Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 16
A Comparison of Clinical Characteristics in Overweight/Obese and Normal Weight Patients with Psoriasis Vulgaris: A Bicentric Retrospective Observational Study
Authors Li L, Liu K, Duan X, Xu L, Yang Q, Liu F
Received 8 March 2023
Accepted for publication 13 May 2023
Published 30 May 2023 Volume 2023:16 Pages 1377—1385
DOI https://doi.org/10.2147/CCID.S411636
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jeffrey Weinberg
Lanzhi Li,1,* Keshuai Liu,1,* Xingwu Duan,1 Limei Xu,2 Qingqi Yang,2 Fang Liu2
1Department of Dermatology, Dongzhimen Hospital, Beijing University of Chinese Medicine, Beijing, People’s Republic of China; 2Department of Dermatology, Air Force Medical Center of PLA, Beijing, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Xingwu Duan, Email [email protected]
Background: Psoriasis is a chronic, inflammatory skin disease that is often accompanied by multiple comorbidities. Obesity is considered an independent risk factor for the development of psoriasis. However, most of the related data are derived from epidemiological studies conducted in the United States of America and Europe. This study aimed to compare the clinical characteristics of patients with psoriasis who are overweight/obese and patients with psoriasis with normal weight in China.
Methods: We reviewed the medical records of 208 patients with psoriasis. Based on their body mass index (BMI), the patients were divided into two groups: patients with psoriasis who were overweight/obese and patients with psoriasis with normal weight.
Results: The most patients enrolled in this study were men (77.40%). Patients with psoriasis who were overweight/obese had a higher mean age, longer disease duration, and significantly higher Psoriasis Area and Severity Index (PASI) values (P=0.032). Additionally, the incidence of fatty liver, hyperlipidemia, hyperuricemia, and abnormal liver function was higher among patients with psoriasis who were overweight/obese (P< 0.05). Linear regression analysis revealed a linear relationship between PASI values and BMI (P=0.016). Moreover, patients with psoriasis who were overweight/obese had significantly higher levels of serum alanine transaminase (ALT), aspartate transaminase (AST), uric acid (UC), total cholesterol (TC), low-density lipoprotein (LDL), and fasting plasma glucose (FPG) (P< 0.05) and lower serum high-density lipoprotein (HDL) levels and absolute lymphocyte count (ALC) (P< 0.05).
Conclusion: Patients with psoriasis who are overweight/obese have more severe psoriatic lesions and metabolic comorbidities. Detailed assessment of the BMI of patients with psoriasis revealed that weight loss may be necessary for patients who are overweight/obese to reduce the risk of metabolic disorders.
Keywords: psoriasis, obesity, metabolic disorders
Introduction
Psoriasis vulgaris is a chronic, inflammatory skin disease characterized by abnormal proliferation and differentiation of keratinocytes. Recent studies have demonstrated that psoriasis vulgaris is associated with comorbidities including cardiovascular disease, obesity, type 2 diabetes mellitus (T2DM), and nonalcoholic fatty liver disease (NAFLD).1,2 As one of the important comorbidities, obesity is considered an independent risk factor for the onset of psoriasis vulgaris.3 With an increase in body mass index (BMI), the prevalence of psoriasis also increases.4,5 A study from the UK and Norway showed that for every 1 kg/m2 increase in BMI in patients with obesity, the incidence of psoriasis increases by 4%.4 Another retrospective study involving 1.5 million people reported that the incidence of psoriasis was significantly higher in individuals with obesity than in individuals with normal weight and increased with an increase in weight.5 Obesity not only worsens the clinical outcome of psoriasis but also increases the likelihood of developing adverse events, such as delayed response to biological therapy6 and increased risk of hyperlipidemia during treatment with methotrexate and acitretin.7,8 However, weight loss could reduce the severity of skin lesions and improve the quality of life of patients with psoriasis.9–13 At present, studies investigating the clinical characteristics of patients with psoriasis who are overweight/obese are lacking.4,5,14,15 Although relevant information is available in several related studies, most data are derived from epidemiological studies conducted in Europe and the USA.16,17 Therefore, this study aimed to investigate the clinical characteristics of Chinese patients with psoriasis to elucidate the relationship between psoriasis and obesity.
