Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 16
A Comparative Study on Adipose-Derived Mesenchymal Stem Cells Secretome Delivery Using Microneedling and Fractional CO2 Laser for Facial Skin Rejuvenation
Authors Yusharyahya SN , Japranata VV , Sitohang IBS , Legiawati L , Novianto E , Suseno LS , Rachmani K
Received 24 December 2022
Accepted for publication 1 February 2023
Published 10 February 2023 Volume 2023:16 Pages 387—395
DOI https://doi.org/10.2147/CCID.S401839
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jeffrey Weinberg
Shannaz Nadia Yusharyahya,1 Valdi Ven Japranata,2 Irma Bernadette S Sitohang,1 Lili Legiawati,1 Endi Novianto,1 Lis Surachmiati Suseno,1 Karin Rachmani1
1Department of Dermatology and Venereology, Faculty of Medicine, Universitas Indonesia - Dr. Cipto Mangunkusumo Hospital, Jakarta Pusat, Indonesia; 2Faculty of Medicine, Universitas Indonesia, Jakarta Pusat, Indonesia
Correspondence: Shannaz Nadia Yusharyahya, Department of Dermatology and Venereology, Faculty of Medicine, Universitas Indonesia - Dr. Cipto Mangunkusumo Hospital, Jalan Diponegoro Nomor 71, Kenari, Senen, Jakarta Pusat, 10430, Indonesia, Tel/Fax +62 21 31935383, Email [email protected]
Background: The efficacy of adipose-derived mesenchymal stem cells (ADMSCs) secretome for skin aging has been established, yet no studies hitherto directly investigated the best administration method for such purpose.
Purpose: We aimed to compare microneedling (MN) versus fractional CO2 laser (FL) as methods of delivery for ADMSCs secretome in the treatment of aging skin.
Patients and Methods: A single-blind, randomized split-face clinical trial was conducted on 30 Indonesian women (aged 35– 59 years old) with signs of facial cutaneous senescence. Their initial aging status was assessed by dermoscopy photoaging scale (DPAS) and Janus-III measurement system. In the second and fourth weeks, all participants were treated with both MN and FL, followed by the application of a four-fold concentrated ADMSC secretome. The assignment of which side of the face received MN or FL was done by computer-based randomization. Skin parameters were reevaluated on the fourth and sixth weeks, along with patient satisfaction, level of comfort, preference for administration techniques, and also adverse events experienced during the study. Appropriate statistical analyses were subsequently performed at a significance level of 0.05.
Results: Significant improvements in total DPAS and wrinkles were found in the MN and FL groups at the end of the trial. In contrast, no statistical differences in all parameters were observed between groups in the fourth and sixth weeks. FL scored higher than MN for satisfaction and preference, but lower in terms of comfort. Pain, burning sensation, and itch were the side effects experienced by subjects upon treatment. Two patients had prolonged reddish skin succeeding FL treatment, which relieved with moisturizer application.
Conclusion: Both MN and FL yielded comparable results for improving several skin aging features. However, subjective preference for ADMSCs secretome administration method may differ when considering satisfaction, comfort, and possible adverse events.
Keywords: adipose-derived mesenchymal stem cells, fractional CO2 laser, microneedling, secretome, skin rejuvenation
A Letter to the Editor has been published for this article.
A Response to Letter by Mrs Rinendyaputri has been published for this article.
Introduction
Aging is an inevitable phenomenon observed in all living organisms, marked by the anatomical and physiological integrity decline from cellular to individual levels, as a direct consequence of the elevated biological lifespan.1 As the largest and most exhibitive organ, skin cosmetically reflects self-confidence and thus, its alterations related to senescence directly impact a personal quality of life. The hallmark of skin aging, which clearly manifests as wrinkles, loss of elasticity, rough-textured appearance, thinning, and dryness, results from cellular and extracellular matrix phenotypic changes due to intrinsic and extrinsic factors.2–4 Various measures have been studied to prevent and reverse the aging process, such as routine application of skin care products, regulating diet intake, limiting sunlight exposure, and aesthetic surgical and non-surgical procedures.4 The current situation conceivably promotes economic growth for the aesthetic cosmeceutical industry.
