 11
A cost-consequence analysis of parecoxib and opioids vs opioids alone for postoperative pain: Chinese perspective
Authors Barra M, Remák E, Liu DD, Xie L, Abraham L, Sadosky AB
Received 10 August 2018
Accepted for publication 3 January 2019
Published 22 February 2019 Volume 2019:11 Pages 169—177
DOI https://doi.org/10.2147/CEOR.S183404
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Prof. Dr. Dean Smith
Magdolna Barra,1 Edit Remák,1 Dong Dong Liu,2 Li Xie,2 Lucy Abraham,3 Alesia B Sadosky4
1Evidera, Budapest, Hungary; 2Health Economics & Outcomes Research, Pfizer Investment Co., Ltd., Beijing, China; 3Health Economics & Outcomes Research, Pfizer Ltd, Tadworth, UK; 4Health Economics & Outcomes Research, Pfizer Inc, New York, NY, USA
Purpose: The use of parecoxib plus opioids for postoperative analgesia in noncardiac surgical patients seems to be cost-saving in Europe due to a reduction in opioid use and opioid-related adverse events. Given the lack of information on postoperative analgesic use in Asia, this study assessed the economic consequences of the addition of parecoxib to opioids vs opioids alone to treat postsurgical pain in China.
Methods: A cost-consequence economic evaluation assessed direct medical costs related to opioid-related clinically meaningful events (CMEs) utilizing dosing information and reported frequency of events from a Phase III, randomized, double-blind, global clinical trial (PARA-0505-069) of parecoxib plus opioids vs opioids alone for 3 days following major orthopedic, abdominal, gynecologic, or noncardiac thoracic surgery requiring general or regional anesthesia. The cost of CMEs was calculated using information on resource utilization and unit costs provided by a panel of clinical experts in China. Sensitivity analyses were performed to test the robustness of the results.
Results: Patients treated with parecoxib plus opioids reported fewer CMEs (mean 0.62 vs 1.04 events per patient [P<0.0001]) compared with opioids alone for the 3-day postoperative period. This suggested a potential savings of 356 Chinese yuan (¥) per patient over the 3 days (total cost of ¥1,418 for parecoxib plus opioids vs ¥1,774 with opioid use alone).
Conclusion: Fewer CMEs with parecoxib plus opioids suggest a reduction in medical resource utilization and reduced costs compared to opioids alone when modeling analgesic use in noncardiac surgery patients in China.
Keywords: multimodal analgesia, parecoxib, opioids, treatment costs
Introduction
Patients undergoing surgery experience postoperative pain. The postoperative pain-related burden of patients includes not only reduced quality of life and interference with daily functioning1 but also has economic consequences in terms of longer recovery times and extended length of stay and accounts for 30% of readmissions.2 Opioids are commonly used for postoperative pain management, but acute opioid treatment is itself associated with several adverse events (AEs), which increase the clinical burden and require additional resource use like diagnostic procedures or laboratory tests.3–5 In recent years, reduced opioid use has become an important focal point in the treatment of pain following surgery.6–8 Multimodal analgesia or the concomitant use of analgesics belonging to different drug classes is associated with less postoperative pain and less opioid consumption than opioids alone. Paracetamol (acetaminophen), non-selective nonsteroidal anti-inflammatory drugs (NSAIDs) and cyclooxygenase-2 (COX-2) inhibitors are commonly used in conjunction with opioids following major noncardiac surgery with the aim of reducing opioid consumption and their associated adverse effects.9 Selective COX-2 inhibitors are especially notable for their opioid-sparing potential.10,11
Parecoxib, a parenteral COX-2 inhibitor, administered in combination with morphine was shown to be safe and effective in reducing postoperative pain and morphine consumption after major noncardiac surgeries in a Phase III, randomized, double-blind, clinical trial.12 The trial found that patients treated with parecoxib plus opioids had lower summed pain intensity (SPI) scores compared with patients receiving opioids alone. Furthermore, parecoxib reduced postoperative opioid-related symptoms compared to opioids administered alone. In a trial,12 patients rated their distress from opioid-related symptoms in terms of frequency (did not have symptom, rarely, occasionally, frequently, almost constantly), severity (did not have symptom, slight, moderate, severe, very severe), and degree of bother on a daily basis, using the validated Opioid-Related Symptom Distress Scale (OR-SDS).13 Ten opioid-related symptoms were assessed: fatigue, drowsiness, inability to concentrate, confusion, dizziness, constipation, itching, difficulty with urination, nausea, and vomiting. Clinically meaningful events (CMEs)14,15 related to opioid use were defined as OR-SDS symptoms judged to be on the upper distress continuum for severity (ie, it was “severe” or “very severe,” with the exception of confusion which was considered to be a CME with a severity between “moderate” and “very severe”).16 Additional analyses of the trial data showed that patients treated with parecoxib and opioids had a significantly lower risk of experiencing opioid-related CMEs than patients treated with opioids alone.14,15
