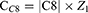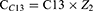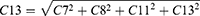Back to Journals » Clinical Ophthalmology » Volume 17
A Comparison of Three Cylindrical Treatment Strategies for Topography-Guided LASIK: Manifest, Topographic, and ZZ VR Cylinders
Authors Zhang J , Zheng L, Zheng C, Sun P
Received 8 March 2023
Accepted for publication 1 May 2023
Published 9 May 2023 Volume 2023:17 Pages 1335—1345
DOI https://doi.org/10.2147/OPTH.S408101
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Jun Zhang, Li Zheng, Chenyao Zheng, Peihong Sun
Department of Ophthalmology, Hangzhou MSK Eye Hospital, Hangzhou, People’s Republic of China
Correspondence: Jun Zhang, Email [email protected]
Purpose: This study was designed to compare the clinical outcomes of three cylindrical treatment strategies using manifest, topographic, and Zhang & Zheng vector-compensated refraction (ZZ VR) cylinders, for topography-guided laser-assisted in situ keratomileusis (LASIK) and to identify the laser programming strategy that optimizes refractive astigmatism outcomes and visual acuity.
Methods: Consecutive patients referred for therapeutic refractive surgery between March and September 2018 at a single center were prospectively analyzed. Using double-masked simple randomization, patients were randomly assigned to undergo treatment based on manifest cylinder, topographic cylinder, and ZZ VR cylinder strategies. Uncorrected distance visual acuity and astigmatic refraction were analyzed preoperatively and 6 months postoperatively.
Results: A total of 138 eyes from 71 patients met the inclusion criteria. The manifest group consisted of 46 eyes in 24 patients, the topographic group consisted of 43 eyes in 22 patients, and the ZZ VR group consisted of 49 eyes in 25 patients. The absolute residual cylindrical refractions at 6 months postoperatively in these three groups were 0.69 ± 0.32 D, 0.58 ± 0.31 D, and 0.42 ± 0.19 D, respectively (P < 0.001; adjusted P < 0.01 for manifest vs ZZ VR, adjusted P = 0.08 for topographic vs ZZ VR). The percentages of postoperative absolute residual cylindrical power within 0.50 D in the manifest, topographic, and ZZ VR groups were 30.4%, 55.8%, and 59.2%, respectively (P = 0.01; adjusted P = 0.06 for manifest vs topographic, adjusted P = 0.02 for manifest vs ZZ VR).
Conclusion: The ZZ VR strategy may achieve better outcomes, as determined by cylindrical correction and visual activity, during topography-guided LASIK.
Clinical Trial Registration Number: ChiCTR1900025779.
Keywords: astigmatism, corneal wavefront aberration, keratomileusis, laser in situ, refraction
Introduction
The primary objective of subjective refraction in refractive surgery is to identify the best combination of spherical and cylindrical lenses to achieve optimal distance visual acuity. Higher-order aberration (HOA) guided keratorefractive surgery based on Zernike polynomials has emerged as a promising approach to improve visual acuity and focus.1 In contrast to optical lens or spectacle glasses correction, however, corneal tissue can only be ablated, not increased, reducing the ability to achieve rational ablation that adheres to the Zernike orthogonal property. Correction of certain higher-order items may lead to changes in lower-order items, complicating the ability to optimize the traditional surgical strategy of HOA plus manifest refraction correction, particularly for eyes with higher HOA. Despite the need to compensate for spherocylindrical components, few studies to date have evaluated their quantitative relationship.2
Topography-guided laser-assisted in situ keratomileusis (LASIK) was used primarily to correct highly irregular corneas by reducing HOA, with any remaining spherocylindrical issues addressed by further treatment.3–5 Because of advances in accuracy and tracking technology,6,7 topography-guided LASIK is now used as a customized keratorefractive surgery in clinical settings for virgin eyes.8–10 It may therefore be clinically significant to minimize residual refraction, particularly cylindrical issues, which can affect both distance and near vision and are difficult to compensate for by accommodation.