Recent technological advancements have enabled researchers to discover the vast advantages of adipose-derived mesenchymal stem cells (ADMSCs) products, which have been regarded as promising candidates for aging therapy. Under conditioned medium in vitro, ADMSCs actively produce growth factors, chemokines, cytokines, and extracellular matrix proteins, collectively known as secretome.5 Previous studies have scientifically uncovered their potential for skin rejuvenation through augmenting collagen synthesis6 and inhibiting fibroblast apoptosis following ultraviolet radiation (photoaging).6,7 Furthermore, relatively lower immunogenicity, and the uncomplicated handling, render ADMSCs secretome more favorable over the cell line for therapeutic purposes.8,9
Apart from the type of ADMSCs-based product, administration techniques are also deemed to affect the outcomes. Microneedle treatment prior to topical ADMSCs secretome administration has been known to enhance macroscopic skin profile, epidermal thickness, skin capacitance, and the amount of dermal collagen and elastic fibers.10,11 However, adverse events have been reported, including pinpoint bleeding, erythema, edema, pain, postinflammatory hyperpigmentation, and tram-track-shaped scar formation.12 Likewise, the utilization of fractional CO2 laser for skin ablation to introduce ADMSCs secretome, albeit expensive, has been demonstrated to decrease transepidermal water loss (TEWL), redness, pigmentation, as well as improve wound healing and hypertrophic scars.13,14 However, to the best of our knowledge, no studies to date have compared the effectiveness, nor the side effects, between the currently accessible methods of microneedling (MN) and fractional CO2 laser resurfacing (FL) for initiating ADMSCs secretome in the management of skin aging.
Methods
The complete protocol for this interventional study was designed based on the Consolidated Standards of Reporting Trials (CONSORT) guideline and was registered at clinicaltrials.gov on 17th August 2022 with the identification number NCT05508191.
Ethical Approval
This clinical trial has passed the ethical review by the Health Research Ethics Committee of the Faculty of Medicine, Universitas Indonesia on 15th August 2022 (registration number KET-837/UN2.F1/ETIK/PPM.00.02/2022) and Dr. Cipto Mangunkusumo Hospital on 9th September 2022 (registration number LB.02.01/2.6.1/957/2022).
Study Population and Sample Selection
In this study, we included Indonesian females aged 35 to 59 years old with apparent facial skin aging, particularly denoted by wrinkles. During the recruitment period, we selected subjects against exclusion criteria, including prior history of hypertrophic scar or keloid formation, autoimmune disorders, malignancies, or hypersensitivity to topical anesthesia and/or retinoic acid. Moreover, applicants who had used products containing retinoic acid in the last 6 months or underwent long-term immunosuppressive medications were also ineligible for this study.
The qualified subjects were comprehensively informed concerning the study protocol and under which circumstances their participation would be revoked, including contracting coronavirus disease 2019 at any time during the trial, deliberately refraining before the trial completion, being absent for more than 2 days from the scheduled appointment, and suffering from major adverse event(s) caused by topical regimes or treatment given during the trial, for instance, allergic drug eruption or severe contact dermatitis. In case of such incident(s) occurred, they would receive proper management. Ultimately, the subjects gave their consent to engage in the six-week clinical trial, which consisted of four meetings each with an interval of 2 weeks.
Preparation of the ADMSCs Secretome
For this clinical study, we used a prototype of allogenic ADMSCs secretome produced by our Stem Cell Medical Technology Integrated Service Installation at Dr. Cipto Mangunkusumo Hospital. The secretome was isolated from ADMSCs cultured in Dulbecco’s modified Eagle’s medium (DMEM) with the maximum passage number of five and the addition of antibiotics for 6 days. Afterward, the stem cell culture was extracted using a filtered syringe with a pore size of 0.22 µm to eliminate the debris. An ultrafiltration centrifugator was utilized to obtain a 4-fold concentrate of ADMSCs secretome, which is further referred to as concentrated secretome. The stabilization test showed that the optimal environmental temperature for storage and thawing was at −20°C and 4°C, respectively. Therefore, the final products were constantly kept at the designated temperature in advance of its usage in our study. In addition, the quality analysis upon our concentrated secretome revealed that it contains these following factors: bone morphogenetic proteins (BMP-2, BMP-7), interleukins (IL-6, IL-10), epidermal growth factor (EGF), beta-nerve growth factor (β-NGF), insulin-like growth factor 1 (IGF-1), hepatocyte growth factor (HGF), platelet-derived growth factor AB (PDGF-AB), fibroblast growth factor 4 (FGF-4), tumor necrosis factor-alpha (TNF-α), and interferon-gamma (IFN-γ).
