Back to Journals » Cancer Management and Research » Volume 13
A Comparison of Different Schemes of Neoadjuvant Chemotherapy Followed by Concurrent Chemotherapy and Radiotherapy for Locally Advanced Cervical Cancer: A Retrospective Study
Authors Tian X, Yang F, Li F, Ran L, Chang J, Li J, Hong W, Shan L, Du Y, Hu L, Mei F, He M, Li Y, Wang H, Zuo K, Zhou B, Chen S, Mao W
Received 14 July 2021
Accepted for publication 21 October 2021
Published 3 November 2021 Volume 2021:13 Pages 8307—8316
DOI https://doi.org/10.2147/CMAR.S328309
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Chien-Feng Li
Xue Tian,1,* Feiyue Yang,2,* Fenghu Li,1 Li Ran,1 Jianying Chang,1 Jiehui Li,1 Wei Hong,3 Lang Shan,1 Yanjun Du,1 Lili Hu,1 Fan Mei,1 Mingyuan He,1 Yongxia Li,1 Heran Wang,1 Kai Zuo,1 Bo Zhou,4 Shuying Chen,1 Wanli Mao1
1Department of Oncology, The Affiliated Hospital of Guizhou Medical University, The Affiliated Cancer Hospital of Guizhou Medical University, Guiyang, 550004, People’s Republic of China; 2Department of Gynecologic Oncology, Guizhou Provincial People’s Hospital, Guiyang, 550004, People’s Republic of China; 3Department of Radiotherapy, The Affiliated Hospital of Guizhou Medical University, The Affiliated Cancer Hospital of Guizhou Medical University, Guiyang, 550004, People’s Republic of China; 4Surgical Department of Gynecological Oncology, The Affiliated Hospital of Guizhou Medical University, The Affiliated Cancer Hospital of Guizhou Medical University, Guiyang, 550004, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Fenghu Li; Li Ran Email [email protected]; [email protected]
Purpose: To examine the clinical significance of unoperated cervical cancer patients treated with different neoadjuvant chemotherapy (NACT) schemes followed by concurrent chemotherapy and radiotherapy (CCRT).
Methods: This retrospective analysis included women with locally advanced cervical cancer treated with NACT-CCRT between September 2011 and September 2014. Neoadjuvant chemotherapy included paclitaxel plus cisplatin (TP group; 62 patients) or paclitaxel plus loplatin (TL group; 58 patients), which were administered three weekly, and cisplatin or loplatin, which were administered weekly for synchronous chemotherapy. External beam radiation therapy (50.4– 56.35 Gy/28 f, 180– 215 cGy/f, 5 f/w) was followed by intracavitary brachytherapy (5 Gy per fraction, mostly 5 fractions, Ir192 based).
Results: One hundred twenty women were included in the analysis. The complete/partial response rate was 99.2% after treatment. The one-year, three-year, and five-year survival rates were 99.2%, 82.5%, and 70.8%, respectively. In the TP and TL groups, the three-year and five-year survival rates were 85.5% vs 77.6% and 75.8% vs 65.5%, respectively, with no significant difference. The 5-year overall survival (OS) rates between patients with stage IIB and stage IIIB disease were not significantly different (69.2% vs 64.7%). In the TP group, grade 3 or 4 digestive reactions were more frequent than those in the TL group. Leukopenia, neutropenia, and thrombocytopenia were more common in the TL group. No significant difference was found in anemia, radiation enteritis, radiation proctitis, or radiation cystitis between the groups.
Conclusion: Lobaplatin may be used as an alternative drug for patients with severe digestive system reactions or contraindications to cisplatin, but hematological toxicity must be considered, particularly in dose-intensive schemes. Neoadjuvant chemotherapy followed by concurrent chemotherapy and radiotherapy (NACT-CCRT) warrants further prospective study in cervical cancer patients with a wide range of tumor invasion (eg, mass size ≥ 5 cm or stage IIIB).
