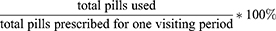Back to Journals » HIV/AIDS - Research and Palliative Care » Volume 14
A Comparison of Adherence and CD4 Cell Count with Respect to Virologic Failure Among HIV-Infected Adults Under Combination Antiretroviral Therapy (cART) at Felege Hiwot Teaching and Specialized Hospital, Bahir Dar, Ethiopia
Authors Tegegne AS , Muluneh MW , Agegn SB , Biresaw HB
Received 1 November 2021
Accepted for publication 13 January 2022
Published 2 February 2022 Volume 2022:14 Pages 33—44
DOI https://doi.org/10.2147/HIV.S346358
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 5
Editor who approved publication: Professor Bassel Sawaya
Awoke Seyoum Tegegne,1 Mitiku Wale Muluneh,2 Setegn Bayabil Agegn,2 Hailegebrael Birhan Biresaw2
1Department of Statistics, Bahir Dar University, Bahir Dar, Ethiopia; 2Department of Statistics, Debre Tabor University, Debre Tabor, Ethiopia
Correspondence: Mitiku Wale Muluneh, Tel + 251-923-23-27-68
, Email [email protected]
Background: Medication adherence plays a significant in the success of combination antiretroviral therapy (cART). Therefore, the current investigation was conducted with the objective of comparing adherence and CD4 cell count with respect to virologic failure among HIV-infected adults under cART.
Methods: A retrospective study design was conducted on 792 randomly selected HIV-infected adult patients who initiated first-line cART enrolled in the first 10 months of 2012 and followed up to August 2018 by using a simple random sampling technique based on their identification number.
Results: The main outcome for the current investigation was the virologic failure which was decreased with successive visits. The area under the receiver operating characteristic curve for adherence and CD4 cell count change were 0.68 and 0.63 with χ2 = 21.2; p-value < 0.001 for the 12-month assessment. Similarly, these areas for the 36th and 60th month assessments were 0.71 and 0.66, with χ2 = 23.2; p-value < 0.001, and 0.73 and 0.71 with χ2 = 24.3; p-value < 0.001 for adherence and CD4 cell count, respectively.
Conclusion: Pill count adherence was more accurate compared to CD4 cell count change for assessing virologic responses. Therefore, because of its easy access, simple use, cost-effectiveness, and accuracy, the adherence to cART was in favor of CD4 cell count change for monitoring the healthcare quality of HIV-infected patients.
Keywords: adherence to cART, CD4 cell count, viral load, HIV, virologic failure, Ethiopia
Background
As the number of HIV-infected patients in combination antiretroviral therapy (cART) increased, searching simple, time- and cost-effective methods for assessing and predicting healthcare quality is crucial. In developed countries, the adherence of pill count and CD4 cell count of HIV-infected patients is assessed considering the amount of viral load using laboratory equipment. However, this might be impractical in resource limited or developing countries because of the unavailability of financial and technical sources.1–3 In its guidelines, the World Health Organization (WHO) recommended, in resource limited settings, to use CD4 cell count for monitoring the healthcare quality of HIV patients in the era of combination Antiretroviral therapy (cART).4 However, this alternative is still difficult to conduct properly for pharmacies where pills to patients at each visiting time can be delivered. Adherence level was measured using pill count, that can be conducted in all clinics (including those without laboratories) and pharmacies, is simple to conduct, and directly measures the healthcare quality, in which providers can intervene.5–7
However, the accuracy of adherence to cART for monitoring the healthcare quality of HIV-infected patients in the era of combination antiretroviral therapy (cART) has not been approved so far.8,9 Previous studies, conducted via two time-points, have shown that adherence has significant intra-individual variability.10 Hence, series assessments with more than two time points may have more prognostic value.11 Therefore, the objective of the current investigation was to check/prove the accuracy of adherence to cART in monitoring the healthcare quality of HIV-infected adults with triangulation of their results to the viral load assessments obtained using laboratory equipment. Hence, the current investigation also aimed to compare adherence and CD4 cell count with respect to virologic failure among HIV-infected adults under cART.
Materials and Methods
Study Design, Period, and Area
The institutional-based retrospective study design was conducted to predict and detect the virologic failure from September 2012 to August 2018. The study was conducted at Felege Hiwot Teaching and Specialized Hospital, Amhara region, in north-western Ethiopia. The hospital is also referral, meaning that different patients fromother district hospitals in Amhara region are referred to this hospital.