Examination of the Participants
At the beginning of the study (week 0), a thorough evaluation of facial skin aging status was conducted on the participants with two modalities, Dermoscopy Photoaging Scale (DPAS) and Janus-III measurement system. A dermoscope was used for DPAS assessment, with which we determined the presence of 11 parameters of skin aging (yellowish papules, solar elastosis, skin atrophy, lentigo, hypopigmented and hyperpigmented macules, telangiectasia, actinic keratosis, senile comedones, superficial wrinkles, deep wrinkles, and criss-cross wrinkles) on four areas: forehead, periorbita, zygoma, and mandible. Every finding on each area had a value of 1; therefore, the maximum possible total DPAS score for each face side was 44. As for the skin analysis using Janus-III, eight variables of cutaneous senescence (wrinkles, pore size, pigmentation, ultraviolet spots, moisture, elasticity, porphyrin, and sebum) were evaluated at each designated area of facial skin. Both examinations were repeated at identical skin areas on the second, fourth, and sixth weeks.
Clinical Trial
Following an early skin evaluation at week 0, the subjects were instructed to perform facial priming by applying a neutral facial cleanser, sunscreen with a sun protection factor (SPF) of 45, and 0.05% retinoic acid cream daily at home until the next appointment (week 2). Upon arrival, they received facial skin anesthesia with a cream consisting of 2.5% lidocaine and 2.5% prilocaine for 30 min, followed by MN treatment on one side of the face and FL on the other half, until diffuse erythema was achieved. The assignment of which side received a specific treatment was determined with computer-based randomization. The investigators evaluating the skin aging parameters were blinded by this randomization until the end of data collection process. Dermapen with 36 fine needles was used for MN in the ordered directions (vertical, horizontal, and diagonal) with a depth of 150 µm, while FL was conducted under the recommended settings (15 mJ energy, 900 µs pulse duration, density level 15, and depth level 2) so they both would produce an equivalent penetrating effect. The endpoint for both methods was diffuse erythema. A total of 3 mL concentrated secretome was then topically administered to both parts of the face in a uniformly distributed manner. The exact procedures were repeated in the fourth week. At the same time, the daily face priming regimen was continued after restricting the usage of facial cleanser for 4 h, sunscreen for 1 day, and retinoic acid cream for 3 days from the secretome application.
At the completion of trial, we required each participant to rate both treatments in terms of satisfaction and comfort using the Likert scale from 0 (unsatisfied) to 10 (extremely satisfied) and pain visual analog scale (VAS) from 0 (no pain) to 10 (extreme pain), respectively. We divided the satisfaction level into three groups: less satisfied (0–3), satisfied (4–6), and very satisfied (7–10); and the pain VAS into four groups: no pain (0), mild (1–3), moderate (4–6), severe (7–10). Any adverse effects during the clinical trial and the subject’s preference for the administration method were recorded.
Data Collection and Analysis
The entire collected data from the investigational study were compiled utilizing the Statistical Package for the Social Sciences (SPSS)® version 23.0. Subsequent analyses were performed on the same software with inferential statistics at a significance level of 0.05. Normally distributed data are reported in mean ± standard deviation, otherwise they are expressed in median (interquartile range).
Clinical Study Statement
This clinical study was conducted according to the Ministry of Health Republic of Indonesia Regulation number 63 (2017) about research involving human subjects (Good Clinical Practice) and adhered to the Declaration of Helsinki (1964).
Results
Participants’ Selection for Clinical Trial
A total of 59 prospective participants registered during the enrollment period (10th and 11th September 2022). Following selection against exclusion criteria and written consent, we included 30 female subjects with a mean age of 47.97 ± 6.03 years old for a single-blind, randomized clinical trial. All were treated with both MN (n = 30) and FL (n = 30) in a split-face manner. None dropped out during the clinical study (12th September 2022 until 26th October 2022), and the collected data were incorporated into the final analysis (Figure 1).