Materials and Methods
This study conformed to the ethical guidelines of the Declaration of Helsinki. The Ethics Committee of Dongzhimen Hospital Affiliated to Beijing University of Chinese Medicine approved the research (Registration number:2022DZMEC-494-01). Patient consent is not required for this retrospective study. We used an electronic medical record database to review the data of patients with psoriasis who visited the dermatology ward of Dongzhimen Hospital of Beijing University of Chinese Medicine and Air Force Medical Center of PLA from January 2022 to December 2022. The diagnosis of all patients with psoriasis was confirmed based on the ICD code in their medical records.
All patients were registered with basic information (gender, age, height, weight, the history of smoking, the history of drinking alcohol, the positive family history of psoriasis, etc.), PASI score, comorbidities, blood tests (WBC, RBC, PLT, ANC, ALC, MONO, CRP, IL-6, ALT, AST, TBiL, UA, LPA, TG, TC, etc.) and abdominal ultrasound examination before starting treatment. We collected the above information and input this data into an Excel table for analysis. The patients were divided into the overweight/obesity and normal weight groups according to their BMI values. Baseline data and serological indicators were compared between the two groups. The diagnostic criteria for obesity were as follows: Given that the World Health Organization (WHO) standards are mainly formulated for European and American countries, we adopted the standards recommended by the Working Group on Obesity of China (WGOC) of the International Life Sciences Institute (ILSI) to define overweight and obesity in Chinese adults and conform to the actual prevalence of obesity in China. 28>BMI≥24 and BMI≥28 were considered overweight and obese, respectively.18
Excel 2016 was used to record the data, and the SPSS Statistics (version 24.0) software was used for statistical analysis. Data conforming to a normal distribution were expressed as mean±standard, whereas those not conforming to a normal distribution were expressed as the median (P25, P75). The t-test was used to compare normally distributed continuous variables between groups. Student’s t-test was used if the distribution was normal and the variance was homogeneous, whereas Welch’s t-test was used if the variance was unequal. The Mann–Whitney U-test was used to compare data that did not conform to a normal distribution, and Pearson’s chi-square test was used to compare dichotomous variables between groups. A p-value of <0.05 indicated statistical significance.
Results
Characteristics of Patients
This retrospective cross-sectional study included 208 patients with psoriasis vulgaris, with an average age of 41.98 ± 17.02 years and a median disease duration of 10 years. The prevalence of overweight and obesity was 36% and 26%, respectively. The patients were divided into the overweight/obesity (BMI≥24; 129 cases) and normal weight (BMI<24; 79 cases) groups according to their BMI values. Age and disease duration were significantly higher in the overweight/obesity group than in the normal weight group (mean age: 44.19±15.46 years versus 38.37±18.84 years, respectively, P=0.022; median duration of disease: 12 years versus 5 years, respectively, P<0.001). Furthermore, most patients were men (male/female patients [M/F]: 161/47; 77.40%/22.60%), and no significant difference was observed in sex distribution between the overweight/obesity (M: 104, 80.6%) and normal weight (M: 57, 72.2%) groups (P=0.156). The PASI scores of patients in the overweight/obesity group were significantly higher than those of patients in the normal weight group (median: 18.90 and 15.90, respectively, P=0.032) (Figure 1). Linear regression analysis revealed that BMI was linearly correlated with PASI scores in all patients (P=0.016). This finding suggests that the severity of psoriasis increases with an increase in BMI (Figure 2). Moreover, no significant differences were observed in the history of drinking alcohol, the history of smoking, psoriasis nail and bunchy hair between the two groups (P>0.05). The basic characteristics of the patients are shown in Table 1.
