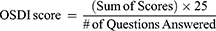Back to Journals » Clinical Ophthalmology » Volume 16
A Comparison of TearCare and Lipiflow Systems in Reducing Dry Eye Disease Symptoms Associated with Meibomian Gland Disease
Authors Holland EJ, Loh J, Bloomenstein M, Thompson V, Wirta D, Dhamdhere K
Received 30 March 2022
Accepted for publication 27 July 2022
Published 30 August 2022 Volume 2022:16 Pages 2861—2871
DOI https://doi.org/10.2147/OPTH.S368319
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Edward J Holland,1 Jennifer Loh,2 Marc Bloomenstein,3 Vance Thompson,4 David Wirta,5 Kavita Dhamdhere6,7
1Cincinnati Eye Institute, Edgewood, KY, USA; 2Loh Ophthalmology Associates, Miami, FL, USA; 3Schwartz Laser Eye Center, Scottsdale, AZ, USA; 4Vance Thompson Vision, Sioux Falls, SD, USA; 5Eye Research Foundation, Newport Beach, CA, USA; 6Sight Sciences, Inc, Menlo Park, CA, USA; 7Mahatma Gandhi Medical College and Research Center, Wardha, India
Correspondence: Kavita Dhamdhere, Tel +1 650-223-4062, Fax +1 877-266-1144, Email [email protected]
Purpose: To compare TearCare and Lipiflow systems in the ability to reduce the symptoms of dry eye disease (DED) associated with meibomian gland dysfunction (MGD).
Methods: In this multicenter, masked, randomized-controlled trial, 235 subjects received a single TearCare treatment (n = 115) or a single LipiFlow treatment (n = 120) and were followed for 1-month post-treatment. DED symptoms were assessed using the Ocular Surface Disease Index (OSDI), Symptom Assessment in Dry Eye (SANDE), and Eye Dryness (ED) questionnaires at baseline and at 1 month. Post-hoc subgroup analysis was conducted on subjects with less severe and more severe gland obstruction determined by baseline meibomian gland secretion score (MGSS).
Results: TearCare system significantly improved total OSDI, SANDE, and ED scores from baseline (p < 0.0001) at 1-month follow-up. Subjects with more severe disease (MGSS < 7) achieved statistically greater reduction with TearCare compared to LipiFlow in total OSDI score (30.4 ± 2.53 and 21.9 ± 2.37, respectively, pANCOVA = 0.0160), OSDI Section B score for quality of vision (5.1 ± 0.48 and 3.6 ± 0.45, respectively, pANCOVA= 0.0206), and SANDE frequency score (51.9 ± 3.70 and 41.5 ± 3.45, respectively, pANCOVA = 0.0455).
Conclusion: TearCare provides significant DED symptom relief at 1 month after a single treatment. Outcomes were consistent in OSDI, SANDE, and ED assessments. In subjects with more severe gland dysfunction, TearCare performed significantly better than LipiFlow in improving quality of vision and overall DED symptom frequency determined by OSDI and SANDE.
Clinical Trial Registration Number: NCT03857919.
Keywords: dry eye disease, meibomian gland deficiency, TearCare procedure
Introduction
Dry eye disease (DED) is a prevalent chronic eye condition affecting between 5% and 34% of the population worldwide, and approximately 1 million to 4 million Americans between 65 and 84 years of age.1,2 This multifactorial disease has considerable socio-economic implications, including daily activities, visual function, social and physical functioning, workplace productivity, and quality of life.3
DED is defined as
a multifactorial disease of the ocular surface characterized by a loss of homeostasis of the tear film, and accompanied by ocular symptoms, in which tear film instability and hyperosmolarity, ocular surface inflammation and damage, and neurosensory abnormalities play etiological roles.4
Based on etiology, DED can be classified as aqueous tear deficiency, evaporative tear deficiency, or a combination of both. According to a report by the Tear Film & Ocular Surface Society Dry Eye Workshop in 2017, evaporative DED is more common than aqueous-deficiency DED.4 Evaporative DED results from excessive loss of water due to exposed ocular surface despite normal lacrimal secretion. Consequently, a vicious cycle is generated, in which tear hyperosmolarity develops and activates inflammation response that contributes to tear film instability, further exacerbating tear hyperosmolarity. Etiologies of evaporative DED include meibomian gland deficiency (MGD), poor lid dynamic, low blink rate, systemic retinoids use, preservatives use, contact lens, vitamin A deficiency, etc. The most common cause for evaporative DED is MGD which causes disruption of the tear lipid layers.5 Chronic glandular inflammation, meibum thickening, gland channel terminal duct obstruction, and glandular atrophy associated with MGD lead to altered meibomian gland secretions and tear film instability. A treatment approach for MGD by restoring meibomian gland function and natural flow of meibum has potential therapeutic relief for DED signs and symptoms.6
Three commonly used products for DED are available in the United States, targeting the inflammatory aspects of the disease: Restasis®, Xiidra®, CEQUA™. However, these currently approved pharmacologic treatments for DED are associated with delayed onset of action for both DED sign and symptom improvements. Clinical experiences reported at least 6 weeks for improved signs and 12 weeks for symptom relief with Xiidra®, 21 weeks for improved signs with Restasis®, and 12 weeks for improved signs with CEQUA™.7–9 It is evident that data for DED symptom improvements with current therapies are limited, and there exists a therapeutic need for faster onset treatments.