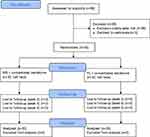 |
Figure 1 The flowchart of this clinical trial according to CONSORT guideline. |
Dermoscopic Appearance and DPAS
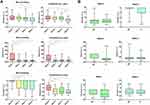
For 6 weeks of trial, we regularly evaluated the qualitative dermoscopic appearance for skin-aging features and the quantitative total DPAS (Figure 2). Subjective improvements of several cutaneous aging signs, particularly hyperpigmented macules and fine wrinkles, were noticed in all observation areas. In addition, a statistically significant reduction of total DPAS was obtained on week 6 of the trial for MN and FL groups, compared to their respective baseline [DPAS MN week 0: 9.00 (4.00) vs DPAS MN week 6: 8.00 (2.25), p<0.01; DPAS FL week 0: 10.00 (4.00) vs DPAS FL week 6: 7.50 (3.00), p<0.01]. On the other hand, the intergroup comparison of DPAS in weeks 4 and 6 showed no significant differences.
Janus-III Measurement System
Correspondingly, we performed intragroup and intergroup comparisons of Janus-III parameters during the trial (Figure 3). No significant disparities existed in MN and FL groups, regarding pore size, pigmentation, ultraviolet spots, moisture, and elasticity. As for wrinkles parameter, both treatments displayed significant reduction on week 6 compared to their corresponding initial status [wrinkle MN week 0: 3.50 (9.25) vs wrinkle MN week 6: −2.50 (7.00), p<0.001; wrinkle FL week 0: 2.5 (8.00) vs wrinkle FL week 6: −1.00 (9.00), p<0.001]. Similar findings were also reported in both groups for sebum [MN week 0: −31.50 (182.75) vs MN week 6: −64.00 (12.50), p<0.001; FL week 0: −35.00 (134.50) vs FL week 6: −62.50 (13.75), p<0.001] and porphyrin [MN week 0: −1.80 ± 16.30 vs MN week 6: −18.70 ± 16.04, p<0.001; FL week 0: 0.00 (25.50) vs FL week 6: −23.50 (17.25), p<0.001] parameters. However, the differences were noticed on week 2 (p<0.01 for sebum and p<0.001 for porphyrin in both groups), showing the influence of retinoic acid priming. Furthermore, comparisons between MN and FL groups on weeks 4 and 6 yielded no marked discrepancies in all parameters.
Adverse Events, Satisfaction and Comfort Level, and the Subjects’ Preference
We also summarize the adverse effects, satisfaction level assessed by the Likert scale, comfort level evaluated with pain VAS, and the subjective preference of MN and FL (Table 1). All subjects reported reddish skin (as expected) in both groups. The erythema subsided within 24 h in 28 subjects (data not shown) and two subjects had prolonged erythema following FL treatment (one patient each on the second and fourth week), which relieved after five and six days of moisturizer application. Pain was experienced by the entire participants when receiving FL treatment and half of them upon MN treatment. Burning sensation following the treatment was described mainly in the FL group (73.3%), but only a fraction in the MN group (13.3%). Regarding satisfaction, many participants were extremely pleased with the results of both treatments (80.0% in MN and 93.3% in FL). In terms of comfort, 24 subjects (80.0%) experienced none to mild pain upon MN treatment, while the same number of participants endured moderate-to-severe pain when treated with FL. Given the outcomes, 20 subjects (66.7%) opted for FL over MN as the preferable ADMSCs secretome delivery method for skin rejuvenation.
 |
Table 1 Assessment Upon Adverse Effects, Satisfaction and Comfort Level, and Subjective Preference of MN and FL Treatment (n = 30) |
Discussion
MN and FL techniques have been utilized in cosmetic dermatology for numerous purposes, including skin aging treatment. Both methods generate vertical microtunnels into the dermis in a controlled manner, thus allowing effective transdermal topical product delivery.15,16 FL is widely recognized for stimulating collagen synthesis and remodeling, permitting aberrant collagen fibers in photoaged skin to reorganize accordingly.16 The present study confirmed the potential of both methods for the application of ADMSCs secretome to reverse skin aging process. Dermoscopic examination of the participants exhibited improvements in fine wrinkles and hyperpigmented macules, and the quantitative approach using modified DPAS from Isik et al17 also displayed significant decrements in MN and FL groups on week 6. Interestingly, we discovered that several subjects had an increased total DPAS at the end of the trial (one subject in MN group and two subjects in FL group), owing to the appearance of telangiectasia, which is evaluated in the calculation of total DPAS. Our first hypothesis of this phenomenon is that telangiectasia existed in the beginning and was previously obscured by hyperpigmented macules. Therefore, when hyperpigmentation partially improved upon treatment, it enabled the visibility of telangiectasia. Another hypothesis is that fibrovascular tissue growth was induced by mechanical and thermal trauma due to MN and FL treatment, resulting in telangiectasia formation in those with genetic predisposition.18 Since telangiectasia manifested in areas with prior pigmentation, the first hypothesis was more plausible.