The standard strategy for topography-guided LASIK involves using clinical refraction to determine ablation data for lower-order aberration (LOA). However, patients with high levels of HOA corneal morphology may experience residual refraction issues without proper spherocylindrical compensation. An alternative approach is topography-modified refraction or Layer Yolked Reduction of Astigmatism, which adjusts the cylindrical ablation data based on anterior corneal topography and reshapes the anterior corneal surface into a regular ellipsoid.11–13 This method, however, may result in high residual refraction issues for patients with high levels of intraocular astigmatism. These concerns may be addressed by using the Zhang & Zheng vector-compensated refraction (ZZ VR) strategy, which calculates the LOA ablation value after spherocylindrical compensation (Figure 1). Because these three approaches use almost identical spherical equivalent correction, the main objectives of this study were to analyze differences among these three approaches in cylindrical correction and visual activity and to identify the laser programming strategy that optimizes refractive astigmatism outcomes and visual acuity.
Materials and Methods
Ethical Statement
The study protocol was approved by the Medical Research Ethics Committee of Hangzhou MSK Eye Hospital (approval number: MSKLL20180906). All procedures in this study adhered to the tenets of the Declaration of Helsinki. All participants were informed about the risks and benefits of the procedure and provided written informed consent. This study was registered in the Chinese Clinical Trials Registry (registration number: ChiCTR 1900025779).
Study Design and Patients
This prospective study analyzed a consecutive series of patients who were referred to our hospital for therapeutic refractive surgery between March and September of 2018 and who underwent a topography-guided LASIK procedure for correcting myopia and astigmatism. Using double-masked simple randomization, patients were randomly assigned to three groups and underwent surgery using manifest cylinder, topographic cylinder and ZZ VR cylinder strategies.
Eyes were included if they were: 1) Naïve to refractive treatment; and 2) Had a transparent corneal optical region. Patients who experienced postoperative complications, such as corneal infections, abnormal intraocular pressure, or diffuse lamellar keratitis, were excluded from the study.
ZZ VR Strategy
The ZZ VR strategy incorporates both clinical refraction and refraction affected by HOA, utilizing vector analysis to determine the predicted therapeutic correction for astigmatic power and axis.
The initial step involved identifying the HOA item ablation that necessitated cylindrical compensation and the HOA item ablation that necessitated spherical compensation. This study focused solely on cylindrical compensated analysis. Because the Zernike polynomials for astigmatism at 45° and 135° and at 0° and 90° contained sin(2θ) and cos(2θ), respectively,14 any HOA polynomial containing sinθ, cosθ, sin(2θ), or cos(2θ) required cylindrical compensation during ablation correction. This was due to the ability to only ablate, not enlarge, corneal tissue, as well as the cosine effect of the excimer laser. Thus, compensations for primary coma and secondary astigmatism were required during third- and fourth-order ablation. Higher-order polynomials were temporarily disregarded, as their influence was significantly reduced by the lower effect of higher orders.
Subsequently, the compensations needed to correct primary coma and secondary astigmatism during ablation were determined. When correcting primary vertical and horizontal comas, the compensations for cylindrical power were CC7 and CC8, respectively, which can be calculated using Equations 1 and 2. When correcting secondary vertical and horizontal astigmatism, the compensations for cylindrical power were CC11 and CC13, respectively, which can be calculated using Equations 3 and 4.
C7 and C8 represent the primary vertical and horizontal coma, respectively, whereas C11 and C13 represent secondary vertical and horizontal astigmatism, respectively, which were addressed as part of the planned ablation. These values were obtained from the Zernike data contained within the ablation pattern of the excimer laser device. Z1 and Z2 were two constants calculated based on the maximum optical path difference of the intersecting meridians with a fixed diameter in the unit quantity of the primary vertical or horizontal coma and the secondary vertical or horizontal astigmatism polynomial, respectively. The corresponding axis positions for CC7, CC8, CC11 and CC13 were 180, 90, 135 and 90 degrees, respectively, with their respective positions denoted as AC7, AC8, AC11, and AC13.
After determining compensation values for the above four groups of astigmatism, compensation of subjective cylindrical refraction was determined by successive vector subtraction of the subjective cylinder, resulting in a predicted cylinder with power CZZ and axis AZZ. Although the detailed derivation has been patented (patent No.: ZL 2019 1 0005564.7, Authorization Announcement No.: CN 109491083 B), the general ZZ VR strategy is open-access and can be found at www.zzcal.com.
Figure 2 shows an illustrative case for a patient with a manifest refraction of −5.75 diopters sphere (DS), −1.25 diopters cylinder (DC) × axis 85, who underwent the four sequential steps of the ZZ VR strategy to achieve a compensated refraction of −6.01 DS, −0.74 DC × axis 90. Notably, the compensated refraction was not adjusted by the nomogram.