Meanwhile, MGD treatment mainly begins from eyelid warming, eyelid massaging, and progresses to the uses of topical lubricants, topical and systemic antibiotics with anti-inflammatory properties, and topical steroids. A number of devices have been developed for eyelid warming to optimize efficacy and compliance with faster onset of action such as LipiFlow and TearCare systems. The LipiFlow Thermal Pulsation System (Johnson & Johnson Vision, Milpitas, CA, USA), indicated for adult patients with MGD, can safely heat the palpebral surfaces of the upper and lower eyelids while applying graded pulsatile pressure to the outer surface. An open label, randomized, crossover study in 139 subjects demonstrated that a single 12-minute treatment with LipiFlow maintained improvement in both signs and symptoms over the 4-week study.10 The TearCare System (Sight Sciences, Menlo Park, CA, USA), a class II exempt device listed with the FDA for the application of warm compress to eyelids, delivers controlled, precise heat to the tarsal plates and underlying meibomian glands for 15 minutes followed by manual mechanical meibomian gland expression. The TearCare system allows subjects to blink naturally thanks to the flexible SmartLid devices applied to the upper and lower eyelid surfaces. The SmartHub component of the TearCare system provided adjustable temperature ranging from 41 to 45 °C for subject comfort.
Previous study in 2017 on 24 subjects demonstrated that the TearCare System significantly improved tear film break-up time (TFBUT), meibomian secretion, corneal and conjunctival staining scores, and dry eye symptom questionnaires at 1-month follow-up and maintained these outcomes out to 6 months11 and 12 months.12 The outcomes were further validated in multicenter studies concluded in 201813 and 2019.14 This report aims to highlight DED symptom improvement efficacy results of TearCare from an unpublished clinical report of OLYMPIA study on 235 subjects and its post-hoc subgroup analysis.
Materials and Methods
Study Design
OLYMPIA study was a randomized, single-masked, multicenter, non-inferiority, post-market study to evaluate the safety and effectiveness of a single TearCare treatment compared to a single LipiFlow treatment for improving signs and symptoms of DED associated with MGD. The study was approved by the institutional review board (Aspire IRB) and conducted in accordance with the Declaration of Helsinki. After explanation of the study was given, subjects with DED who were ≥22 years of age and provided informed consent were randomized 1:1 to receive one in-office treatment of either the TearCare system or LipiFlow Thermal Pulsation on study day 0. Subjects could not be masked due to apparent treatment design. Subjects must have Ocular Surface Disease Index (OSDI) score of 23–79, TFBUT of ≤7 seconds in both eyes, and best corrected visual acuity of 20/100 or better in both eyes. At least 15 glands in each lower eyelid should be expressible, and total Meibomian Gland Secretion Score (MGSS) must be ≤12 in each eye.
Study staff performing endpoint assessments were masked to the subject’s treatment arm. The study comprised 4 visits: Baseline, Treatment (if not at Baseline Visit), Day 1, Week 2, and Month 1. Within the 1-month follow-up, subjects refrained from using any dry eye drops, lubricants, or other type of dry eye treatment (eg warm compresses, prescription medication, etc.). If they required “rescue therapy” for relieve symptoms, they could use the same type of drops or lubricants they were using prior to the study and recorded any use in the Dry Eye Lubricant/Drop Log.