Keywords: cervical cancer, neoadjuvant chemotherapy, survival benefit, side effects
Introduction
Cervical cancer is the most common gynecologic malignancy in China.1,2 Women in some areas do not consider cervical cancer screening; thus, most Chinese patients are diagnosed at advanced stages.3 Concurrent chemoradiotherapy (CCRT) is the recommended initial therapy for locally advanced cervical cancer (patients with stage IB2-IVA) according to the National Comprehensive Cancer Network (NCCN) Clinical Practice Guidelines in Oncology. However, not all hospitals are equipped with radiation equipment; thus, neoadjuvant chemotherapy (NACT) followed by radical surgery or NACT followed by CCRT (received at a qualified superior hospital) may be used as an alternative.
Despite the high incidence of cervical cancer in China, hospitals have limited capacity. Some patients are hospitalized with obvious pain or vaginal bleeding. These patients may need to wait a long time for synchronous radiotherapy and chemotherapy. Previous studies have reported that a delay in treatment greater than 64 days is associated with a 5-year mortality risk.4 Several randomized trials have shown improved cure rates in the treatment of cervical cancer by adding cisplatin to local radiotherapy. In February 1999, the National Cancer Institute (NCI) made a clinical announcement concerning the role of cisplatin.5 However, some patients cannot tolerate the severe gastrointestinal toxicity and nephrotoxicity of cisplatin therapy, while others have contraindications due to impaired renal function.6–8 Thus, second- and third-generation platinum analogs, such as lobaplatin, with reduced gastrointestinal toxicity have been proposed.9 Lobaplatin, a diastereometric mixture of platinum (II) complexes containing a 1,2-diamino-methylcyclobutaneplatinum (II)-lactate, is a representative third-generation platinum drug that shows incomplete cross-resistance to cisplatin. It interferes with the progression of the tumor cell cycle by obstructing DNA replication and transcription.10 Compared with cisplatin, lobaplatin is less toxic, more soluble and stable in water. The common side effects of lobaplatin are neutrophil suppression, thrombocytopenia, anemia and digestive tract toxicity. Many clinical trials suggest the effectiveness of lobaplatin in treating various cancers, including breast cancer and small cell lung cancers.11,12 In the present study, we retrospectively analyzed and compared the toxicity, efficacy and long-term survival of lobaplatin-based combined chemotherapy with cisplatin-based combined chemotherapy in cervical cancer patients from two medical institutions.
Materials and Methods
Patients
This retrospective analysis of a prospectively collected database included 120 women with bulky stage I (IB2) or locally advanced cervical cancer (FIGO, IIB–IVA).13 Thirteen patients (aged 18 to 75 years) were admitted to the Affiliated Cancer Hospital of Guizhou Medical University and the Guizhou Provincial People’s Hospital between January 2012 and June 2014. Cancer (squamous cell carcinoma or adenocarcinoma of the cervix) was confirmed by biopsy. The exclusion criteria were as follows: contraindications to radiation or chemotherapy; previous oncologic treatment such as surgery, radiation or chemotherapy; an absolute neutrophil count <1500/µL; a platelet count <100,000/µL; a hemoglobin level <80 g/L; an Eastern Cooperative Oncology Group (ECOG) performance status >2; significant renal, hepatic or hematological impairment; severe medical complications. Patients were treated with NACT comprising two to four cycles (mostly two cycles) of three weekly paclitaxel plus cisplatin (TP group) or paclitaxel plus lobaplatin (TL group) followed by CCRT. Their detailed records were available for analysis.
Treatment
NACT was administered as an injection of paclitaxel 135–175 mg/m2 (d1), cisplatin 60–80 mg/m2 (d2), or lobaplatin 30 mg/m2 (d2). Chemotherapy was repeated after 3 weeks after checking the complete blood count, liver function tests, and renal function before every cycle of chemotherapy. Two to four cycles of NACT were administered before evaluating the therapeutic effect using Response Evaluation Criteria in Solid Tumors (RECIST)14 by imaging examination and colposcopy. Common Terminology Criteria for Adverse Events (CTCAE)15 toxicity criteria were used to document hematological toxicities.