 |
Table 1 Basic Characteristics of the Included Patients |
 |
Figure 1 Comparison of PSAI scores between the two groups before treatment. *P<0.05. |
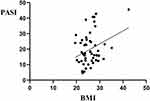 |
Figure 2 Linear correlation analysis of BMI and PASI scores. |
Analysis of Comorbidities and Hematological Indicators
A total of 176 patients underwent ultrasound examination. The results revealed that 85.7% (78 cases) of patients with psoriasis who were overweight/obese and 14.3% (13 cases) of patients with psoriasis with normal weight had fatty liver (P<0.001). The incidence of hyperlipidemia (P=0.011), hyperuricemia (P<0.001), and abnormal liver function (P=0.036) was significantly higher in the overweight/obesity group than in the normal weight group; however, the incidence of hypertension (P=0.06), T2DM (P=0.406), and other complications was not significantly different between the two groups. In terms of hematological indices, RBC count (P=0.000), ALT levels (P=0.001), AST levels (P=0.021), UA levels (P=0.000), TC levels (P=0.041), LDL levels (P=0.000) and FPG levels (P=0.036) were higher in the overweight/obesity group than in the normal weight group, whereas HDL levels (P=0.024) and ALC (P=0.047) were lower in the overweight/obesity group than in the normal weight group. Moreover, no significant difference was observed in complement C3 levels (P=0.070), complement C4 levels (P=0.882), IL-6 levels (P=0.645), C-reactive protein levels (P=0.178), and lipoprotein A levels (P=0.822) between the two groups (Table 2).
 |
Table 2 Complications and Hematological Indices |
Discussion
As a chronic inflammatory skin disease, psoriasis is often accompanied by multiple systemic comorbidities. Obesity is one of the common comorbidities and an independent risk factor for psoriasis.10 Some studies have suggested that patients with psoriasis have a higher risk of developing obesity than those without psoriasis (OR=1.66), and the risk of obesity is significantly higher in patients with severe psoriasis than in patients with mild psoriasis.19 Additionally, obesity could increase the risk of psoriasis.20 As a low-grade chronic inflammatory organ, the adipose tissue of patients with obesity contains various immune cells. Inflammatory cells and factors are closely related to the occurrence and development of obesity and psoriasis.21–25 In this study, psoriatic lesions were more severe in patients who were overweight/obese, and the severity of lesions increased with an increase in BMI in all patients. These results are consistent with those of previous studies.26 A reason underlying this phenomenon is that the imbalance between pro-inflammatory and anti-inflammatory factors in patients who are overweight/obese could result in chronic inflammation. Several studies have demonstrated that the adipose tissue of patients with obesity secretes more pro-inflammatory cytokines (TNF-α, IL-6, IFN-γ, IL-17A, leptin, visfatin, and chemokines)27 and less anti-inflammatory cytokines (adiponectin).28 These inflammatory factors can activate the inflammatory pathway and upregulate the secretion of IL-6, IL-8, and other pro-inflammatory cytokines, leading to increased inflammation.23 In a study on mouse models of psoriasis with obesity fed a high-fat diet (HFD), activation of the NLRP3 inflammasome induced the production of pro-inflammatory cytokines, such as IL-17A, IL-1β, and IL-18, in innate lymphoid cells (ILCs) and macrophages, thereby aggravating psoriasis.22 Leptin can stimulate the proliferation of keratinocytes29 and is highly expressed in the serum30 and skin lesions of patients with psoriasis.31 Visfatin is elevated in the serum of patients with psoriasis and is positively correlated with PASI scores.32 Visfatin and TNF-α can synergistically stimulate keratinocytes to produce chemokines and antimicrobial peptides via the NF-kB and STAT3 pathways.33 The levels of the anti-inflammatory factor adiponectin are negatively correlated with PASI scores and the expression of IL-6 and TNF-α.34 Adiponectin may exert anti-inflammatory effects by inhibiting the expression of adhesion molecules induced by TNF-α and the production of IFN-γ.35 The above-mentioned studies suggest that psoriasis and obesity share a common inflammatory pathway, which may explain the effects of obesity on the development of psoriasis; however, the molecular mechanisms underlying this inflammatory pathway remain unclear.