Resource use and costs associated with postoperative pain treatment may be influenced by a number of factors: the choice of analgesic medication may impact pain intensity and supplemental opioid use, and opioid use in turn may impact the frequency of patient-reported opioid-related CMEs. The economic impact of adding parecoxib to opioid treatment against opioid-only analgesia was evaluated in the UK17 and Greece18 by using a cost-consequence model, which incorporated resource use, costs, and clinical outcomes for 3 days following noncardiac surgery. The reduction in opioid-associated CMEs with parecoxib12,14,15 resulted in shorter hospital stays and a reduced need for physician and nurse time in the models. Subsequently, the excess drug cost of adding parecoxib to opioid treatment was offset by the decrease in CME-related costs, which suggested cost savings for both the UK and Greek health care systems compared with opioid use alone. However, country health care systems differ widely in several ways, including resource use and cost of medical services. For example, in China, the cost of a hospital day on a general ward (¥1132.40,19 which equates to £129) is much lower than the £355 average cost of a hospital day in the UK.20 Therefore the impact of parecoxib treatment and the potential cost savings due to opioid sparing may not be as pronounced in China as in other countries. Furthermore, there is a paucity of information on the cost of medical resources in a postoperative setting in China. The aim of this study was to conduct a similar evaluation in Europe, estimating and comparing the economic impact of the addition of parecoxib to opioid treatment vs opioids only for noncardiac surgical pain management from a Chinese perspective.
Methods
The costs and consequences of the use of parecoxib with opioids as postoperative pain relief were evaluated based on a randomized, double-blind, placebo-controlled Phase III trial (Trial 069).12 The trial assessed the analgesic efficacy and safety of intravenous parecoxib, followed by oral valdecoxib, vs placebo, in addition to opioid analgesia for treating postoperative pain in patients undergoing major orthopedic, abdominal, gynecologic, or noncardiac thoracic surgery.12 Parecoxib sodium is a water-soluble pro-drug of valdecoxib which is a poorly water soluble COX-2 inhibitor. Parecoxib is administered during a time period where patients would have difficulty managing oral medications. Eligible patients were men and women, aged 18–80 years, with an American Society of Anesthesiologists physical status classification of I–III,21 who were expected to require opioids for postoperative pain. The parecoxib group received an initial parenteral dose of 40 mg parecoxib on the day of surgery, followed by 20 mg parecoxib every 12 hours for 3 days, and then 20 mg valdecoxib orally every 12 hours until day 10; the control group received matching placebo throughout the 10-day period. In addition to parecoxib/valdecoxib or placebo, both the patient groups had access to the standard regimen of intravenous opioids and oral narcotics as patient-controlled analgesia throughout the course of the trial. Each patient’s supplemental opioid consumption was recorded daily in morphine equivalents over the study period.
Treatment efficacy was evaluated through pain intensity experienced by patients in the postoperative period.12 Throughout the study period, patients recorded their current pain level using a 4-point scale (0=none, 1=mild, 2=moderate, 3=severe). SPI scores, ranging from 0 (no pain) to 72 (severe pain throughout the day), were calculated from the five categorical pain intensity assessments recorded each day. The pre-specified AEs (cardiovascular events, renal failure or dysfunction, upper gastrointestinal events, and surgical wound events) in the trial were similar between the treatment arms. The number of patients reporting one CME, two or more CMEs, occurring simultaneously were determined for each study day.
The model
A decision analytic model, with a time horizon of 3 days postsurgery corresponding to the timing of parecoxib use in the trial, was used to assess the differences between parecoxib plus standard opioid therapy and standard opioid therapy alone, in terms of clinical outcomes, resource utilization associated with postoperative opioid-related CMEs, and costs. The model included information on the number of patients taking pain medications, their respective doses, the number of CMEs occurring and daily CME-associated resource use. The model also examined whether two or more CMEs occurred simultaneously in patients.