Study Participants
All HIV-positive people starting their cART at Felege Hiwot Teaching and Specialized Hospital, Bahir Dar, Ethiopia under follow-up during September 2012 to August 2018 were the study population.
Eligibility Criteria
Patients whose were aged at least 18 years, whose baseline CD4 cell count was at most 200 cells/mL, and those patients with WHO stage IV and patients who started their cART were included under the current investigation. Patients who had at least one undetectable viral load after initiation of cART were included in this study. However, HIV-positive children and adults who had follow-ups <6 months in the hospital were excluded in this study.
Study Variables
The primary variable of interest for this investigation was the amount of viral load (copies/milliliter (mL)). Hence, the amount of viral load ≥1,000 copies /mL of blood were indicators of treatment failure or lack of virologic response.
Operational Definition
Good adherence to ART is a patient taking ≥95% of the recommended regime, fair adherence to treatment is defined as a patient taking the drug between 85% and 95% of the recommended regimen, and poor adherence to treatment is defined as a patient taking <85% of the recommended regimen.12
Data Collection
The data related to patients’ socio-demographic and clinical performance were recorded and collected by health staff after detailed orientations about the variables included under investigation. In collecting/recording performance of CD4 cell count change on viral load results, the health staff were oriented to use the same laboratory equipment for all patients to reduce the variation of accuracy of laboratory equipment. In the case of pill count adherence data, the adherence level was calculated by the medical staff using the formula;13–15
This helps to categorize patients as adherent or non-adherent.16 Patients whose adherence level was ≥95% of the prescribed medication were categorized as adherent, otherwise, they were categorized as non-adherent. Therefore, in the current investigation, 95% was considered as the cut-off point for positive predictive values (PPVs) and negative predictive values (NPVs).17
Data Analysis
Potential socio-demographic variables were assessed using non-parametric data analysis (median and Wilcoxon sign rank test for continuous predictors and chi-square test for categorical data). The overall accuracy of the two comparable approaches, CD4 cell count change, and adherence were demonstrated using a ROC curve with the inclusion of the 95% confidence interval (CI). In this process, large areas in ROC are considered as evidence of greater accuracy or the ability to indicate patients with virologic failure. The area under ROC had been denoted to be C, which stands for concordance statistics.18 In the case of binary responses, Concordance (C) statistics are the proportion of patients with event to those without event such that patients with event had great predicted probability of the outcome.19 The C-statistics were compared by applying the chi-square tests in SPSS version 23. The sensitivities for virologic failure of the current investigation were analyzed based on the level of 1,000 copies/mL.20
For the test of characteristics and percentage of each patient, the characteristics of adherence to cART and CD4 cell count alone were compared to each other. The predictive power of adherence to cART was done for 3 months, before CD4 cell count and viral load assessments. Similarly, the predictive power of CD4 cell count and adherence were assessed using positive and negative predictive values and sensitivity and specificity.17
Results
There were 792 adults who initiated cART and all the necessary information available in the ART database was included in the current study. However, the participants at each visit decreased from visit to visit and participants were recorded as 792 patients at the 1st time point (12th month), 740 patients at the 2nd time point (36th month), and 710 patients at the 3rd time point (60th month). Potential predictor variables for virologic failure are indicated in Table 1. The median and interquartile range for CD4 cell count change were smaller for those patients who did not have full follow-ups. The number of patients who got Virologic failure for the three time points (1st, 2nd, and 3rd time points) were 428 (54%), 420 (56.8%), and 418 (58.8%), respectively. This indicates that the reduced number of CD4 cell counts at each time point was highly associated with an increase in number for the virologic failure. The increased CD4 cell count changes were significantly smaller for those experiencing virologic failure, as indicated in Table 1 for 1st, 2nd, and 3rd time points (Table 1).
 |
Table 1 Characteristic of Patients with Virologic Failure at 1st, 2nd, and 3rd Time Points after Commencement of cART |
The areas under the curves for two responses are indicated in Figures 1–Figure 3 for the 1st, 2nd, and 3rd time point, respectively. Hence, the results in this investigation indicate that, using area under ROC curve, adherence had a larger area as compared to CD4 cell count. The larger area under the curve is evidence that it is more accurate to predict patients with virologic failure as compared to the others.