In this study, patients with psoriasis were found to have dyslipidemia of varying degrees, which is consistent with the results of previous studies. A systematic review that included 25 observational studies demonstrated that psoriasis is significantly associated with dyslipidemia, and the incidence of hyperlipidemia is significantly increased in patients with psoriasis.36 Furthermore, compared with the normal weight group, the overweight/obesity group had a higher incidence of hyperlipidemia (P=0.011) and significantly higher total cholesterol levels (P=0.041). Additionally, the levels of LDL and HDL, important indicators of lipid metabolism, were significantly abnormal. LDL levels were higher (P=0.000) and HDL levels were lower (P=0.024) in the overweight/obesity group than in the normal weight group. These results are consistent with those of a previous study from the UK.37 Elevated levels of pro-inflammatory cytokines such as IL-17A and IL-6 can lead to an increase in LDL-L levels and a decrease in HDL-L levels, which may be caused by the enhancement of HDL catabolism owing to the acute inflammatory reaction of hepatocytes caused by IL-17A and IL-6.38 Widawski et al reported a higher BMI in patients with hyperuricemia (30.9 versus 28.7 kg/m2, P=0.015) in a retrospective two-center case–control study on psoriatic arthritis.39 Another study from China reported that hyperuricemia was associated with a higher BMI, and the prevalence of hyperuricemia was higher in patients with psoriasis who were overweight/obese than in those with normal weight.40 Consistently, in this study, the incidence of hyperuricemia was significantly different between the overweight/obesity and normal weight groups (P<0.001). Increased body weight may be a major factor involved in the elevation of serum urate levels in psoriasis patients.41 NAFLD is one of the most common comorbid systemic diseases in patients with psoriasis, and several previous studies have validated that psoriasis is associated with NAFLD (OR=1.96).42 In the present study, the incidence of fatty liver and liver dysfunction was higher in the overweight/obesity group than in the normal weight group, which is consistent with the results of a cross-sectional study from Iran.43
Furthermore, the levels of FPG, TC, and LDL were higher and those of HDL were lower in the overweight/obesity group than in the normal weight group. Significant differences were observed between the two groups, and these differences may be attributed to the disorder of glucose and lipid metabolism caused by insulin resistance. Studies have demonstrated that Th17 cells in the peripheral blood of patients with psoriasis can downregulate the expression of the transcription factor Foxp3 by secreting cytokines such as IL-17A, which inhibits the expression of the insulin gene and leads to a decrease in insulin secretion.44 Additionally, obesity can induce insulin resistance through the secretion of adipose tissue factors, further exacerbating glucose and lipid metabolism disorders.45 In this study, absolute lymphocyte counts were lower in the overweight/obesity group than in the normal weight group, suggesting a decrease in immune function in patients in the overweight/obesity group, which may be related to the inflammatory status of obesity. However, no significant changes were observed in complement C3 and C4 levels between the two groups, which may be attributed to the sample size and warrants further investigation. Additionally, patients with psoriasis who were overweight/obese were older and had a longer disease duration, which may be attributed to the poor outcomes of psoriasis owing to overweight/obesity and the recurrence of the disease.
We also observed in this study that the proportion of male patients with psoriasis who were overweight/obese was higher (M/F: 104/25). We speculate that this difference is related to lifestyle habits. On the one hand, men have a higher consumption of smoking and alcohol; on the other hand, women may be protected by estrogen. Studies have demonstrated that the incidence of metabolic syndrome is significantly lower in premenopausal women than in men but higher in postmenopausal women than in men.46 Additionally, no difference was observed in the psoriasis nail and bunchy hair between the two groups, which may be related to personal factors such as negligence in assessment by medical workers.