RT was administered approximately three weeks after completing the last cycle of neoadjuvant chemotherapy. The chemotherapy regimen of CCRT treatment was cisplatin 40 mg/m2 or lobaplatin 30 mg/m2 weekly according to NACT. External beam radiation therapy (EBRT) was administered using a cobalt 60 teletherapy machine. Regarding radiotherapy technology, 26 patients received 3-dimensional conformal radiation therapy (3D-CRT), 91 patients received intensity modulated radiotherapy (IMRT), and 3 patients received image guided radiotherapy (IGRT). Regarding cases with large masses, lymph node metastasis in the drainage area or eccentric growth of the mass before radiotherapy, the dose coverage of brachytherapy was not sufficient; thus, the radiotherapy physician would boost the dose of the tumor mass or lymph node metastasis locally accordingly. The gross tumor volume (GTV) dose of 50.4–56.35 Gy and clinical target volume (CTV) dose of 50.4 Gy in 28 fractions in 6 weeks were administered at a dose of 180–215 centigray per fraction daily, 5 days a week. During EBRT or after a gap of 1 to 2 weeks, the patients were subjected to intracavitary brachytherapy (5 Gy per fraction, mostly 5 fractions, Ir192 based). Approximately 1/5 of the patients received 1–3 cycles of chemotherapy using paclitaxel plus cisplatin or lobaplatin after CCRT. The RTOG toxicity criterion16 was used for radiation-induced toxicities.
Follow Up
Patients were followed up at an outpatient clinic from September 2011 to February 2019 every 3 months during the first two years, every 6 months for 3–5 years, and every year after 5 years. OS was defined as the time from diagnosis to death or the last follow-up. Patients with progressive/recurrent disease were offered palliative chemotherapy or symptomatic treatment depending on the ECOG performance status.
Statistical Analysis
The statistical software SPSS 24.0 was used for statistical analysis.17 If the measurement data conformed to a normal distribution, they were displayed as means ± standard deviation; if the data did not conform to a normal distribution, they were displayed as median values (range). Piece data were displayed as n (%). t-test was used to determine the significance of the measurement data between the groups. Chi-squared/Fisher’s exact test was used to determine the significance of the study parameters on a categorical scale between two groups. The Kaplan–Meier method was used to calculate survival curves. The survival differences between the groups were compared by the log rank test. A p value < 0.05 was considered statistically significant.
Results
Patient Demographics
Among 154 patients who met the inclusion criteria, 34 patients were excluded because they did not receive complete treatment or a complete review because of factors other than toxicity and side effects or were lost to follow-up. Finally, 120 patients were included in the study. They were from the Affiliated Cancer Hospital of Guizhou Medical University of Guizhou Provincial People’s Hospital. The baseline characteristics are shown in Table 1. The median age was 50 (29–72) years, and 96% had squamous cell cancers. Most of the cases were either FIGO stage IIB (55%) or IIIB (28.3%) (Table 1). No significant difference was found between the TP group (62 patients) and TL group (58 patients) or between the <5 cm group (50 patients) and the ≥5 cm group (70 patients) at baseline.
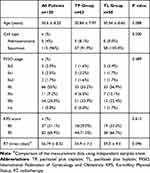 |
Table 1 Baseline Characteristics |
Efficacy
The proportion of those with a complete response (CR)/partial response (PR) was 81.7% at the end of NACT and 99.2% after completing CRT. A complete response (CR) was observed in 9 women after NACT and in 63 patients 12 weeks after completing CRT. No patient progressed after treatment. Chi-squared/Fisher’s exact test was used to determine the significance of the study parameters on a categorical scale between two groups.
We also performed subgroup analysis based on the chemotherapy regimen (Table 2). The RRs (response rates, CR+PR) after neoadjuvant chemotherapy in the TP and TL groups were 82.2% and 81.0%, respectively, with no significant difference (χ2=0.030 p=0.403). In the paclitaxel plus cisplatin (TP) group and paclitaxel plus lobaplatin (TL) group, the RRs after all treatments were 98.4% and 100%, respectively, with no significant difference (χ2=0.000; p=0.991) (Table 2). The response rates (CR+PR) after all treatments in the ≥ 5 cm and <5 cm groups were 98.6% and 100%, respectively, with no significant difference (χ2=0.174; p=0.677).