Manifest Group and Topographic Group
In the manifest group, the source of cylindrical refraction was the manifest refraction. In the topographic group, the cylindrical refraction was determined by the measured data provided by the excimer device software (EX500 SP3, Alcon, Fort Worth, TX, USA).
Data Collection
Patient data were collected, including pre-operative baseline characteristics and topography (Topolyzer Vario, Alcon). The manifest refraction was determined by combining subjective and objective methods (ARK-1, Nidek, Aichi, Japan), and the axial length was obtained by biometry (IOLMaster 700, Carl Zeiss, Jena, Germany). Cylindrical refraction within a 4-mm zone was obtained using the wavefront aberration device (WaveLight Analyzer, Alcon) and visual activity was measured preoperatively and 6 months postoperatively. The primary coma (C7 and C8), secondary astigmatism (C11 and C13), and HOA value within a 5-mm zone of the cornea were determined by corneal topography (Sirius 3.7, CSO, Florence, Italy), both before and 6 months after the operation. Subjective refraction, topography, and wavefront aberration were assessed by a single optometrist with more than 5 years of experience.
Alpins vector analysis, which calculated the residual vector cylindrical refraction, was performed to determine whether the residual vector cylindrical refraction might offer a more intuitive reflection of astigmatism correction than the absolute value of residual cylindrical refraction. The methodology involved summing all the astigmatism vectors, determining the mean direction based on the sum, and calculating the mean power by dividing the total sum by the summation result.15
The total value of HOA compensated by ZZ VR was calibrated based on C7, C8, C11, and C13, as described in Equation 5:
HOA comprised of C7, C8, C11, and
Statistical Analysis
Continuous variables were expressed as mean ± standard deviation (SD), and categorical variables were reported as frequency (percentage). Because the Kolmogorov–Smirnov test showed that the absolute residual astigmatism in each group did not conform to a Gaussian distribution, parameters across groups were compared by Kruskal–Wallis H-tests. The frequencies of absolute residual cylinder ≤0.50 D were compared by the chi-square test, followed by pairwise comparisons using the Bonferroni method. The associations between absolute residual cylinder and HOA comprised of C7, C8, C11, and C13 were determined by calculating Spearman correlation coefficients. All statistical analyses were performed using SPSS 19.0 (IBM, Armonk, NY, USA), with two-sided P values <0.05 defined as statistically significant. The minimum sample size was estimated by PASS 15 (NCSS, LLC. Kaysville, UT, USA).
Results
Characteristics of the Patients
This study included 146 eyes of 75 patients who met the eligibility criteria. Of these, six eyes of three patients were excluded due to abnormal intraocular pressure and two eyes of one patient were excluded due to diffuse lamellar keratitis. The manifest group included 46 eyes of 24 patients, the topographic group included 43 eyes of 22 patients, and the ZZ VR group included 49 eyes of 25 patients. Table 1 displays the characteristics of each group.
 |
Table 1 Baseline Characteristics of the Study Subjects |
Residual Cylinder Analysis
The mean absolute residual cylindrical refraction 6 months after surgery was 0.74±0.30 D in the manifest group, 0.64±0.33 D in the topographic group, and 0.44±0.22 D in the ZZ VR group (P < 0.001; adjusted P < 0.01 for manifest vs ZZ VR, adjusted P = 0.01 for topographic vs ZZ VR). The percentages of postoperative absolute residual cylinder within 0.50 D were 30.4%, 51.2%, and 59.2% in the manifest, topographic, and ZZ VR groups, respectively (P =0.02; adjusted P = 0.04 for manifest vs topographic, adjusted P = 0.01 for manifest vs ZZ VR) (Table 2, Figure 3). The Alpins vector analysis method showed that the mean vector residual cylinders 6 months postoperatively using the manifest, topographic, and ZZ VR strategies were −0.58@11°±0.56 D, −0.15@13°±0.71 D, and −0.22@3°±0.45D, respectively (Figure 4).
 |
Table 2 Residual Cylinder Analysis 6 Months After Surgery |
 |
Figure 3 Scatter plot showing absolute residual astigmatism in the manifest, topographic and ZZ VR groups. |
 |
Figure 4 Scatter plot showing the residual astigmatism as a vector in the (A) manifest group (B) topographic group, and (C) ZZ VR group. |
Visual Activity Analysis
Figure 5 shows a histogram of the postoperative uncorrected distance visual acuity, and Figure 6 shows a histogram of the difference between postoperative uncorrected distance visual acuity and preoperative corrected distance visual acuity.