In conclusion, considering the crosstalk between psoriasis and obesity, we recommend weight loss as one of the interventions for patients with psoriasis who are overweight/obese. Healthy dietary patterns such as the Mediterranean diet have been reported to be beneficial not only for weight loss but also for the alleviation of psoriasis.47 A low-calorie ketogenic diet (VLCKD) may reduce the severity of psoriasis by reducing weight and chronic inflammation.48 There is no unanimous opinion on the type and duration of exercise for patients with psoriasis with obesity, and because strenuous exercise may cause some damage to the joints of patients with psoriasis who are overweight/obese, moderate-intensity aerobic exercise, such as calisthenics, jogging, and ball games, may be more appropriate. Aerobic training burns more energy than resistance training49 and is enjoyable and acceptable to patients.50 Based on the combined analysis of data from several studies, patients with psoriasis vulgaris with obesity should adhere to aerobic exercise 3-5 times per week, with a duration of ≥30 minutes each time, to lose approximately 1 kg per month on the basis of diet management.51 This study has the following limitations: (1) this study had a retrospective design, and there may be potential information bias; (2) this study had a cross-sectional design; therefore, we could not accurately determine the causal relationship between obesity and psoriasis.
Conclusion
This study suggests that over 60% patients with psoriasis vulgaris suffer from overweight/obesity. Patients with higher BMI values tend to have more severe skin lesions and metabolic disorders. Therefore, the BMI of patients with psoriasis should be monitored and lifestyle changes, such as dietary habits and exercise patterns, should be promptly implemented to improve patient outcomes.
Ethics Approval
This study was approved by the Ethics Committee of Dongzhimen Hospital Affiliated to Beijing University of Chinese Medicine (Registration number:2022DZMEC-494-01). This retrospective study conformed to the ethical guidelines of the Declaration of Helsinki, and patients’ privacy and personal identity information are protected. Exemption from informed consent will not have any adverse impact on patients’ health and rights. Therefore, the patient consent is not required for this retrospective study.
Acknowledgments
Lanzhi Li and Keshuai Liu are co-first authors for this study.
Funding
This work was supported by National Natural Science Foundation of China (Num: 82074436).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Griffiths CEM, Armstrong AW, Gudjonsson JE, Barker JNWN. Psoriasis. Lancet. 2021;397(10281):1301–1315. doi:10.1016/S0140-6736(20)32549-6
2. Yamazaki F. Psoriasis: comorbidities. J Dermatol. 2021;48(6):732–740. doi:10.1111/1346-8138.15840
3. Snekvik I, Smith CH, Nilsen TIL, et al. Obesity, waist circumference, weight change, and risk of incident psoriasis: prospective data from the HUNT study. J Invest Dermatol. 2017;137(12):2484–2490. doi:10.1016/j.jid.2017.07.822
4. Budu-Aggrey A, Brumpton B, Tyrrell J, et al. Evidence of a causal relationship between body mass index and psoriasis: a Mendelian randomization study. PLoS Med. 2019;16(1):e1002739. doi:10.1371/journal.pmed.1002739
5. Norden A, Rekhtman S, Strunk A, Garg A. Risk of psoriasis according to body mass index: a retrospective cohort analysis. J Am Acad Dermatol. 2022;86(5):1020–1026. doi:10.1016/j.jaad.2021.06.012