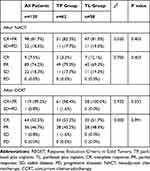 |
Table 2 Tumor Response Using RECIST 1.0 Criteria After Neoadjuvant Chemotherapy and at the End of Treatment |
Survival Analysis
The one-year, three-year, and five-year survival rates were 99.2%, 82.5%, and 70.8%, respectively. The Log rank test was used to compare the two groups in survival analysis. In the paclitaxel plus cisplatin (TP) and paclitaxel plus lobaplatin (TL) groups, the three-year and five-year survival rates were 85.5% and 77.6% (χ2=1.177; p=0.278) and 75.8% and 65.5% (χ2=1.424, p=0.233), respectively, with no significant difference. Similarly, no significant difference was found in overall survival between the two groups (p=0.426) (Figure 1, Table 3).
 |
Table 3 Three- and Five-Year Survival Rates of Different Stages, Chemotherapy Regimens, or Tumor Sizes and Efficacy After All Treatments |
 |
Figure 1 Kaplan–Meier plots for OS (overall survival) between patients with different chemotherapy regimens (A), tumor stages (B), and tumor masses (C). |
In the ≥ 5 cm and <5 cm groups, the five-year survival rates were 84.0% and 61.4% (χ2=6.906; p=0.009), respectively, and the difference was significant. However, no significant difference was found in the overall survival rates between the groups (χ2=1.795; p=0.180) (Figure 1, Table 3).
Subgroup analysis showed that the OS between stage IIB and stage IIIB was not significantly different (p=0.521). The 3-year and 5-year survival rates were 83.1% vs 70.6% (χ2=2.730; p=0.098) and 69.2% vs 64.7% (χ2=0.597; p=0.440), respectively (Figure 1, Table 3). After treatment, 63 patients achieved CR, and 56 patients reached PR, but no significant difference was found in the 3-year survival rate (p=0.098), 5-year survival rate (p=0.682), or overall survival (p=0.149).
Adverse Events
In the TP group, grade 3 or 4 digestive reactions were more frequent than those in the TL group (54.3% vs 1.8%, respectively; p=0.000). Leukopenia, neutropenia and thrombocytopenia were more common in the TL group than in the TP group (leukopenia: 55.2% vs 30.6%, respectively, p=0.007; neutropenia: 48.3% vs 30.6%, respectively, p=0.048; thrombocytopenia: 44.8% vs 1.6%, respectively, p=0.000). No significant difference was found in anemia, radiation enteritis, radiation proctitis, or radiation cystitis between the TP and TL groups (p>0.05).
No grade 3/4 toxicity was observed in radiation proctitis, radiation cystitis, or radiation enteritis during treatment (NACT/CCRT) or follow-up. The incidence rates of grade 3 or 4 toxicity were as follows: digestive tract reaction, 26.5%; leukopenia, 42.5%; neutropenia, 39.2%; thrombocytopenia, 22.5%; hemoglobin, 9.2% (Table 4).
 |
Table 4 Grade 3 or 4 Adverse Events (Classified According to NCI CTCAE V4.0 and RTOG Criteria), Based on the Worst Grade for Each Patient and Each Type of Toxicity |
The incidence of radiation enteritis, radiation proctitis, and radiation cystitis (classified according to RTOG criteria) are shown in Table 4. No difference was found between different chemotherapy regimens and different tumor sizes.
Discussion
A meta-analysis of 21 randomized trials suggested that NACT does not affect the OS in patients with cervical cancer.18 The study also suggested that a short cycle length or an intensive dose greater than 25 mg/m2 per week of platinum may improve the outcome. Trials with a cycle length of p14 days were associated with an improvement in the OS of approximately 7% at five years.18 A Phase II trial of neoadjuvant chemotherapy followed by chemoradiation in locally advanced cervical cancer reported a comparatively ideal 3-year OS in 71% and 86% of patients with stage III and IVA tumors, respectively.19 Another retrospective study compared the data from 612 patients and reported that neoadjuvant chemotherapy followed by concurrent chemotherapy and radiotherapy (NACT-CCRT) improved the 5-year DFS compared with concurrent radiotherapy and chemotherapy (41.8% vs 58.3%; p = 0.001).20 Harsh reported the 5-year results of induction chemotherapy followed by concurrent chemoradiation in cervical carcinoma. The 5-year OS was 84.0% for stage IIA, 79.7% for stage IIB, 67.6% for stage IIIA, 48.4% for stage IIIB, and 28.6% for stage IV-A disease.21 In our study, the overall one-year, three-year, and five-year survival rates were 99.2%, 82.5%, and 70.8%, respectively. Until our last follow-up, less than 50% of the patients died; thus, the median survival was not calculated. Regardless of such favorable results, in the present study, no control group treated with CCRT could be used for comparison. Consequently, we could not compare whether neoadjuvant chemotherapy could improve the disease control rate and survival.