 |
Figure 1 The area under the ROC for CD4 cell count and adherence at the 1st time point. |
 |
Figure 2 The area under the ROC for CD4 cell count and adherence at the 2nd time point. |
 |
Figure 3 The area under the ROC for CD4 cell count and adherence at the 3rd time point. |
Areas under the curves (AUCs) for the first time points were 0.68, 95% CI=0.57–0.73 for adherence and 0.63, 95% CI=0.35–0.72 for CD4 cell count, with χ2=21.2. The area under the curves for adherence at the 2nd time point was 0.71, 95% CI=0.54–0.75 and that of CD4 cell count was 0.66, 95% CI=0.54–0.75 with χ2=23.2. Similarly, for the 3rd time point, the area under the curve for adherence was 0.73, 95% CI=0.67–0.85 and 0.71, 95% CI=0.64–0.82 for CD4 cell count with χ2=24.3. The values 25, 50, and 100 increased as the number of visits increased, indicating that overall accuracy increased (Table 2).
 |
Table 2 Test Characteristics for CD4 Cell Count Change in Identifying Patients with Virologic Failure after Commencement of cART Assessed at 1st, 2nd, and 3rd Time Points |
In Table 3, the cut-off point for adherence level for PPVs was 95% and for NPVs was 95%. Comparing the pairwise results in Table 2 for CD4 cell count and Table 3 for adherence to cART, based on the cut-off points at each of the three time points, adherence to cART was more accurate compared to that of CD4 cell count. Hence, the power of identifying patients with virologic failure using adherence to cART was more accurate compared to CD4 cell count change. The use of an adherence threshold alone for 95% at the 1st, 2nd, and 3rd time points after initiation of cART resulted in NPVs of 0.89 (0.83–0.93), 0.92 (0.85–0.96), and 0.96 (0.88–0.98), respectively. Moreover, 0.79 (0.65–0.85), 0.73 (0.66–0.82), and 0.71 (0.62–0.82) of all patients without virologic failure satisfied the combined criterion at the 1st, 2nd and 3rd time points, respectively (Table 3).
 |
Table 3 Test Characteristics of Adherence to cART Level in Identifying Patients with Virologic Failure after Commencement of cART Assessed at 1st, 2nd, and 3rd Time-Points |
Table 4 indicates that CD4 cell count and adherence were strongly associated with breakthrough viremia. Hence, there was no significant difference between adherence to cART and CD4 cell count change from maximum treatment values to follow-ups in identifying patients with breakthrough viremia (AUCs, 0.66 [0.55–0.74] against 0.68 [0.57–0.73] with χ2=0.4, p-value =0.67). To compare the predictive power of adherence with the combination of adherence and CD4 cell count data, tabulating the PPVs and NPVs were crucial. Similarly, tabulating sensitivity and specificity for adherence lonely and a combination of adherence and CD4 cell data were also important.
 |
Table 4 Test Characteristics for CD4 Cell Count Change and Adherence Levels for Identifying Patients with Breakthrough Virologic Failure after First Visiting Time |
As the time point for viral load assessment increased, both the PPVs and percentage of patients with PPVs increased, which implies that the power of identifying patients with virologic failure increased as time points increased. The result also indicates that the power of PPVs for patients with virologic failure using the combined CD4 cell count change and adherence to cART at their reference values (CD4 cell count ≤50% and adherence <85%) were approximately the same; (0.59 (0.43–0.68) against 0.62 (0.54–0.73)) (Table 5).
 |
Table 5 PPVs of CD4 Cell Count and Adherence Level for Assessment of Virologic Failure at the 1st, 2nd, and 3rd Time Points |
Adherence and CD4 cell combined data used a cut-off-point of 95%, the values were reduced when CD4 cell count and adherence combined data were used at each of the time points (refer to Table 6). Hence, the power of point estimation for positive predictive values or negative predictive values using adherence lonely (Table 3) was in favor of those combined CD4 cell and adherence data (Table 6). In Table 6, CD4 cell count reduced to ≤40% and adherence reduced to ≤30% have p-values <0.05; hence, they had a high power of identifying patients with breakthrough viremia. However, the p-values for the other comparable response, adherence to cART were all significant except adherence level ≤100%. The significant p-values were evidence of their accuracy in identifying patients with breakthrough viremia. This procedure also implies that adherence to cART was accurate compared to CD4 cell count.
 |
Table 6 NPVs for Combined CD4 Cell Count and Adherence Level for Virologic Failure at the 1st, 2nd, and 3rd Time-Points |
Discussion
The result of the current investigation showed that adherence levels measured using pill count were more accurate compared to CD4 cell count change for identifying patients with virologic failures in the assessment of the healthcare quality of HIV-infected patients in the era of antiretroviral therapy. This result was consistent in evaluation of the healthcare quality of patients at three time points after initiation of cART. The results obtained in these assessments were independent on the level of viremia/virologic failures.