 |
Figure 5 Histogram showing the distribution of postoperative uncorrected distance visual acuity. |
 |
Figure 6 Histogram showing the distribution of the differences between postoperative uncorrected distance visual acuity and preoperative corrected distance visual acuity. |
HOA and Correlation Analysis
The absolute residual cylinder of the manifest group was significantly correlated with the HOA consisting of C7, C8, C11, and C13 (P=0.003). In contrast, the absolute residual cylinders of the topographic and ZZ VR groups were not significantly correlated with the HOA consisting of C7, C8, C11, and C13 (P >0.05 each, Table 3).
 |
Table 3 Correlations Between High-Order Aberration and Absolute Residual Cylinder |
Discussion
The findings on residual cylinder and visual activity reveal that the ZZ VR strategy offers greater clinical advantages than the other strategies. Moreover, the results of the correlation analysis provide indirect support for the error sources analysis of the three strategies. Additionally, although the mean difference among strategies may not have indicated clinical advantages, as the differences were ≤0.50 D, the comparison of dispersion showed greater agreement with the aim of this study, of identifying the optimal strategies.
HOAs are a crucial concern in refractive surgery, as they can affect postoperative visual quality.16–18 The development of incidental HOA after corneal ablation can cause glare, halos, haze, or starbursts.19 Due to technological advances, the FDA has approved topographic-guided LASIK, which can reduce the postoperative induction of HOA in treatment-naïve eyes.20
Surgical strategies, including the manifest and topographic strategies, have been proposed to improve postoperative visual activity.11,21 The manifest strategy, however, may overcorrect cylindrical refraction affected by HOA, whereas the topographic strategy may underestimate the effects of the posterior corneal surface and intraocular lens. These limitations have led to the development of several optimized topographic strategies, including the 50% and 75% topographic strategies, and a topographic strategy involving the removal of the posterior corneal cylinder. In agreement with previous findings,11 the results of the present study suggest that the topographic strategy was superior to the manifest strategy, as indicated by the percentage of absolute residual cylindrical power within 0.50 D.
The ZZ VR strategy, which was based on the Zernike HOA formula and the limitations of the excimer laser, included an assessment of the impact of HOA on subjective refraction. This strategy included individual calculation of the compensation for each Zernike item. The polynomial function of each Zernike term, which was related to the difference in optical path between opposite points, was subsequently analyzed. If Differences in the number of opposite points with a 90-degree azimuth difference under the same intercept may indicate the induction of new astigmatism after correction. Therefore, only the corrections of coma-like and high-order-astigmatism-like HOA would induce astigmatism, allowing the axial orientation of new astigmatism to be calculated. Higher Zernike order was associated with reduced impact on vision. Therefore, to simplify clinical applications, Zernike terms of order 5 and above were excluded, enabling compensation only for primary coma and second-order astigmatism. Pupil size can affect the magnitude of astigmatism, with the present study assuming that astigmatism in the 3 mm area of the corneal apex was the most significant. Using computer-aided technology, the results can be obtained quickly through the website www.zzcal.com.
The rationale for utilizing cylindrical refraction in a 4-mm zone, as measured by the wavefront aberration device, was to provide an accurate reflection of refraction while minimizing the refractive step to 0.01 D.22 This approach resulted in a reduced system error compared with the 0.125-D error typical of computer or subjective refraction.
Several factors primarily affect the ZZ VR strategy when determining the effect of HOA on subjective refraction. First, errors can arise from acquiring HOA, as these measurements rely on corneal topography or tomography devices, such as the Placido disk and Scheimpflug technology. These devices may not exhibit any differences for treatment-naïve corneas, but special corneas require optimization and attention.23–25 Second, errors from other HOAs can affect subjective refraction, but only primary coma and secondary astigmatism participate in compensation in the ZZ VR strategy. Third, the ZZ VR strategy assumes that excimer ablation will reduce, not increase, ocular primary coma and secondary astigmatism within an effective optical zone. Fourth, the calculation of refraction may be influenced by HOA within the diameter of individual pupils. Fifth, the ZZ VR strategy compensates for intraocular astigmatism by introducing corneal astigmatism, making it particularly relevant for elderly patients with specific clinical needs. In addition, the biomechanical changes and epithelial remodeling issues of the cornea after surgery have not yet been predicted or compensated for.26
The current study had several limitations. First, the ZZ VR strategy may not be effective for irregularities in the optical area, such as eccentric ablation, where planned corrections of HOA may have little impact on subjective refraction. Second, the small sample size of this single-center trial may limit the generalizability of the findings. Additionally, bilateral analysis did not rule out factors of symmetry.27 Lastly, clinical results were not available for correction of patients with hyperopia, suggesting the need for further research prior to the widespread clinical adoption of this technology.