6. Hung YT, Lin YJ, Chiu HY, Huang YH. Impact of previous biologic use and body weight on the effectiveness of guselkumab in moderate-to-severe plaque psoriasis: a real-world practice. Ther Adv Chronic Dis. 2021;12:20406223211046685. doi:10.1177/20406223211046685
7. Shan J, Zhang J. Impact of obesity on the efficacy of different biologic agents in inflammatory diseases: a systematic review and meta-analysis. Joint Bone Spine. 2019;86(2):173–183. doi:10.1016/j.jbspin.2018.03.007
8. Menter A, Korman NJ, Elmets CA, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: section 4. Guidelines of care for the management and treatment of psoriasis with traditional systemic agents. J Am Acad Dermatol. 2009;61(3):451–485. doi:10.1016/j.jaad.2009.03.027
9. Mahil SK, McSweeney SM, Kloczko E, McGowan B, Barker JN, Smith CH. Does weight loss reduce the severity and incidence of psoriasis or psoriatic arthritis? A critically appraised topic. Br J Dermatol. 2019;181(5):946–953. doi:10.1111/bjd.17741
10. Jensen P, Skov L. Psoriasis and obesity. Dermatology. 2016;232(6):633–639. doi:10.1159/000455840
11. Polo TCF, Corrente JE, Miot LDB, Papini SJ, Miot HA. Dietary patterns of patients with psoriasis at a public healthcare institution in Brazil. An Bras Dermatol. 2020;95(4):452–458. doi:10.1016/j.abd.2020.02.002
12. Paroutoglou K, Papadavid E, Christodoulatos GS, Dalamaga M. Deciphering the association between psoriasis and obesity: current evidence and treatment considerations. Curr Obes Rep. 2020;9(3):165–178. doi:10.1007/s13679-020-00380-3
13. Kaushik SB, Lebwohl MG. Psoriasis: which therapy for which patient: psoriasis comorbidities and preferred systemic agents. J Am Acad Dermatol. 2019;80(1):27–40. doi:10.1016/j.jaad.2018.06.057
14. Setty AR, Curhan G, Choi HK. Obesity, waist circumference, weight change, and the risk of psoriasis in women: nurses’ health study II. Arch Intern Med. 2007;167(15):1670–1675. doi:10.1001/archinte.167.15.1670
15. Barros G, Duran P, Vera I, Bermúdez V. Exploring the links between obesity and psoriasis: a comprehensive review. Int J Mol Sci. 2022;23(14):7499. doi:10.3390/ijms23147499
16. Jaacks LM, Vandevijvere S, Pan A, et al. The obesity transition: stages of the global epidemic. Lancet Diabetes Endocrinol. 2019;7(3):231–240. doi:10.1016/S2213-8587(19
17. Ortega FB, Lavie CJ, Blair SN. Obesity and cardiovascular disease. Circ Res. 2016;118(11):1752–1770. doi:10.1161/CIRCRESAHA.115.306883
18. Chen C, Lu FC. Department of disease control ministry of health, PR China. The guidelines for prevention and control of overweight and obesity in Chinese adults. Biomed Environ Sci. 2004;17:1–36.
19. Armstrong AW, Harskamp CT, Armstrong EJ. The association between psoriasis and obesity: a systematic review and meta-analysis of observational studies. Nutr Diabetes. 2012;2(12):e54. doi:10.1038/nutd.2012.26
20. Han JH, Lee JH, Han KD, et al. Increased risk of psoriasis in subjects with abdominal obesity: a nationwide population-based study. J Dermatol. 2019;46(8):695–701. doi:10.1111/1346-8138.14939
21. Chehimi M, Vidal H, Eljaafari A. Pathogenic role of IL-17-producing immune cells in obesity, and related inflammatory diseases. J Clin Med. 2017;6(7):68. doi:10.3390/jcm6070068