While both treatments failed to show significant results on pore size, pigmentation, ultraviolet spots, moisture, and elasticity, we found marked diminution of wrinkles assessed with Janus-III measurement system, compared with their respective initial status. These findings corroborate the notion that MN and FL facilitate ADMSCs secretome penetration into the skin, which exerts its anti-wrinkle effects by upregulating procollagen type I production and inhibiting matrix metalloproteinase 1 (MMP-1) secretion responsible for the degradation of collagen fibers.19 When compared to their baselines, an apparent reduction of sebum and porphyrin was also noticed in the week 6 in both MN and FL groups. However, significant changes were noticeable in week 2 in both groups, when the subjects had undergone retinoic acid priming and had yet to be treated with MN or FL and ADMSCs secretome. This is understandable since retinoic acid has been extensively known to reduce sebocyte proliferation and differentiation, as well as sebum production,20 which further interrupts the growth of Cutibacterium acnes, a porphyrin-producing commensal flora found in human skin.21
Intergroup comparison of all variables on weeks 4 and 6 did not show any discrepancies, indicating that both MN and FL are objectively equipotent for ADMSCs secretome administration to alleviate aging skin. Nevertheless, when possible adverse events, satisfaction, and comfort were considered, that was not the case. Apart from diffuse erythema, which was the supposedly expected outcome prior to ADMSCs secretome application in this study, the subjects reported more side effects (prolonged erythema, pain, burning sensation, and itch) upon FL treatment compared to MN. These findings were consistent with a previous study by Li et al, who reported increased skin sensitivity to pain and persistent erythema following FL treatment.22 Pooja et al also outlined transient erythema and edema after FL treatment, while pain and discomfort may be experienced for several hours succeeding MN treatment.23 In addition, the participants suffered higher pain intensity when subjected to FL compared to MN (p<0.001). Satisfaction level was similar in both groups (p=0.103), but two-thirds of the subjects chose FL over MN for skin aging treatment, despite adverse events and pain. When inquired about their preference for FL, the participants felt that the face side treated with FL was more “voluminous” than the portion subjected to MN. Theoretically, this may be explained by the photothermolysis effect of FL in creating arrays of microthermal zones on the skin, which are potentially filled with collagen during the healing process24 –a feature that is not present with MN treatment.
Our findings in clinical practice imply that one may select either MN or FL for skin aging treatment with ADMSCs secretome, particularly for total DPAS and wrinkles reduction. MN may be an economical yet effective approach for such purpose. FL, albeit expensive, has an added advantage of photothermolysis effect. Nonetheless, we realize that the relatively short duration (6 weeks) of our trial serves as a limitation for this study, since we cannot extrapolate our findings for a longer term. On top of that, although we demonstrated how to do face priming and provided a diary to record their skincare routine at home, we cannot be certain of the quantity of retinoic acid used, the distribution of topical products utilized on their face, and the amount of time spent in outdoor, which potentially affect the final outcomes.
The discoveries in the present study may also prompt future research in exploring other substrates and modalities for skin rejuvenation, such as platelet-rich plasma (PRP) and radiofrequency (RF) therapy. As an autologous preparation isolated from centrifuged blood, PRP contains an immense amount of growth factors and biologically active proteins (similar to ADMSCs secretome), which in turn promotes neoangiogenesis and collagen synthesis for wound healing.25 PRP has been studied for extenuating skin aging parameters through direct topical application, intradermal injection, or an adjuvant to MN and FL.26 For instance, Banihashemi et al reported a significant improvement upon skin wrinkles and periorbital dark circles with intradermal injection of PRP.27 Moreover, RF is a therapeutic approach in which electromagnetic current is utilized to produce a sufficient thermal energy for soft and connective tissue remodeling without causing tissue injury (as seen in FL).28 Compared to other techniques, RF has been used in aesthetic dermatology for improving skin aging through collagen fibers production, contraction, and thickening.29 However, direct comparison of these substrates/modalities with ADMSCs secretome, MN, or FL in the treatment of skin aging is yet to be elucidated.