In this study, DED symptoms were assessed as secondary endpoints using the Eye Dryness (ED) score, mean change from baseline in OSDI score, and mean change from baseline in Symptom Assessment in Dry Eye (SANDE) scores.
Symptom Endpoint Assessments
OSDI
The OSDI questionnaire is a valid and reliable instrument for measuring dry eye symptoms (normal, mild to moderate, and severe) and its effect on vision-related functions. The investigator asked subjects 12 questions which were categorized into three subsections: Section A (ocular symptoms), Section B (vision-related functions), and Section C (environmental triggers). The OSDI was assessed on a scale of 0 to 100 with higher score representing greater disability. The 5-unit scale for responses to the OSDI was given by the following: 0 = None of the time, 1 = Some of the time, 2 = Half of the time, 3 = Most of the time, and 4 = All of the time. The total OSDI score was calculated by the following:
Note that the number of questions answered in the denominator should exclude those questions with a response of “N/A.”
Based on the calculated OSDI score, the severity of the subject’s dry eye symptoms was classified as Normal (0–12), Mild (13–22), Moderate (23–32), and Severe (33 or higher).
SANDE
The SANDE questionnaire is an instrument containing two questions to assess frequency and severity of DED symptoms. It is useful to detect changes in symptoms over time. Each item was assessed on a 100 mm Visual Analog Scale (VAS) ranging from Never to All the time for frequency assessment and from Very comfortable to Very severe for severity assessment. Subjects were asked to respond by placing a vertical line across the horizontal line of the VAS. SANDE Score (ranging from 0 to 100) is the distance (in mm) between the left end of the scale and the subject’s response.
Eye Dryness Score
The Eye Dryness (ED) Score measures the subject’s level of discomfort related to eye dryness ranging from No Discomfort to Maximal Discomfort. Subjects were asked to respond by placing a vertical line across the horizontal line of the VAS. The ED Score (ranging from 0 to 100) is the distance (in mm) between the left end of the scale and the subject’s response.
Meibomian Gland Score
The Meibomian Gland Secretion Score (MGSS) is an assessment of the quality of the secretion produced by the meibomian glands in the lower eyelids. This assessment was performed by the masked assessor.
Quality of secretions of 5 central glands in the lateral, central and temporal thirds of the lower eyelids; a total of 15 glands per eye was graded using the method described by Korb and Blackie.15 The part of the instrument’s contact surface was placed onto the skin immediately inferior to the eyelashes of the lower eyelid so that the long dimension is parallel to the eyelid margin. Once full contact was achieved between the instrument and the skin immediately below the lash line of the lower lid, the shaft of the instrument was rotated downward approximately 15 to 45 degrees. Then, the shaft was depressed midway (~3 mm) and the lower eyelid margin was rolled slightly outward. The instrument was held in place over each third of the lid for a minimum of 10 and a maximum of 15 seconds while grading the quality of secretion of the 5 glands in the center of the instrument (15 glands total per eye) as per the following scale described by Lane et al:16
- 0= nothing
- 1= toothpaste
- 2= cloudy
- 3= clear
From this assessment the total MGSS was computed as: Sum of the grade (0–3) for each of the 15 glands. Range for this score is 0–45.
Statistical Analyses
The analysis of symptoms assessments was conducted on all randomized subjects and two post-hoc subgroups stratified by median baseline MGSS reflecting the severity of meibum and meibomian gland dysfunction at baseline: one group with MGSS above the median (≥7) representing less severe dysfunction and one with MGSS below the median (<7) indicating more severe dysfunction. The DED symptom endpoints were measured on per-subject basis and analyzed via a paired t-test and baseline-adjusted analysis of covariance (ANCOVA) to evaluate change from baseline to 1-month post-treatment and difference between two treatment groups.
Results
Baseline Characteristics
This study was conducted at 10 multispecialty eye care facilities across the United States between March 2019 and February 2020. The analysis population comprised 235 subjects with 115 subjects in the TearCare group and 120 subjects in the LipiFlow group. The distribution of gender and race was similar across treatment groups. There were no distinguished differences between the study groups as well as the subgroups stratified by MGSS (Table 1). For DED baseline symptoms, the TearCare group and LipiFlow group had similar baseline mean OSDI score (51.7 ± 14.79 and 51.7 ± 15.27, respectively), mean total SANDE score (70.6 ± 16.78 and 77.2 ± 15.82, respectively), and mean ED score (69.9 ± 20.15 and 71.9 ± 18.48, respectively).
 |
Table 1 Demographics of TearCare and LipiFLow Groups |
The subgroup population including subjects with more severe disease determined by baseline MGSS <7 comprised 56 subjects in the TearCare group and 65 subjects in the LipiFlow group. The subgroup population of subjects with less severe dry eye disease (baseline MGSS ≥7) included 59 subjects in the TearCare group and 55 subjects in the LipiFlow group.