In some studies, platinum, 5-fluorouracil combined with cisplatin administered weekly, Taxol-ifosfamide-cisplatin, or paclitaxel combined with cisplatin showed tolerable side effects. The platinum-based compound cisplatin is the most widely used first-generation drug approved by the FDA to treat solid tumors.22–24 Two Phase III studies have shown that combination therapy is superior to cisplatin alone in the response rate and median progression-free survival when treating patients with recurrent or persistent squamous cell carcinoma of the cervix.25,26 Additionally, cisplatin-containing chemoradiotherapy for cervical cancer showed a reduction in the recurrence risk of 40–60%.27 However, cisplatin therapy has also been associated with severe gastrointestinal toxicity and nephrotoxicity.6–8 Thus, second- and third-generation platinum analogs, such as lobaplatin, with reduced gastrointestinal toxicity have been proposed.9 In the current study, neoadjuvant therapy was combination chemotherapy, and cisplatin or loplatin alone chemotherapy was used during concurrent radiotherapy. Our data showed no difference in the response rates (100% vs 98.4%; p>0.5), five-year survival rates (63.8% vs 75.8%; p>0.5), or overall survival (p=0.426) between the TL group (paclitaxel plus loplatin) and TP group (paclitaxel plus cisplatin). Because of the differences in whether patients receive neoadjuvant chemotherapy, neoadjuvant chemotherapy regimens, chemotherapy cycles, tumor stage or proportion and many other factors, comparing the advantages and disadvantages of the scheme in the present study with those reported in other studies is challenging.
In a pilot study,28 grade 3–4 neutropenia was present in 29% of patients during CCRT after NACT, a value that is lower than our study’s data. A systematic review of CCRT trials reported 27.6% hematologic toxicity, while most of the included trials used combined chemotherapy (with or without platinum).29 Duenas-Gonzalez found grade 3 and 4 toxicities in 46.3% of patients with stage IIB to IVA carcinoma of the cervix treated with gemcitabine plus cisplatin chemoradiotherapy followed by BCT adjuvant gemcitabine/cisplatin chemotherapy.30 In the present study, grade 3 or 4 digestive reaction was obviously more frequent in the TP group, while leukopenia, neutropenia, and thrombocytopenia were more common in the TL group. This finding suggests that for some patients with severe digestive system reactions or cisplatin contraindications, lobaplatin may be a good alternative choice. However, in our study, the incidence of grade 3–4 leukopenia in the paclitaxel combined with lobaplatin group was as high as 55.2%, and the incidence of grade 3–4 thrombocytopenia was 44.8%, which may increase medical costs and prolong the total treatment time, suggesting that lobaplatin should only be used as an alternative. Additionally, the incidence of hematological toxicity in the TL group may be related to its intensive dose. If the dose is reduced to 30 mg/m2 per cycle and 3 weeks are considered a cycle, the hematological toxicity may be reduced.
A previous study reviewed a database of 131 women with FIGO IIIB cervical cancer who were treated by definitive radiotherapy. Eighty-nine of them received concurrent chemotherapy. Ultimately, the five-year overall survival (OS) rate was 52.4%.31 In this study, the 5-year OS of stage IIIB was 64.7%. This improved survival rate may be related to whether patients receive neoadjuvant chemotherapy. A randomized controlled trial reported that 183 stage IIB cervical cancer patients received neoadjuvant chemotherapy plus concomitant chemoradiation, and the 5-year DFS rate was 79.3%.32 At our center, the 5-year overall survival rate of stage IIB cervical cancer patients was 69.2%. The rate of neoadjuvant chemotherapy in our study was less than that in a previous study, possibly reducing survival. Another survey revealed that when all disease stages are evaluated together, the 5-year survival rate is less than 60% in low-income countries compared with 70% in high-income nations.33 In our study, no difference was observed in long-term survival between stage IIB and IIIB patients. We speculate that NACT-CCRT for a wide range of tumors, such as FIGO IIIB stage cervical cancer, may improve survival. This hypothesis must be confirmed in prospective studies.