22. Nakamizo S, Honda T, Adachi A, et al. High fat diet exacerbates murine psoriatic dermatitis by increasing the number of IL-17-producing γδ T cells. Sci Rep. 2017;7(1):14076. doi:10.1038/s41598-017-14292-1
23. Pestel J, Chehimi M, Bonhomme M, Robert M, Vidal H, Eljaafari A. IL-17A contributes to propagation of inflammation but does not impair adipogenesis and/or insulin response, in adipose tissue of obese individuals. Cytokine. 2020;126:154865. doi:10.1016/j.cyto.2019.154865
24. Chehimi M, Robert M, Bechwaty ME, et al. Adipocytes, like their progenitors, contribute to inflammation of adipose tissues through promotion of Th-17 cells and activation of monocytes, in obese subjects. Adipocyte. 2016;5(3):275–282. doi:10.1080/21623945.2015.1134402
25. Qi Y, Liu W, Wang X, et al. Adipose-derived mesenchymal stem cells from obese mice prevent body weight gain and hyperglycemia. Stem Cell Res Ther. 2021;12(1):277. doi:10.1186/s13287-021-02357-y
26. Ko SH, Chi CC, Yeh ML, Wang SH, Tsai YS, Hsu MY. Lifestyle changes for treating psoriasis. Cochrane Database Syst Rev. 2019;7(7):CD011972. doi:10.1002/14651858.CD011972.pub2
27. Lynch M, Ahern T, Sweeney CM, et al. Adipokines, psoriasis, systemic inflammation, and endothelial dysfunction. Int J Dermatol. 2017;56(11):1103–1118. doi:10.1111/ijd.13699
28. Versini M, Jeandel PY, Rosenthal E, Shoenfeld Y. Obesity in autoimmune diseases: not a passive bystander. Autoimmun Rev. 2014;13(9):981–1000. doi:10.1016/j.autrev.2014.07.001
29. Tadokoro S, Ide S, Tokuyama R, et al. Leptin promotes wound healing in the skin. PLoS One. 2015;10(3):e0121242. doi:10.1371/journal.pone.0121242
30. Zhu KJ, Zhang C, Li M, Zhu CY, Shi G, Fan YM. Leptin levels in patients with psoriasis: a meta-analysis. Clin Exp Dermatol. 2013;38(5):478–483. doi:10.1111/ced.12171
31. Xue K, Liu H, Jian Q, et al. Leptin induces secretion of pro-inflammatory cytokines by human keratinocytes in vitro--a possible reason for increased severity of psoriasis in patients with a high body mass index. Exp Dermatol. 2013;22(6):406–410. doi:10.1111/exd.12162
32. Okan G, Baki AM, Yorulmaz E, Doğru-Abbasoğlu S, Vural P. Serum visfatin, fetuin-A, and pentraxin 3 levels in patients with psoriasis and their relation to disease severity. J Clin Lab Anal. 2016;30(4):284–289. doi:10.1002/jcla.21850
33. Kanda N, Hau CS, Tada Y, Tatsuta A, Sato S, Watanabe S. Visfatin enhances CXCL8, CXCL10, and CCL20 production in human keratinocytes. Endocrinology. 2011;152(8):3155–3164. doi:10.1210/en.2010-1481
34. Shibata S, Saeki H, Tada Y, Karakawa M, Komine M, Tamaki K. Serum high molecular weight adiponectin levels are decreased in psoriasis patients. J Dermatol Sci. 2009;55(1):62–63. doi:10.1016/j.jdermsci.2009.02.009
35. Wang ZV, Scherer PE. Adiponectin, the past two decades. J Mol Cell Biol. 2016;8(2):93–100. doi:10.1093/jmcb/mjw011
36. Ma C, Harskamp CT, Armstrong EJ, Armstrong AW. The association between psoriasis and dyslipidaemia: a systematic review. Br J Dermatol. 2013;168(3):486–495. doi:10.1111/bjd.12101
37. Xiao Y, Jing D, Tang Z, et al. Serum lipids and risk of incident psoriasis: a prospective cohort study from the UK biobank study and Mendelian randomization analysis. J Invest Dermatol. 2022;142(12):3192–3199.e12. doi:10.1016/j.jid.2022.06.015
38. Mohamed-Ali V, Goodrick S, Rawesh A, et al. Subcutaneous adipose tissue releases interleukin-6, but not tumor necrosis factor-alpha, in vivo. J Clin Endocrinol Metab. 1997;82(12):4196–4200. doi:10.1210/jcem.82.12.4450