DED Symptom Improvements
Raw results data of groups and subgroups on the total scores of each questionnaire are presented in Table 2.
 |
Table 2 Differences in the Improvement in Symptoms at 1 Month from Baseline in TearCare and LipiFlow Groups and Less Severe and More Severe Disease Subgroups |
OSDI
In all randomized subjects, both TearCare and LipiFlow procedures demonstrated statistically significant improvements compared to baseline in MGD-related symptoms of DED as measured by OSDI 1-month post-procedure (p < 0.0001). The reduction from baseline OSDI score as expressed by least-squared (LS) mean and standard errors (SE) was 29.1 ± 1.62 for subjects treated with TearCare (95% CI = [25.9, 32.2]) and 24.4 ± 1.59 for subjects treated with LipiFlow (95% CI = [21.3, 27.6]). The reduction from baseline total OSDI score achieved with TearCare was significantly greater than the reduction achieved with LipiFlow (LS mean difference = 4.6 ± 2.27, 95% CI = [0.1, 9.1], pANCOVA = 0.0433) (Figure 1). Analysis on each OSDI subsection revealed similar significant results favoring TearCare treatment in improving OSDI Section A (ocular symptoms). TearCare reduced OSDI Section A score by 5.1 ± 0.31 (95% CI = [4.5, 5.7]), and LipiFlow reduced OSDI Section score by 4.1 ± 0.31 (95% CI = [3.4, 4.7]. The treatment difference between the TearCare group and LipiFlow group was 1.0 ± 0.44 (95% CI = [0.2, 1.9], pANCOVA = 0.0201) (Figure 2A).
In the subgroup analysis, TearCare demonstrated greater treatment effect in total OSDI score at 1 month with statistical significance in subjects experiencing more severe meibum and meibomian gland dysfunction at baseline (MGSS <7) in comparison to LipiFlow (pANCOVA = 0.0160) (Figure 1). At 1-month follow-up visit, the reduction from baseline in total OSDI score was 30.4 ± 2.53 (95% CI = [25.4, 35.4]) for subjects treated with TearCare and 21.9 ± 2.37 (95% CI = [17.2, 26.6]) for subjects treated with LipiFlow. For the same subgroup, TearCare also performed significantly better than LipiFlow in improving OSDI Section B score that assessed visual functions specifically (LS mean difference = 1.5 ± 0.65, pANCOVA = 0.0206). The reduction from baseline in OSDI Section B score was 5.1 ± 0.48 (95% CI = [4.2, 6.1]) for the TearCare group and 3.6 ± 0.45 (95% CI = [2.7, 4.5]) for the LipiFlow group (Figure 2B). Specifically, in comparison to LipiFlow, TearCare provided better improvements in the following functions: driving at night (LS mean difference = 0.5 ± 0.23, 95% CI = [0.0, 0.9], pANCOVA = 0.0458), working with a computer or bank machine (LS mean difference = 0.4 ± 0.21, 95% CI = [0.0, 0.8], pANCOVA = 0.0333), and watching TV (LS mean difference = 0.4 ± 0.16, 95% CI = [0.1, 0.8], pANCOVA = 0.0076) (Figure 3).
In the subjects with less severe MGSS ≥7, TearCare and LipiFlow were equivalent in significantly improving total OSDI score and each OSDI subsection’s score from baseline (p < 0.0001).
SANDE
In all randomized subjects, TearCare and LipiFlow were equivalent in improving the total SANDE score as well as separate SANDE score for severity and frequency from baseline (p < 0.0001). Similar results were obtained for the subgroup of subjects with less severe disease (MGSS ≥7).