The analysis was conducted from a third-party payer perspective and reported direct medical costs to the Chinese health care system. The mean cost of opioid medications was calculated using morphine equivalents. The impact of treating CMEs was evaluated on resource use items including days in an intensive care unit (ICU) and general ward, and physician and nurse time (in hours). The impact of concurrent CMEs was assessed through a multiplier comparing resource use associated with two or more concurrent CMEs in a single patient to resource use occurring in two (or more) separate patients. Multipliers differed based on the number of CMEs (two CMEs vs three or more CMEs), but were assumed to be the same for all the ten opioid-related AEs. For example, it was assumed that 3 minutes of additional physician contact would be required due to constipation and 7 minutes of additional physician contact due to confusion if they occurred in separate patients. If a patient presented with both severe confusion and constipation at the same time, a multiplier of 0.8 would indicate that the additional time a physician needs to spend with the patient presenting with both confusion and constipation would be 8 minutes (8 minutes = 0.8 * (3 + 7) minutes), where for costing purposes the model would allocate 0.8 * 3 minutes = 2.4 minutes to co-occurring constipation and 0.8 * 7 minutes = 5.6 minutes to co-occurring confusion. SPI scores were taken from Trial 069 for the 3 days when patients were administered postsurgical analgesia.
A one-way deterministic sensitivity analysis was performed by modifying all parameters ±20% to test which influenced the results the most. A probabilistic analysis was undertaken to assess the complete impact of parameter uncertainty on the outcomes, and recommendations for the distribution of assumptions were followed to reflect the mathematical properties of the specific inputs. Accordingly, SPI scores and drug doses were assumed to follow a normal distribution, the proportion of patients taking medications and experiencing single or concurrent CMEs was assumed to follow beta distributions, and costs and resource use were assumed to follow gamma distributions.22
Model inputs
Daily SPI scores, number of patients experiencing CMEs occurring either alone or concurrently, and use of pain medication were based on Trial 069.12 Due to the lack of published information on the impact of CMEs on resource use in China, a questionnaire-based survey of local experts was conducted to characterize local treatment patterns for CMEs and associated costs of CME treatment. The panel comprised four Chinese surgeons from different districts in China covering both Eastern and Western provinces. The questionnaire provided a description of what constitutes a CME and focused on the additional resource use associated with the treatment of CMEs, and included length of stay in ICUs and general hospital wards, number of contacts with medical staff, and number and types of tests or procedures and medication(s) needed to treat CMEs. Panel members were asked to estimate the multiplier for length of hospital stay, physician time, and nurse time associated with a patient experiencing concurrent CMEs. Interviews were conducted individually by telephone, and the experts were remunerated for their participation in the study. The interviews to collect resource use from experts did not require ethics approval as they did not contain any comparisons, no patients were involved, and the only new data came from clinicians talking about treatment practices.
The cost of ICU (¥1,942/day), general hospital stay (¥1,132/day), physician (¥3.90/hour), and nurse hours (¥1.50/hour) were estimated based on data reported in the database of China Health Insurance Research Association for the year 2016. Due to the lack of publicly available sources for the costs for other resource use items in China, unit costs of other items listed in the questionnaire were obtained from the panelists.
Results
Safety and efficacy outcomes of the clinical trial have been reported previously.12,14,15 Parecoxib reduced SPI scores over 3 days by 21.6 (59.2 vs 80.8 for opioids alone, P<0.01). This was also associated with a reduction in the need for supplemental opioid analgesia and, subsequently, a reduction in the frequency of opioid-related CMEs (on average 0.62 CMEs with parecoxib plus opioids vs 1.04 CMEs with opioids alone for a 3-day period). The largest absolute reductions were observed for fatigue (from 0.187 to 0.090 events per patient over 3 days) and drowsiness (from 0.171 to 0.93), while the largest relative reductions were observed for confusion (63%, from 0.088 to 0.032) and concentration problems (56%, from 0.105 to 0.046) as shown in Figure 1.
  | Figure 1 Number of CMEs reported by patients over 3 days. Abbreviation: CME, clinically meaningful event. |
Recently, subpopulation analyses have confirmed that these results are consistently observed across subpopulations defined according to surgery types too.23–25
Resource use and cost estimates of CMEs
Additional costs per CME per patient varied between ¥575 for constipation and ¥4722 for confusion (Table 1). More costly CMEs were associated with lengthened ICU and general ward stays, as well as increased physician and nurse time. Due to the high cost of additional time spent in the ICU and general ward, the differences between individual physician responses can mostly be explained by whether or not additional ICU or general ward time for the CME was indicated. Two concurrent CMEs resulted in decreased resource use and associated costs beyond what was expected for individual CME events, but three or more concurrent CMEs increased costs for drowsiness, confusion, dizziness, and vomiting.