The results obtained in this investigation were consistent with some previous studies,10 but contradicted one other previous study,21 and the findings obtained in the current investigation were important for resource limited settings, especially in developing countries.
The results of pill count adherence done before 3 months of viral load assessment were more accurate compared to CD4 cell count change conducted simultaneously with viral load assessment in identifying patients with virologic failure. This cost-effective and at least as accurate as CD4 cell count change for viral load assessment was not seen by the previous investigators in the study area22 and many of them recommended CD4 cell count change as an alternative approach for viral load assessment. However, CD4 cell count change cannot be assessed in pharmacies where they can deliver pills for patients. Therefore, pill count adherence in the cART program can be considered as an alternative method for viral load assessments in monitoring healthcare quality of HIV-infected patients in the era of antiretroviral therapy. Patients who got virologic failure in turn lead to immunologic decline and poor longevity.23 Importantly, unlike CD4 cell count detection of patients in viral load assessment, adherence to cART results was consistent in different assessments of time points. This result was consistent with findings obtained in previous research.22 The results of the current investigation also indicated that assessment of healthcare quality using adherence lonely done early aids in preventing virologic failure and assures the longevity of patients with less-expensive first-line cART, and this contrasts with assessments of health status of HIV-infected patients using CD4 cell count change, which detects patients with virologic failure after the occurrence of the event.24
This result could be important for pharmacies and clinics (with no laboratory equipment) who are delivering pills for HIV-infected patients. Even clinicians who are performing viral load assessment for all patients in a tiresome way could use an adherence approach to guide decision–making on timing these tests. To apply adherence to the cART approach in assessing the health status of the patients, the healthcare service providers should inform the patients not to discard the unused pills and advise them to bring bottles to the healthcare delivery center. The health staff should also count the unused pills for calculation of adherence. One other method of applying adherence was using predictive values where it is more applicable in settings that the assays are available to some extent. In this approach, the high negative predictive values indicated that there was a relatively large group of patients with low probability of having virologic failure.25
The combined use of adherence result with one threshold result of CD4 cell count was also used for assessment of health status of patients. The estimation accuracy of adherence to cART for virologic failure in the current investigation was consistent with results presented from two other studies.26,27 Since the inclusion and exclusion criteria for the current investigation were strict, we did not feel that significant bias resulted from patient exclusions.27,28
The adherence calculation was based on pill counts, and patients receiving cART at free clinics should submit/bring back all unused pills. In addition, alternative measures of adherence, including subjective measures that were not available in current investigation,29 should be examined for its accuracy.
Patterns of adherence may influence the risk of virologic failure differently when patients initiate therapy compared to when their viral loads are suppressed below quantifiable levels. Since nearly 25% of all drug resistance occurs in patients with high levels of adherence,30 it would have been interesting to explore the relationship between virologic failure, resistance, and diagnostic accuracy of adherence in this setting.
Although CD4 counts inform when to start antiretroviral therapy and when to stop prophylaxis for opportunistic infections, these data may be used as additional information for revision of guidelines based on previous studies.31,32
Limitations of the Study
The current study was not without limitations. One of the limitations is that the research was conducted on a single treatment site. Including other treatment sites might include additional information about the predictive values of the two comparables (CD4 cell count and adherence to pill count). In this study, since we used secondary data, either we failed to capture some important variables due to missing records or the variables may not be required for patient care purposes.
Conclusion
As a conclusion, adherence to cART assessed using AUC for ROC curves was more accurate in detecting current virologic failure during the initial year after commencement of cART. In detecting current breakthrough viremia, adherence was accurate compared to CD4 cell count. Additionally, adherence to cART had better prediction ability for patients with virologic failure as compared to the CD4 cell count change. This result indicated that the potential of adherence in prediction of patients with virologic failure before it occurred. Therefore, adherence to cART is simple, accurate, and cost-effective for monitoring healthcare quality for HIV-infected patients in the era of antiretroviral therapy. In monitoring healthcare quality for patients in the era of cART, in resource-limited settings, pill count adherence as an alternative approach should be taken into consideration. Patients should take their medication as prescribed and consult their clinician when needed. Health professionals should monitor and evaluate the recording systems and improve health information system. Furthermore, the health staff should also count the unused pills for calculation of adherence.