Analysis in subgroup of subjects with more severe disease (MGSS <7) showed notable results. These subjects achieved higher numerical reduction of 48.4 ± 3.55 (95% CI = [41.4, 55.4]) in total SANDE score with TearCare than with LipiFlow (39.3 ± 3.31 and 95% CI = [32.8, 45.9]) although the difference was not statistically significant (pANCOVA = 0.0673). However, significantly better improvement was observed with TearCare in comparison to LipiFlow in this subgroup when SANDE frequency score was evaluated separately. The mean reduction from baseline in SANDE frequency score was 51.9 ± 3.70 (95% CI = [44.5, 59.2]) for the TearCare group and 41.5 ± 3.45 (95% CI = [34.7, 48.3]) for the LipiFlow group with LS mean difference = 10.4 ± 5.13 and pANCOVA = 0.0455 (Figure 4). In terms of SANDE severity score, although statistically significant difference was not established between the two treatment groups (pANCOVA = 0.1028), these subjects trended toward better improvement using TearCare treatment with numerical mean reduction of 44.6 ± 3.60 (95% CI = [37.5, 51.8]) compared to 36.5 ± 3.36 (95% CI = [29.8, 43.2]) with LipiFlow treatment.
Eye Dryness Score
Significant improvements from baseline in ED scores (p < 0.0001) were demonstrated for all randomized subjects, subgroup with less severe disease (MGSS ≥7), and subgroup with more severe disease (MGSS <7). TearCare and LipiFlow showed no statistically significant difference between two groups. In the subgroup with more severe disease (MGSS <7), although the difference between two treatment groups was not significant, the treatment effect seemed to trend in favor of TearCare with LS mean difference of 8.9 ± 4.87 (95% CI = [0.8, 18.5] and p-value of 0.0713.
Discussion
The results presented in this report showed that a single treatment of LipiFlow and TearCare can significantly improve DED symptoms in adult patients with MGD with added benefits from TearCare in certain populations. Subjects treated with TearCare achieved similar reduction compared to the active-control, LipiFlow, in total OSDI score, total SANDE score, and ED score for all randomized subjects. Post-hoc subgroup analysis in the less severe group and more severe group based on median MGSS provided further understanding of the treatment effect favoring TearCare for DED symptom improvement. Subjects with more severe meibomian gland dysfunction achieved greater significant improvements in total OSDI, OSDI subsection focusing on quality of vision, and SANDE frequency score with TearCare in comparison to LipiFlow. Results of other assessments including OSDI subsections on ocular symptoms and environmental triggers, total SANDE score, SANDE severity score, and ED score also trended toward more reduction with TearCare although statistical significance was not attained. These results suggested that TearCare not only is beneficial for all patients regardless of MGD severity but also can offer additional meaningful DED symptom relief to the subjects with more severe blockages in the meibomian gland. For subjects with less severe blockage in the meibomian gland, both LipiFlow and TearCare presented as effective treatments since the two devices significantly reduced the total OSDI score, OSDI subsection scores, total SANDE score, and SANDE score for severity and frequency from baseline.
Maintaining a healthy lipid layer plays a critical role in preventing evaporative DED.17,18 The TearCare system delivers precise heat to the tarsal plates of upper and lower eyelids and meibomian glands with the SmartHub controller, and facilitates the manual expression of subjects’ meibomian glands with its Clearance Assistant units to enhance the natural flow of lipids. LipiFlow system uses a Console and a single-use sterile device called Activator to apply heat to the palpebral surfaces of the upper and lower eyelids directly over the meibomian glands after a local anesthetic drop is instilled. Simultaneously, graded pulsatile pressure is applied to the outer eyelid surfaces, thereby expressing the meibomian glands in closed eyes during heating. The TearCare system delivers to the outer eyelid surfaces and thus allows normal blinking with its SmartLid units and does not use an initial anesthetic drop. Both systems utilize heat and pressure applications as the mechanism of action; however, all applications are embedded in one system with LipiFlow while with TearCare, there is an additional unit used to further express the meibomian gland manually by clinicians. The mechanisms described in both devices helps tear film to recover as the gland blockage is cleared. Consequently, DED improves as demonstrated by positive outcomes in DED symptom assessments presented here and also in DED sign assessments such as TFBUT, fluorescein staining, etc. in earlier studies.11,12
This study provided reliable data in terms of symptom evaluations with different assessment tools. Previous efficacy data on symptom improvements were limited as the majority of currently available DED treatments such as Restasis®, Xiidra®, and CEQUA™ were mostly approved based on sign efficacy endpoints.7–9 Here, symptom improvements are highlighted across multiple reliable assessment measurements. Consistent results supporting TearCare efficacy were observed in not only ED score, overall SANDE score, and OSDI but also in each subsection of the SANDE questionnaire (frequency and severity) and OSDI questionnaire (ocular symptoms, vision-related functions, environmental triggers) for both the analysis in all randomized subjects and in subgroups. Utilizing multiple questionnaires offers additional benefits because besides symptom assessment, and some questionnaires such as OSDI also incorporate quality of life measures that can capture patient perspectives on treatment effectiveness more comprehensively. Such questionnaires are highly encouraged as the US Federal Food and Drug Administration promoted the use of patient-reported outcomes to support clinical trials and product submissions. Based on this report, TearCare successfully provided clinically meaningful improvements in both dry eye symptoms and vision-related functions to have positive impact on patients’ lives.