Conclusions
The findings of this study showed that the ZZ VR strategy may achieve better outcomes in cylindrical correction and visual activity during topography-guided LASIK than the manifest and topographic strategies.
Abbreviations
HOA, higher-order aberration; LOA, lower-order aberration; ZZ VR, Zhang & Zheng vector-compensation refraction; DS, diopters sphere; DC, diopters cylinder; SD, standard deviation.
Data Sharing Statement
The data supporting the findings of this study are available from the corresponding author upon reasonable request.
Ethics Approval and Informed Consent
The study protocol was approved by the Medical Research Ethics Committee of Hangzhou MSK Eye Hospital (approval number: MSKLL20180906). All procedures in this study adhered to the tenets of the Declaration of Helsinki. All participants were informed about the risks and benefits of the procedure and provided written informed consent. This study was registered in the Chinese Clinical Trials Registry (registration number: ChiCTR 1900025779).
Funding
This study was supported by the Zhejiang Province Medical and Health Science and Technology Project (Grant No. 2020167858).
Disclosure
All authors state that they do not have any conflicts of interest.
References
1. Tiwari NN, Sachdev GS, Ramamurthy S, Dandapani R. Comparative analysis of visual outcomes and ocular aberrations following wavefront optimized and topography-guided customized femtosecond laser in situ keratomileusis for myopia and myopic astigmatism: a contralateral eye study. Indian J Ophthalmol. 2018;66(11):1558–1561. doi:10.4103/ijo.IJO_507_18
2. Zhou W, Stojanovic A, Utheim TP. Assessment of refractive astigmatism and simulated therapeutic refractive surgery strategies in coma-like-aberrations-dominant corneal optics. Eye Vis. 2016;3:13. doi:10.1186/s40662-016-0044-8
3. Reinstein DZ, Gobbe M, Archer TJ, Youssefi G, Sutton HF. Stromal surface topography-guided custom ablation as a repair tool for corneal irregular astigmatism. J Refract Surg. 2015;31(1):54–59. doi:10.3928/1081597X-20141218-06
4. Holland S, Lin DT, Tan JC. Topography-guided laser refractive surgery. Curr Opin Ophthalmol. 2013;24(4):302–309. doi:10.1097/ICU.0b013e3283622a59
5. Ghoreishi M, Naderi beni A, Naderi Beni Z. Visual outcomes of topography-guided excimer laser surgery for treatment of patients with irregular astigmatism. Lasers Med Sci. 2014;29(1):105–111. doi:10.1007/s10103-013-1282-9
6. Motwani M, Pei R. The use of WaveLight contoura to create a uniform cornea: 6-month results with subjective patient surveys. Clin Ophthalmol. 2018;12:1559–1566. doi:10.2147/OPTH.S175661
7. Zhang J, Zheng L, Zhao X, Xu Y, Lin H, Mao H. Contoura矫正屈光不正伴轻度角膜不规则散光的临床效果 [Clinical study of the contoura automatic location tracking system for correction of ametropia with mild irregular astigmatism]. Chin J Optom Ophthalmol Vis Sci. 2018;20(4):216–221. Chinese.
8. Wallerstein A, Caron-Cantin M, Gauvin M, Adiguzel E, Cohen M. Primary topography-guided LASIK: refractive, visual, and subjective quality of vision outcomes for astigmatism ≥2.00 diopters. J Refract Surg. 2019;35(2):78–86. doi:10.3928/1081597X-20181210-01
9. Durrie D, Stulting RD, Potvin R, Petznick A. More eyes with 20/10 distance visual acuity at 12 months versus 3 months in a topography-guided excimer laser trial: possible contributing factors. J Cataract Refract Surg. 2019;45(5):595–600. doi:10.1016/j.jcrs.2018.12.008
10. De Stefano VS, Meister C, Ehlke GL, Krueger RR. Analysis of planning strategies in primary eyes gaining a line or more of visual acuity after topography-guided laser in situ keratomileusis. J Cataract Refract Surg. 2019;45(3):321–327. doi:10.1016/j.jcrs.2018.10.040
11. Kanellopoulos AJ. Topography-modified refraction (TMR): adjustment of treated cylinder amount and axis to the topography versus standard clinical refraction in myopic topography-guided LASIK. Clin Ophthalmol. 2016;10:2213–2221. doi:10.2147/OPTH.S122345
12. Motwani M. The use of WaveLight® contoura to create a uniform cornea: the LYRA protocol. Part 1: the effect of higher-order corneal aberrations on refractive astigmatism. Clin Ophthalmol. 2017;11:897–905.