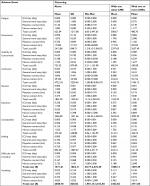  | Table 1 Resource use and total mean cost per CME Abbreviations: CME, clinically meaningful event; hrs, hours; ICU, intensive care unit; SD, standard deviation; ¥, Chinese yuan. |
Cost consequences of different analgesic strategies
Due to the lower incidence of opioid-related CMEs, modeling the use of parecoxib in addition to opioids suggested reductions in the duration of hospital stays and need for medical staff time compared with opioid use alone. The additional drug cost of parecoxib use in China was ¥401 per patient on average over 3 days and mirrored the actual use in the clinical trial. However, the additional cost of parecoxib is predicted to be offset by reduced costs associated with the reduced use of opioids and reduced cost of opioid-related CMEs. As shown in Table 2, the model suggests that the combination of parecoxib with opioids results in an overall cost saving of ¥356 per patient over the 3 days of postoperative analgesia.
The ten most influential variables identified by the one-way deterministic sensitivity analysis are presented graphically in Figure 2 depicting the difference between the total cost of treatment with parecoxib plus opioids vs opioids after changing inputs of the model one by one by ±20% of their base case value. The results were most sensitive to the proportion of patients taking opioids in the opioid only arm, the probability of CMEs co-occurring in the opioid only arm, and the cost of parecoxib. However, even after reducing opioid use in the opioid only arm by 20% on all 3 days and increasing the probability of CMEs co-occurring by 20% at the same time, the parecoxib plus opioid therapy arm was still predicted to remain cost-saving compared with the opioid-only arm, although the savings were reduced to ¥21.60 per patient.
  | Figure 2 Tornado plot for deterministic sensitivity analysis. Abbreviation: CME, clinically meaningful event. |
Given the large uncertainty surrounding the treatment patterns and costs of CMEs, a probabilistic analysis was also undertaken taking into account the variability between responses of the expert panel regarding additional costs of CMEs for individual resource items (variability as shown in Table 1) and the variability of pain medication use and occurrence of CMEs as reported previously from the pivotal trial.12,14,15 The probabilistic estimate of the cost-saving provided by parecoxib was ¥374, with a 95% CI of between parecoxib costing more than opioids alone by ¥66 and savings of up to ¥1,030 (Table 2). Modeling parecoxib use with opioids suggested savings in total costs over the 3-day period in 93.8% of the simulations.
Discussion
In a postoperative setting, modeling the clinical outcomes for parecoxib and opioids was more effective in reducing pain scores than opioids alone.12 This study suggests that the combination of parecoxib and opioids is less costly in a Chinese setting by reducing the need for supplemental opioid use and subsequently the occurrence of CMEs. Therefore, use of parecoxib for postoperative analgesia in China may be a clinically relevant and cost-saving analgesic strategy for noncardiac surgical patients when considering longer term economic consequences as well as immediate drug costs. Results from the expert panel interviews suggest that the costs of managing opioid-related CMEs are high; even a single CME is costly compared to the prices of the drugs themselves. Assessing the value of multimodal analgesia, postsurgery should be considered not only because of improved pain management, but for the potential to decrease hospital length of stay and physician and nurse time associated with opioid-related CMEs.
The modeling results appear robust. Modifying all input parameters by ±20% and varying seven of the ten most influential variables in a direction not favoring parecoxib did not alter the suggestion that the combination of parecoxib and opioids compared with opioids alone is cost saving. The probabilistic analysis showed a >90% probability of parecoxib being cost-saving. The finding that parecoxib plus opioid use may be cost-saving compared with opioids alone is consistent with the findings in the UK17 and Greece,18 despite the differences in the costs of hospital stays and medical staff time between the health care systems. Parecoxib’s impact on costs is likely due to the reduced need for opioids and, subsequently, the reduced occurrence of CMEs. The added cost of treatment of CMEs is lower in China compared to the UK and Greece, nonetheless the reduction in CMEs was sufficient to compensate for the additional cost of parecoxib in China.