In addition, delayed onset of action of several months associated with currently available anti-inflammatory treatments is the most prevalent factor leading to low treatment satisfaction in both physicians and patients.19,20 A study in 2020 reported that only 10% and 48% of physicians were satisfied with the onset of action for cyclosporine ophthalmic emulsion 0.05% and lifitegrast 5% ophthalmic solution, respectively.18 Similarly, patients also perceived low satisfaction with onset of action for current treatments: only 51% of patients on cyclosporine ophthalmic emulsion and 63% of patients on lifitegrast ophthalmic solution reported being satisfied.19 Low level of satisfaction can negatively affect overall treatment compliance and effectiveness. Therefore, DED treatment options with faster onset are needed. In this study, TearCare demonstrated remarkable onset of action for symptom relief at 1-month follow-up. This result aligns well with previously reported efficacy studies in TearCare. Prior studies proved TearCare to be more effective than warm compress in improving both DED signs and symptoms at 1-month timepoint and maintaining such effects for up to 6 and 12 months.11,12 An exploratory study in 2020 described significant improvements in OSDI achieved with TearCare as early as at 1-week follow-up visit and continued to improve further at 1-month visit.13 Early onset of action is a notable advantage that sets TearCare apart as an effective treatment option for DED. One limitation of this current study is the short follow-up duration of 1 month.
In summary, both LipiFlow and the TearCare system consistently improved DED symptoms assessed by total OSDI, SANDE, and Eye Dryness score from baseline. Furthermore, the subject population with severe gland obstructions and in need for a better gland clearance, achieved greater meaningful symptom relief with TearCare in comparison to LipiFlow. In subjects with more severe meibomian gland dysfunction, TearCare provided better improvements in overall symptom relief, vision-related functions, and DED symptom frequency expressed by total OSDI score, OSDI Section B score, and SANDE frequency score. With the benefits of fast action onset and excellent efficacy in symptomatic relief, the TearCare system presents as a compelling option for DED treatment among currently available treatments.
Abbreviations
OSDI, Ocular Surface Disease Index; TBUT, Tear-film Break-Up Time; MGSS, Meibomian Gland Secretion Score; MGD, Meibomian Gland Dysfunction; CI, Confidence Intervals; DED, Dry Eye Disease; SANDE, Symptom Assessment In Dry Eye.
Data Sharing Statement
The authors do not intend to share participant-level data.
Acknowledgments
The authors would like to acknowledge Garrick Wallstrom, PhD at Statistics & Data Corporation for statistical analyses support.
Funding
This study was funded by Sight Sciences, Inc.
Disclosure
Kavita Dhamdhere is an employee of Sight Sciences, Inc. Jennifer Loh reports grants from Sight Sciences, during the conduct of the study; consultant and/or speaker for Johnson and Johnson, Sun Ophthalmics, Allergan, Kala Pharmaceuticals, and Imprimis, outside the submitted work. Vance Thompson and David Wirta reports personal fees/grants for research from TearClear, during the conduct of the study. The authors report no other conflicts of interest in this work.
References
1. Fiscella RG. Understanding dry eye disease: a managed care perspective. Am J Manag Care. 2011;17(Suppl 16):S432–439.
2. Messmer EM. The pathophysiology, diagnosis, and treatment of dry eye disease. Dtsch Arztebl Int. 2015;112(5):
3. Pflugfelder SC. Prevalence, burden, and pharmacoeconomics of dry eye disease. Am J Manag Care. 2008;14(3 Suppl):S102–106.