Theoretically, neoadjuvant chemotherapy decreases the proportion of hypoxic cells and decreases the tumor size, improving the success rate of surgery and sensitivity to radiotherapy.34,35 However, to date, only a few reports have demonstrated survival differences in patients with different tumor sizes. In our study, the response rates (CR+PR) after treatment in patients with a mass size ≥ 5 cm and <5 cm were 98.5% and 100%, respectively, without a significant difference. Additionally, no significant difference was found in overall survival between the groups with different mass sizes. Therefore, our institution is conducting a randomized controlled study to explore whether neoadjuvant chemotherapy can improve the efficacy and survival of large mass cervical cancer. The preliminary research data are expected to be published in 2022.
In our study, lobaplatin was intensively administered and associated with relatively obvious hematological toxicity; however, these side effects were tolerated after treatment, and the treatment time was not significantly prolonged. Furthermore, no significant difference was observed in the survival rate compared with cisplatin when combined with paclitaxel to treat locally advanced cervical cancer.
This study has a few limitations. First, it is retrospective with a relatively small sample size, which may generate some bias. Additionally, no comparison was conducted with concurrent chemoradiotherapy cases as a control. Therefore, the advantages and disadvantages of these two chemotherapy regimens should be further addressed in future prospective studies.
Conclusion
For patients with severe digestive system reactions or contraindications to cisplatin, lobaplatin can be used as an alternative drug. However, hematological toxicity must be considered, particularly in dose-intensive schemes. However, the value of this therapeutic pattern in clinical practice warrants in-depth exploration and larger prospective clinical studies.
Abbreviations
NACT, neoadjuvant chemotherapy; CCRT, concurrent chemotherapy and radiotherapy; RT, radiotherapy; 3D-CRT, 3-dimensional conformal radiation therapy; IMRT, intensity modulated radiotherapy; IGRT, image guided radiotherapy; GTV, gross tumor volume; CTV, clinical target volume; TP, paclitaxel plus cisplatin; TL, paclitaxel plus loplatin; OS, overall survival; NCCN, National Comprehensive Cancer Network; FIGO, International Federation of Gynecology and Obstetrics; KPS, Karnofsky Physical Status; ECOG, Eastern Cooperative Oncology Group; RECIST, Response Evaluation Criteria in Solid Tumors; CTCAE, Common Terminology Criteria for Adverse Events; RTOG, radiotherapy cooperation group; CR, complete response; PR, partial response; RR, response rate; SR, survival rate; DFS, disease-free survival; FDA, Food and Drug Administration; BCT, Brachytherapy; NCI, National Cancer Institute.
Data Sharing Statement
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request. However, the authors do not intend to share any data besides what is included in the manuscript.
Ethics Approval and Informed Consent
We have obtained permission from the dataset owners (the Affiliated Cancer Hospital of Guizhou Medical University and the Guizhou Provincial People’s Hospital) to use the information in databases/repositories for the current retrospective study. We confirmed that the data were anonymized or maintained with confidentiality. This study followed the guidelines outlined in the Declaration of Helsinki.
Consent for Publication
This study is a retrospective and comprehensive data analysis. There is no information to identify the corresponding patient in this study.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
Xue Tian and Feiyue Yang are co-first authors in this study. The authors report no conflicts of interest in this work.
References
1. Chen W, Zheng R, Zeng H, Zhang S. The incidence and mortality of major cancers in China, 2012. Chin J Cancer. 2016;35(1):73. doi:10.1186/s40880-016-0137-8
2. Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65(2):87–108.
3. Goss PE, Strasser-Weippl K, Lee-Bychkovsky BL, et al. Challenges to effective cancer control in China, India, and Russia. Lancet Oncol. 2014;15(5):489–538.