The analysis is based on the outcomes of a single trial. This was the pivotal Phase III trial conducted in patients undergoing noncardiac surgeries. A recent comprehensive review of all parecoxib trials reported consistent outcomes across all trials, regardless of the populations or comparators included.26 Analyses of subpopulations of the main trial also showed very similar outcomes regardless of surgery type.23–25 The trial that the present analysis was based on included 113 centers from 14 different countries. Although China was not among the trial countries, no differences in terms of treatment efficacy were reported across countries.12 This suggests that the results of the main trial may be generalizable across all populations. The trial analyzed hospital length of stay according to surgery type and geographical location, but not according to the occurrence of CMEs (data on file). Although the parecoxib group had a slightly shorter length of stay, the difference was not statistically significant. Due to the trial protocol mandating close follow-up of all participants, one would not expect the difference in terms of hospital length of stay to be as pronounced in the clinical trial as it may be in real life. No information on medical staff time was collected in the trial.
The economic outcomes depend very much on the treatment patterns associated with each CME as well as the unit cost of each resource use item. One limitation of this study is the lack of published resource use associated with CMEs. In the absence of reliable data sources, our study relied on a panel of clinical experts from different regions of China. Their responses to items in the questionnaire varied with respect to treatment practices regarding how CMEs would be managed both in terms of diagnostic tests, imaging procedures used, as well as in terms of the estimated impact on hospital length of stay and medical staff time. Similar to the economic study conducted in the UK, extended hospital stay – including time in general wards and ICUs – accounted for the largest proportion of total average costs for all CMEs.17
However, the probabilistic model took into account the inter-clinician variability in indicated resource use. The finding that time spent with patients presenting with two CMEs is shorter compared with time spent with two separate patients differs from the finding in the UK, where co-occurrence of CMEs always increased costs,17 but seems logical. There may be efficiencies in examining and talking to a single patient about their symptoms vs doing the same for two separate patients. At the same time, the fact that a patient reports a higher number of co-occurring CMEs may warrant a more detailed examination of the symptoms, thereby explaining the higher resource use associated with some of the CMEs co-occurring with two or more other CMEs. Future research is warranted to confirm the actual units of resource use in noncardiac surgical patients with opioid-related CMEs and the impact of co-occurrence.
Another limitation is that the study assessed only opioid-related CMEs. As reported in the clinical trial,12 additional AEs were observed beyond those related to opioids; however, the differences were minimal between treatment arms and therefore not included in the model based on the assumption that inclusion in the model would not have influenced the incremental costs between treatments. A final limitation is that Asia was not represented among the 14 countries in the pivotal trial, potentially biasing clinical outcomes.
Conclusion
Modeling the use of opioid-sparing therapies like parecoxib for postoperative analgesia suggests reduced health resource utilization and costs compared with opioids inform a Chinese perspective.
Acknowledgments
This study was funded by Pfizer Inc. We would like to acknowledge the help of Ms Yuxin Li, who conducted the clinician interviews and translated the resource use questionnaires into Chinese and the answers into English.
Author contributions
All authors contributed to data analysis, drafting and revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
LA, ABS, and DDL are employees and shareholders of Pfizer Ltd, Pfizer Inc, and Pfizer Investment Co., Ltd., respectively. LX was an employee and shareholder of Pfizer Investment Co., Ltd. at the time of the study. ER and MB were employees of Evidera at the time of the study, who were paid consultants to Pfizer Inc in connection with the development of this manuscript. The authors report no other conflicts of interest in this work.