13. Motwani M. The use of WaveLight® contoura to create a uniform cornea: the LYRA protocol. Part 2: the consequences of treating astigmatism on an incorrect axis via excimer laser. Clin Ophthalmol. 2017;11:907–913. doi:10.2147/OPTH.S133840
14. McAlinden C, McCartney M, Moore J. Mathematics of Zernike polynomials: a review. Clin Exp Ophthalmol. 2011;39(8):820–827. doi:10.1111/j.1442-9071.2011.02562.x
15. Alpins N. Astigmatism analysis by the Alpins method. J Cataract Refract Surg. 2001;27(1):31–49. doi:10.1016/S0886-3350(00)00798-7
16. Smadja D, Reggiani-Mello G, Santhiago MR, Krueger RR. Wavefront ablation profiles in refractive surgery: description, results, and limitations. J Refract Surg. 2012;28(3):224–232. doi:10.3928/1081597X-20120217-01
17. Liang J, Williams DR. Aberrations and retinal image quality of the normal human eye. J Opt Soc Am A. 1997;14(11):2873–2883. doi:10.1364/JOSAA.14.002873
18. Zhang J, Zheng L, Zhao X, Sun Y, Feng W, Yuan M. Corneal aberrations after small-incision lenticule extraction versus Q value-guided laser-assisted in situ keratomileusis. Medicine. 2019;98(5):e14210. doi:10.1097/MD.0000000000014210
19. Halliday BL. Refractive and visual results and patient satisfaction after excimer laser photorefractive keratectomy for myopia. Br J Ophthalmol. 1995;79(10):881–887. doi:10.1136/bjo.79.10.881
20. Jung JH, Jeong SJ, Kim JH, et al. Inactivation of HDAC3 and STAT3 is critically involved in 1-stearoyl-sn-glycero-3-phosphocholine-induced apoptosis in chronic myelogenous leukemia K562 cells. Cell Biochem Biophys. 2013;67(3):1379–1389. doi:10.1007/s12013-013-9670-0
21. Alpins NA. New method of targeting vectors to treat astigmatism. J Cataract Refract Surg. 1997;23(1):65–75. doi:10.1016/S0886-3350(97)80153-8
22. Yan S, Yabo Y, Qing F. Comparison of the refraction results measured with MEL-70 wavefront analyzer and classical methods. Rec Adv Ophthalmol. 2005;25(1):54–56.
23. Rio-Cristobal A, Martin R. Corneal assessment technologies: current status. Surv Ophthalmol. 2014;59(6):599–614. doi:10.1016/j.survophthal.2014.05.001
24. de Jong T, Sheehan MT, Dubbelman M, Koopmans SA, Jansonius NM. Shape of the anterior cornea: comparison of height data from 4 corneal topographers. J Cataract Refract Surg. 2013;39(10):1570–1580. doi:10.1016/j.jcrs.2013.04.032
25. Savini G, Carbonelli M, Sbreglia A, Barboni P, Deluigi G, Hoffer KJ. Comparison of anterior segment measurements by 3 scheimpflug tomographers and 1 Placido corneal topographer. J Cataract Refract Surg. 2011;37(9):1679–1685. doi:10.1016/j.jcrs.2011.03.055
26. De Bernardo M, Pagliarulo S, Rosa N. Unexpected ocular morphological changes after corneal refractive surgery: a review. Front Med. 2022;9:1014277. doi:10.3389/fmed.2022.1014277
27. Cione F, De Bernardo M, Capasso L, Rosa N. Total keratometry in intraocular lens power calculations in eyes with previous laser refractive surgery: comment. Clin Exp Ophthalmol. 2021;49(1):87–88. doi:10.1111/ceo.13883
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.