4. Nascimento MI, Silva GA. [Effect of waiting time for radiotherapy on five-year overall survival in women with cervical cancer, 1995–2010]. Cad Saude Publica. 2015;31(11):2437–2448. Portuguese. doi:10.1590/0102-311X00004015
5. Thomas GM. Improved treatment for cervical cancer–concurrent chemotherapy and radiotherapy. N Engl J Med. 1999;340(15):1198–1200. doi:10.1056/NEJM199904153401509
6. Shahid F, Farooqui Z, Khan F. Cisplatin-induced gastrointestinal toxicity: an update on possible mechanisms and on available gastroprotective strategies. Eur J Pharmacol. 2018;827:49–57. doi:10.1016/j.ejphar.2018.03.009
7. Harmers FP, Gispen WH, Neijt JP. Neurotoxic side-effects of cisplatin. Eur J Cancer. 1991;27(3):372–376. doi:10.1016/0277-5379(91)90549-S
8. Pabla N, Dong Z. Cisplatin nephrotoxicity: mechanisms and renoprotective strategies. Kidney Int. 2008;73(9):994–1007. doi:10.1038/sj.ki.5002786
9. Wang JQ, Wang T, Shi F, et al. A randomized controlled trial comparing clinical outcomes and toxicity of lobaplatin- versus cisplatin-based concurrent chemotherapy plus radiotherapy and high-dose-rate brachytherapy for FIGO stage II and III cervical cancer. Asian Pac J Cancer Prev. 2015;16(14):5957–5961. doi:10.7314/APJCP.2015.16.14.5957
10. McKeage MJ. Lobaplatin: a new antitumour platinum drug. Expert Opin Investig Drugs. 2001;10(1):119–128. doi:10.1517/13543784.10.1.119
11. Li F, Wang B, He M, et al. Pilot study of docetaxel combined with lobaplatin or gemcitabine for recurrent and metastatic breast cancer. Medicine. 2019;98(52):e18513. doi:10.1097/MD.0000000000018513
12. Chen J, Liu B, Zhang F, Cui W, Zhang P. Pharmacokinetics and safety of lobaplatin plus etoposide in Chinese men older than 65 years with extensive-stage small cell lung cancer: a phase II clinical trial. Cancer Chemother Pharmacol. 2019;84(1):73–81. doi:10.1007/s00280-019-03828-z
13. Pecorelli S. Revised FIGO staging for carcinoma of the vulva, cervix, and endometrium. Int J Gynaecol Obstet. 2009;105(2):103–104. doi:10.1016/j.ijgo.2009.02.012
14. Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228–247. doi:10.1016/j.ejca.2008.10.026
15. National Cancer Institute. Common terminology criteria for adverse events (CTCAE) v4.0; 2009. Available from: http://evs.nci.nih.gov/ftp1/CTCAE/About.html.
16. Cox JD, Stetz J, Pajak TF. Toxicity criteria of the Radiation Therapy Oncology Group (RTOG) and the European Organization for Research and Treatment of Cancer (EORTC). Int J Radiat Oncol Biol Phys. 1995;31(5):1341–1346. doi:10.1016/0360-3016(95)00060-C
17. IBM SPSS Statistics for Windows, Version 24.0. Armonk, NY: IBM Corp; 2016. Available from: https://www-01.ibm.com/support/docview.wss?uid=swg21476197.
18. Neoadjuvant Chemotherapy for Locally Advanced Cervical Cancer Meta-analysis Collaboration. Neoadjuvant chemotherapy for locally advanced cervical cancer: a systematic review and meta-analysis of individual patient data from 21 randomised trials. Eur J Cancer. 2003;39(17):2470–2486.
19. de Azevedo C, Thuler LCS, de Mello MJG, et al. Phase II trial of neoadjuvant chemotherapy followed by chemoradiation in locally advanced cervical cancer. Gynecol Oncol. 2017;146(3):560–565. doi:10.1016/j.ygyno.2017.07.006
20. Narayan S, Sharma N, Kapoor A, et al. Pros and cons of adding of neoadjuvant chemotherapy to standard concurrent chemoradiotherapy in cervical cancer: a regional cancer center experience. J Obstet Gynaecol India. 2016;66(5):385–390. doi:10.1007/s13224-015-0698-5
21. Harsh KK, Kapoor A, Paramanandhan M, et al. Induction chemotherapy followed by concurrent chemoradiation in the management of different stages of cervical carcinoma: 5-year retrospective study. J Obstet Gynaecol India. 2016;66(5):372–378. doi:10.1007/s13224-015-0699-4
22. Buda A, Fossati R, Colombo N, et al. Randomized trial of neoadjuvant chemotherapy comparing paclitaxel, ifosfamide, and cisplatin with ifosfamide and cisplatin followed by radical surgery in patients with locally advanced squamous cell cervical carcinoma: the SNAP01 (Studio Neo-Adjuvante Portio) Italian Collaborative Study. J Clin Oncol. 2005;23(18):4137–4145.