4. Craig JP, Nichols KK, Akpek EK, et al. TFOS DEWS II definition and classification report. Ocul Surf. 2017;15(3):276–283. doi:10.1016/j.jtos.2017.05.008
5. Schaumberg DA, Nichols JJ, Papas EB, Tong L, Uchino M, Nichols KK. The international workshop on meibomian gland dysfunction: report of the subcommittee on the epidemiology of, and associated risk factors for, MGD. Invest Ophthalmol Vis Sci. 2011;52(4):1994–2005. doi:10.1167/iovs.10-6997e
6. Geerling G, Baudouin C, Aragona P, et al. Emerging strategies for the diagnosis and treatment of meibomian gland dysfunction: proceedings of the OCEAN group meeting. Ocul Surf. 2017;15(2):179–192. doi:10.1016/j.jtos.2017.01.006
7. Mah F, Milner M, Yiu S, Donnenfeld E, Conway TM, Hollander DA. PERSIST: physician’s evaluation of Restasis((R)) satisfaction in second trial of topical cyclosporine ophthalmic emulsion 0.05% for dry eye: a retrospective review. Clin Ophthalmol. 2012;6:1971–1976. doi:10.2147/OPTH.S30261
8. Tauber J, Karpecki P, Latkany R, et al. Lifitegrast ophthalmic solution 5.0% versus placebo for treatment of dry eye disease: results of the randomized Phase III OPUS-2 study. Ophthalmology. 2015;122(12):2423–2431. doi:10.1016/j.ophtha.2015.08.001
9. CEQUA. CEQUA (cyclosporine ophthalmic solution) 0.9% [drug label]. Sun Pharmaceuticals; 2018.
10. Lane SS, DuBiner HB, Epstein RJ, et al. A new system, the LipiFlow, for the treatment of meibomian gland dysfunction. Cornea. 2012;31(4):396–404. doi:10.1097/ICO.0b013e318239aaea
11. Badawi D. A novel system, TearCare((R)), for the treatment of the signs and symptoms of dry eye disease. Clin Ophthalmol. 2018;12:683–694. doi:10.2147/OPTH.S160403
12. Badawi D. TearCare((R)) system extension study: evaluation of the safety, effectiveness, and durability through 12 months of a second TearCare((R)) treatment on subjects with dry eye disease. Clin Ophthalmol. 2019;13:189–198. doi:10.2147/OPTH.S191588
13. Karpecki P, Wirta D, Osmanovic S, Dhamdhere K. A prospective, post-market, multicenter trial (CHEETAH) suggested TearCare((R)) system as a safe and effective blink-assisted eyelid device for the treatment of dry eye disease. Clin Ophthalmol. 2020;14:4551–4559. doi:10.2147/OPTH.S285953
14. Gupta PK, Holland EJ, Hovanesian J, et al. TearCare for the treatment of meibomian gland dysfunction in adult patients with dry eye disease: a masked randomized controlled trial. Cornea. 2021. doi:10.1097/ICO.0000000000002837
15. Korb DR, Blackie CA. Meibomian gland diagnostic expressibility: correlation with dry eye symptoms and gland location. Cornea. 2008;27(10):1142–1147. doi:10.1097/ICO.0b013e3181814cff
16. Lane SS, DuBiner H, Epstein RJ, et al. A new system, the LipiFlow, for the treatment of meibomian gland dysfunction. Cornea. 2012;31(4):396–404.
17. Bron AJ, de Paiva CS, Chauhan SK, et al. TFOS DEWS II pathophysiology report. Ocul Surf. 2017;15(3):438–510. doi:10.1016/j.jtos.2017.05.011
18. bron AJ, Tiffany JM. The contribution of meibomian disease to dry eye. Ocul Surf. 2004;2(2):149–165. doi:10.1016/S1542-0124(12)70150-7
19. White DE, Zhao Y, Jayapalan H, Machiraju P, Periyasamy R, Ogundele A. Physician satisfaction with anti-inflammatory topical medications for the treatment of dry eye disease. Clin Ophthalmol. 2020;14:931–938. doi:10.2147/OPTH.S237832
20. White DE, Zhao Y, Jayapalan H, Machiraju P, Periyasamy R, Ogundele A. Treatment satisfaction among patients using anti-inflammatory topical medications for dry eye disease. Clin Ophthalmol. 2020;14:875–883. doi:10.2147/OPTH.S233194
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.