Data Sharing Statement
The data will be available upon request from the corresponding author.
Ethical Approval and Consent to Participate
Bahir Dar University Ethical Review Board approved the study as per the Declaration of Helsinki. Ethical clearance was obtained from Bahir Dar University, College of Science, and Ethical Review Committee with Ref≠ BDU/017/2020. Informed consent was waived by the review committee as all the data source of patients’ medical record numbers was anonymously registered using codes without personal identifiers such as the names of patients. The individual patients were not subject to any harm as far as confidentiality was kept.
Acknowledgment
The authors would like to thank the Amhara National Regional State Health Bureau, and staff of Felege Hiwot Teaching and Specialized Hospital, Ethiopia, for the data they supplied for this research to be successful. The authors also acknowledge Prof. Abiy Yigzaw for his language editing and Prof. Getu Degu for his constructive comments on the medical outcomes and medical terminologies.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising, or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that they have no competing interests in this work.
References
1. Lichtfuss GF, Hoy J, Rajasuriar R, Kramski M, Crowe SM, Lewin SR. Biomarkers of immune dysfunction following combination antiretroviral therapy for HIV infection. Biomark Med. 2011;5(2):171–186. doi:10.2217/bmm.11.15
2. Langebeek N, Gisolf EH, Reiss P, et al. Predictors and correlates of adherence to combination antiretroviral therapy (ART) for chronic HIV infection: a meta-analysis. BMC Med. 2014;12(1):1–14.
3. Sabin CA. Do people with HIV infection have a normal life expectancy in the era of combination antiretroviral therapy? BMC Med. 2013;11(1):1–7. doi:10.1186/1741-7015-11-251
4. Welles S, Jackson J, Yen-Lieberman B, et al. Prognostic value of plasma human immunodeficiency virus type 1 (HIV-1) RNA levels in patients with advanced HIV-1 disease and with little or no prior Zidovudine therapy. Clin Infect Dis. 1996;174(4):696–703. doi:10.1093/infdis/174.4.696
5. Aidala AA, Wilson MG, Shubert V, et al. Housing status, medical care, and health outcomes among people living with HIV/AIDS: a systematic review. Am J Public Health. 2016;106(1):e1–e23. doi:10.2105/AJPH.2015.302905
6. Group SfMoATS. CD4+ count–guided interruption of antiretroviral treatment. N Engl j Med. 2006;2006(355):2283–2296.
7. Nachega JB, Hislop M, Dowdy DW, Chaisson RE, Regensberg L, Maartens G. Adherence to nonnucleoside reverse transcriptase inhibitor–based HIV therapy and virologic outcomesEffect of NNRTI–based HIV therapy adherence on HIV-1 RNA. Ann Intern Med. 2007;146(8):564–573. doi:10.7326/0003-4819-146-8-200704170-00007
8. Gross R, Bilker WB, Friedman HM, Strom BL. Effect of adherence to newly initiated antiretroviral therapy on plasma viral load. Aids. 2001;15(16):2109–2117. doi:10.1097/00002030-200111090-00006
9. Bangsberg DR. Less than 95% adherence to nonnucleoside reverse-transcriptase inhibitor therapy can lead to viral suppression. Clin Infect Dis. 2006;43(7):939–941. doi:10.1086/507526
10. Bisson GP, Gross R, Bellamy S, et al. Pharmacy refill adherence compared with CD4 count changes for monitoring HIV-infected adults on antiretroviral therapy. PLoS Med. 2008;5(5):e109. doi:10.1371/journal.pmed.0050109
11. Nachega JB, Hislop M, Dowdy DW, et al. Adherence to highly active antiretroviral therapy assessed by pharmacy claims predicts survival in HIV-infected South African adults. J Acquir Immune Defic Syndr. 2006;43(1):78–84. doi:10.1097/01.qai.0000225015.43266.46
12. Deribew A, Biadgilign S, Berhanu D, et al. Capacity of health facilities for diagnosis and treatment of HIV/AIDS in Ethiopia. BMC Health Serv Res. 2018;18(1):1–8. doi:10.1186/s12913-018-3347-8
13. Bangsberg DR, Moss AR, Deeks SG. Paradoxes of adherence and drug resistance to HIV antiretroviral therapy. J Antimicrob Chemother. 2004;53(5):696–699. doi:10.1093/jac/dkh162