39. Widawski L, Fabacher T, Spielmann L, et al. Psoriatic arthritis with hyperuricemia: more peripheral, destructive, and challenging to treat. Clin Rheumatol. 2022;41(5):1421–1429. doi:10.1007/s10067-022-06061-x
40. Zhao Z, Cai L, Zhang S, et al. Effects of secukinumab and Adalimumab on serum uric acid level in patients with plaque psoriasis. Chin Med J. 2022;135(12):1438–1443. doi:10.1097/CM9.0000000000002130
41. Wang J, Chen S, Zhao J, et al. Association between nutrient patterns and hyperuricemia: mediation analysis involving obesity indicators in the NHANES. BMC Public Health. 2022;22(1):1981. doi:10.1186/s12889-022-14357-5
42. Bellinato F, Gisondi P, Mantovani A, Girolomoni G, Targher G. Risk of non-alcoholic fatty liver disease in patients with chronic plaque psoriasis: an updated systematic review and meta-analysis of observational studies. J Endocrinol Invest. 2022;45(7):1277–1288. doi:10.1007/s40618-022-01755-0
43. Abedini R, Salehi M, Lajevardi V, Beygi S. Patients with psoriasis are at a higher risk of developing nonalcoholic fatty liver disease. Clin Exp Dermatol. 2015;40(7):722–727. doi:10.1111/ced.12672
44. Shao S, He F, Yang Y, Yuan G, Zhang M, Yu X. Th17 cells in type 1 diabetes. Cell Immunol. 2012;280(1):16–21. doi:10.1016/j.cellimm.2012.11.001
45. Pérez-García A, Torrecilla-Parra M, Fernández-de Frutos M, Martín-Martín Y, Pardo-Marqués V, Ramírez CM. Posttranscriptional regulation of insulin resistance: implications for metabolic diseases. Biomolecules. 2022;12(2):208. doi:10.3390/biom12020208
46. Krakowiak J, Raczkiewicz D, Humeniuk E, Wdowiak A, Wróbel A, Bojar I. Metabolic syndrome, BMI, and polymorphism of estrogen receptor-α in peri- and post-menopausal Polish women. Metabolites. 2022;12(8):673. doi:10.3390/metabo12080673
47. Korovesi A, Dalamaga M, Kotopouli M, Papadavid E. Adherence to the Mediterranean diet is independently associated with psoriasis risk, severity, and quality of life: a cross-sectional observational study. Int J Dermatol. 2019;58(9):e164–e165. doi:10.1111/ijd.14523
48. Castaldo G, Rastrelli L, Galdo G, Molettieri P, Rotondi Aufiero F, Cereda E. Aggressive weight-loss program with a ketogenic induction phase for the treatment of chronic plaque psoriasis: a proof-of-concept, single-arm, open-label clinical trial. Nutrition. 2020;74:110757. doi:10.1016/j.nut.2020.110757
49. Donnelly JE, Blair SN, Jakicic JM, et al. American College of Sports Medicine Position Stand. Appropriate physical activity intervention strategies for weight loss and prevention of weight regain for adults [published correction appears in Med Sci Sports Exerc. 2009 Jul;41(7):1532]. Med Sci Sports Exerc. 2009;41(2):459–471. doi:10.1249/MSS.0b013e3181949333
50. Vella CA, Taylor K, Drummer D. High-intensity interval and moderate-intensity continuous training elicit similar enjoyment and adherence levels in overweight and obese adults. Eur J Sport Sci. 2017;17(9):1203–1211. doi:10.1080/17461391.2017.1359679
51. Johns DJ, Hartmann-Boyce J, Jebb SA, Aveyard P; Behavioural Weight Management Review Group. Diet or exercise interventions vs combined behavioral weight management programs: a systematic review and meta-analysis of direct comparisons. J Acad Nutr Diet. 2014;114(10):1557–1568. doi:10.1016/j.jand.2014.07.005
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