23. Yang Z, Chen D, Zhang J, et al. The efficacy and safety of neoadjuvant chemotherapy in the treatment of locally advanced cervical cancer: a randomized multicenter study. Gynecol Oncol. 2016;141(2):231–239. doi:10.1016/j.ygyno.2015.06.027
24. Benedetti Panici P, Bellati F, Pastore M, et al. An update in neoadjuvant chemotherapy in cervical cancer. Gynecol Oncol. 2007;107(1 Suppl 1):S20–S22. doi:10.1016/j.ygyno.2007.07.041
25. Moore DH, Blessing JA, McQuellon RP, et al. Phase III study of cisplatin with or without paclitaxel in stage IVB, recurrent, or persistent squamous cell carcinoma of the cervix: a gynecologic oncology group study. J Clin Oncol. 2004;22(15):3113–3119. doi:10.1200/JCO.2004.04.170
26. Monk BJ, Sill MW, McMeekin DS, et al. Phase III trial of four cisplatin-containing doublet combinations in stage IVB, recurrent, or persistent cervical carcinoma: a Gynecologic Oncology Group study. J Clin Oncol. 2009;27(28):4649–4655. doi:10.1200/JCO.2009.21.8909
27. Whitney CW, Sause W, Bundy BN, et al. Randomized comparison of fluorouracil plus cisplatin versus hydroxyurea as an adjunct to radiation therapy in stage IIB-IVA carcinoma of the cervix with negative para-aortic lymph nodes: a Gynecologic Oncology Group and Southwest Oncology Group study. J Clin Oncol. 1999;17(5):1339–1348. doi:10.1200/JCO.1999.17.5.1339
28. Singh RB, Chander S, Mohanti BK, et al. Neoadjuvant chemotherapy with weekly paclitaxel and carboplatin followed by chemoradiation in locally advanced cervical carcinoma: a pilot study. Gynecol Oncol. 2013;129(1):124–128. doi:10.1016/j.ygyno.2013.01.011
29. Kirwan JM, Symonds P, Green JA, et al. A systematic review of acute and late toxicity of concomitant chemoradiation for cervical cancer. Radiother Oncol. 2003;68(3):217–226. doi:10.1016/S0167-8140(03)00197-X
30. Dueñas-González A, Zarbá JJ, Patel F, et al. Phase III, open-label, randomized study comparing concurrent gemcitabine plus cisplatin and radiation followed by adjuvant gemcitabine and cisplatin versus concurrent cisplatin and radiation in patients with stage IIB to IVA carcinoma of the cervix. J Clin Oncol. 2011;29(13):1678–1685. doi:10.1200/JCO.2009.25.9663
31. Kuroda Y, Murakami N, Morota M, et al. Impact of concurrent chemotherapy on definitive radiotherapy for women with FIGO IIIb cervical cancer. J Radiat Res. 2012;53(4):588–593. doi:10.1093/jrr/rrs010
32. Gupta S, Maheshwari A, Parab P, et al. Neoadjuvant chemotherapy followed by radical surgery versus concomitant chemotherapy and radiotherapy in patients with stage IB2, IIA, or IIB squamous cervical cancer: a randomized controlled trial. J Clin Oncol. 2018;36(16):1548–1555. doi:10.1200/JCO.2017.75.9985
33. Ginsburg O, Bray F, Coleman MP, et al. The global burden of women’s cancers: a grand challenge in global health. Lancet. 2017;389(10071):847–860. doi:10.1016/S0140-6736(16)31392-7
34. Park DC, Kim JH, Lew YO, Kim DH, Namkoong SE. Phase II trial of neoadjuvant paclitaxel and cisplatin in uterine cervical cancer. Gynecol Oncol. 2004;92(1):59–63. doi:10.1016/j.ygyno.2003.09.015
35. Dueñas-Gonzalez A, López-Graniel C, González-Enciso A, et al. A phase II study of multimodality treatment for locally advanced cervical cancer: neoadjuvant carboplatin and paclitaxel followed by radical hysterectomy and adjuvant cisplatin chemoradiation. Ann Oncol. 2014;50(4):877–887.
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