References
Beauregard L, Pomp A, Choinière M. Severity and impact of pain after day-surgery. Can J Anaesth. 1998;45(4):304–311. | ||
Koo PJ. Addressing stakeholders’ needs: economics and patient satisfaction. Am J Health Syst Pharm. 2007;64(6_Supplement_4):S11–S15. | ||
Elia N, Lysakowski C, Tramèr MR. Does multimodal analgesia with acetaminophen, nonsteroidal antiinflammatory drugs, or selective cyclooxygenase-2 inhibitors and patient-controlled analgesia morphine offer advantages over morphine alone? Meta-analyses of randomized trials. Anesthesiology. 2005;103(6):1296–1304. | ||
Fabi DW. Multimodal analgesia in the hip fracture patient. J Orthop Trauma. 2016;30(Suppl 1):S6–S11. | ||
Minkowitz HS, Gruschkus SK, Shah M, Raju A. Adverse drug events among patients receiving postsurgical opioids in a large health system: risk factors and outcomes. Am J Health Syst Pharm. 2014;71(18):1556–1565. | ||
Kehlet H, Jensen TS, Woolf CJ. Persistent postsurgical pain: risk factors and prevention. Lancet. 2006;367(9522):1618–1625. | ||
Redan JA, Wells T, Reeder S, McCarus SD. Reducing opioid adverse events: a safe way to improve outcomes. Surg Technol Int. 2016;28:101–109. | ||
Wang L, Johnston B, Kaushal A, Cheng D, Zhu F, Martin J. Ketamine added to morphine or hydromorphone patient-controlled analgesia for acute postoperative pain in adults: a systematic review and meta-analysis of randomized trials. Can J Anaesth. 2016;63(3):311–325. | ||
Chou R, Gordon DB, de Leon-Casasola OA, et al. Management of postoperative pain: a clinical practice guideline from the American pain Society, the American Society of regional anesthesia and Pain medicine, and the American Society of Anesthesiologists’ committee on regional anesthesia, executive Committee, and administrative Council. J Pain. 2016;17(2):131–157. | ||
Lu X, Li K. [Multimodal effect of celecoxib on the perioperative analgesia in orthopaedic surgery]. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2009;34(8):815–819. Chinese | ||
Puura A, Puolakka P, Rorarius M, Salmelin R, Lindgren L. Etoricoxib pre-medication for post-operative pain after laparoscopic cholecystectomy. Acta Anaesthesiol Scand. 2006;50(6):688–693. | ||
Nussmeier NA, Whelton AA, Brown MT, et al. Safety and efficacy of the cyclooxygenase-2 inhibitors parecoxib and valdecoxib after noncardiac surgery. Anesthesiology. 2006;104(3):518–526. | ||
Apfelbaum JL, Gan TJ, Zhao S, Hanna DB, Chen C. Reliability and validity of the perioperative opioid-related symptom distress scale. Anesth Analg. 2004;99(3):699–709. | ||
Katz J, Ferrante M, Boye M, Brown M. Treatment with parenteral parecoxib followed by oral valdecoxib after major general surgery reduces opioid consumption and opioid-related adverse effects. Eur J Anaesthesiol. 2005;22(Supplement 34):185. | ||
Katz JFF, Neumann J, Rowinski W. Parenteral parecoxib followed by oral valdecoxib after major general surgery reduces opioid consumption and opioid-related symptoms. Value in Health. 2007;3(8):241. | ||
Chan KS, Chen WH, Gan TJ, et al. Development and validation of a composite score based on clinically meaningful events for the opioid-related symptom distress scale. Qual Life Res. 2009;18(10):1331–1340. | ||
Muszbek N, Choy A, Remak E. Cost-consequence analysis of adjunctive use of the COX-2 inhibitor parecoxib compared to opioids alone after non-cardiac surgery, in the United Kingdom. Gazz Med Ital. 2013;172(11):835–844. | ||
Athanasakis K, Petrakis I, Vitsou E, Pimenidou A, Kyriopoulos J. A cost-effectiveness analysis of parecoxib in the management of postoperative pain in the Greek health care setting. Clin Ther. 2013;35(8):1118–1124. | ||
Ministry of Health of the People’s Republic of China. China Health Statistical Yearbook 2013. Beijing: Peking Union Medical College Publishing House; 2013. | ||
Department of Health [webpage on the Internet]. NHS reference costs 2014 to 2015; 2015. Available from: https://www.gov.uk/government/publications/nhs-reference-costs-2014-to-2015. Accessed August 1, 2016. | ||
Keats AS. The ASA classification of physical status – a recapitulation. Anesthesiology. 1978;49(4):233–235. | ||
Briggs A, Claxton K, Schulper M. Decision Modelling for Health Economic Evaluation. New York, NY: Oxford University Press, Inc; 2006. | ||
Diaz-Borjon E, Torres-Gomez A, Essex MN, et al. Parecoxib provides analgesic and Opioid-Sparing effects following major orthopedic surgery: a subset analysis of a randomized, placebo-controlled clinical trial. Pain Ther. 2017;6(1):61–72. | ||
Parsons B, Zhu Q, Xie L, Li C, Cheung R. Effects of parecoxib on postoperative pain and opioid-related symptoms following gynecologic surgery. J Pain Res. 2016;9:1101–1107. | ||
Essex MN, Xu H, Parsons B, Xie L, Li C. Parecoxib relieves pain and has an opioid-sparing effect following major gastrointestinal surgery. Int J Gen Med. 2017;10:319–327. | ||
Schug SA, Parsons B, Li C, Xia F. The safety profile of parecoxib for the treatment of postoperative pain: a pooled analysis of 28 randomized, double-blind, placebo-controlled clinical trials and a review of over 10 years of postauthorization data. J Pain Res. 2017;10:2451–2459. |
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