14. Walsh JC, Pozniak AL, Nelson MR, Mandalia S, Gazzard BG. Virologic rebound on HAART in the context of low treatment adherence is associated with a low prevalence of antiretroviral drug resistance. J Acquir Immune Defic Syndr. 2002;30(3):278–287. doi:10.1097/00126334-200207010-00003
15. Sethi AK, Celentano DD, Gange SJ, Moore RD, Gallant JE. Association between adherence to antiretroviral therapy and human immunodeficiency virus drug resistance. Clin Infect Dis. 2003;37(8):1112–1118. doi:10.1086/378301
16. Grossberg R, Gross R. Use of pharmacy refill data as a measure of antiretroviral adherence. Curr HIV/AIDS Rep. 2007;4(4):187–191. doi:10.1007/s11904-007-0027-4
17. Trevethan R. Sensitivity, specificity, and predictive values: foundations, pliabilities, and pitfalls in research and practice. Front Public Health. 2017;5:307. doi:10.3389/fpubh.2017.00307
18. Simoni JM, Kurth AE, Pearson CR, Pantalone DW, Merrill JO, Frick PA. Self-report measures of antiretroviral therapy adherence: a review with recommendations for HIV research and clinical management. AIDS Behav. 2006;10(3):227–245. doi:10.1007/s10461-006-9078-6
19. Nieuwkerk PT, Oort FJ. Self-reported adherence to antiretroviral therapy for HIV-1 infection and virologic treatment response: a meta-analysis. J Acquir Immune Defic Syndr. 2005;38(4):445–448. doi:10.1097/01.qai.0000147522.34369.12
20. Moore DM, Mermin J, Awor A, Yip B, Hogg RS, Montaner JS. Performance of immunologic responses in predicting viral load suppression: implications for monitoring patients in resource-limited settings. J Acquir Immune Defic Syndr. 2006;43(4):436–439. doi:10.1097/01.qai.0000243105.80393.42
21. Efron B, Tibshirani R. Bootstrap methods for standard errors, confidence intervals, and other measures of statistical accuracy. Stat Sci. 1986;1:54–75.
22. Hanley JA, McNeil BJ. The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology. 1982;143(1):29–36. doi:10.1148/radiology.143.1.7063747
23. Efron B. Better bootstrap confidence intervals. J Am Stat Assoc. 1987;82(397):171–185. doi:10.1080/01621459.1987.10478410
24. Fullman N, Yearwood J, Abay SM, et al. Measuring performance on the Healthcare Access and Quality Index for 195 countries and territories and selected subnational locations: a systematic analysis from the Global Burden of Disease Study 2016. Lancet. 2018;391(10136):2236–2271.
25. Dunbar-Jacob J, Rohay JM. Predictors of medication adherence: fact or artifact. J Behav Med. 2016;39(6):957–968. doi:10.1007/s10865-016-9752-8
26. Collaboration ATC. Prognostic importance of initial response in HIV-1 infected patients starting potent antiretroviral therapy: analysis of prospective studies. Lancet. 2003;362(9385):679–686. doi:10.1016/S0140-6736(03)14229-8
27. Arnsten JH, Demas PA, Farzadegan H, et al. Antiretroviral therapy adherence and viral suppression in HIV-infected drug users: comparison of self-report and electronic monitoring. Clin Infect Dis. 2001;33(8):1417–1423. doi:10.1086/323201
28. Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. JAMA. 2000;283(15):2008–2012. doi:10.1001/jama.283.15.2008
29. Bisson G, Gross R, Gaolathe T, et al. Diagnostic characteristics of CD4 cell count response in predicting virologic response in HIV-infected patients initiating HAART in Botswana. Pharmacoepidemiol Drug Saf. 2006;15:S67–S68.
30. Coetzee D, Hildebrand K, Boulle A, et al. Outcomes after two years of providing antiretroviral treatment in Khayelitsha, South Africa. Aids. 2004;18(6):887–895. doi:10.1097/00002030-200404090-00006
31. Initiative TMb, World Health Organization. Scaling up antiretroviral therapy in resource-limited settings: treatment guidelines for a public health approach; 2004.
32. Curran J, Debas H, Arya M, Kelley P, Knobler S, Pray L. Scaling up antiretroviral therapy in resource-limited settings: treatment guidelines for a Public Health Approach; 2005.
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.