Conclusion
ADMSC's secretome application following MN or FL yielded similar results for attenuating several skin aging features, specifically total DPAS and wrinkles, and satisfaction level after 6 weeks of treatment. FL resulted in more adverse effects, including prolonged erythema, pain, burning sensation, and itch, compared to MN, yet it did not influence the subjective preference towards FL. In light of these results, dermatologists are free to choose either MN or FL to deliver secretome, considering that not every clinic could provide FL due to high cost, while MN may be available in all settings.
Data Sharing Statement
Due to the subjects’ data during the clinical trial are properties of Dr. Cipto Mangunkusumo Hospital health medical records and referring to the Ministry of Health Republic of Indonesia Regulation number 18 (2022) about health information system, the authors are unable to share individual participant data.
Acknowledgments
Our deepest gratitude goes to Prof. Jeanne Adiwinata Pawitan; Isabella Kurnia Liem; Ines Soepinarko Putri; and Tri Kurniawati from Stem Cell Medical Technology Integrated Service Installation - Dr. Cipto Mangunkusumo Hospital for providing the ADMSCs secretome used in this study. We also thanked Bianca Christabel Sudarma; Maria Olivia Angeline Wijanto; Syifa Larasati; and Noer Kamila for assisting with the clinical trial; Prof. Muchtarruddin Mansyur for the statistical analysis; and Stephen Akihiro Wirya for proofreading the manuscript.
Disclosure
This clinical study received a grant from the Kedaireka-Matching Fund 2022 program, which was supported by the Ministry of Education, Culture, Research, and Technology, Republic of Indonesia and PT. Kimia Farma, Tbk with contract number 271/PKS/WRIII-DISTP/UI/2022. Shannaz Nadia Yusharyahya has an intellectual property rights (Indonesia) patent pending to (on progress). Lili Legiawati has an intellectual property right (Indonesia) patent pending to (on progress). The authors report no other conflicts of interest in this work.
References
1. Estebsari F, Dastoorpoor M, Khalifehkandi ZR, et al. The concept of successful aging: a review article. Curr Aging Sci. 2020;13(1):4–10. doi:10.2174/1874609812666191023130117
2. Zhang S, Duan E. Fighting against skin aging: the way from bench to bedside. Cell Transplant. 2018;27(5):729–738. doi:10.1177/0963689717725755
3. Trojahn C, Dobos G, Lichterfeld A, Blume-Peytavi U, Kottner J. Characterizing facial skin ageing in humans: disentangling extrinsic from intrinsic biological phenomena. Biomed Res Int. 2015;2015:318586. doi:10.1155/2015/318586
4. Rodan K, Fields K, Majewski G, Falla T. Skincare bootcamp: the evolving role of skincare. Plast Reconstr Surg Glob Open. 2016;4(12 Suppl):e1152. doi:10.1097/GOX.0000000000001152
5. Balasubramanian S, Thej C, Walvekar A, et al. Evaluation of the secretome profile and functional characteristics of human bone marrow mesenchymal stromal cells-derived conditioned medium suggest potential for skin rejuvenation. J Cosmet Dermatol Sci Appl. 2017;7(1):99.
6. Damayanti RH, Rusdiana T, Wathoni N. Mesenchymal stem cell secretome for dermatology application: a review. Clin Cosmet Investig Dermatol. 2021;14:1401–1412. doi:10.2147/CCID.S331044
7. Xia J, Minamino S, Kuwabara K, Arai S. Stem cell secretome as a new booster for regenerative medicine. Biosci Trends. 2019;13(4):299–307. doi:10.5582/bst.2019.01226
8. Vizoso FJ, Eiro N, Cid S, Schneider J, Perez-Fernandez R. Mesenchymal stem cell secretome: toward cell-free therapeutic strategies in regenerative medicine. Int J Mol Sci. 2017;18(9):1852. doi:10.3390/ijms18091852
9. Putri WE, Endaryanto A, Rantam FA, Prakoeswa CR. Mesenchymal stem cells-conditioned medium (SECRETOME) in skin aging: a systematic review. Int J Pharm Res. 2021;13(2):613–635.
10. El-Domyati M, Moftah NH, Nasif GA, Ameen SW, Ibrahim MR, Ragaie MH. Facial rejuvenation using stem cell conditioned media combined with skin needling: a split-face comparative study. J Cosmet Dermatol. 2020;19(9):2404–2410. doi:10.1111/jocd.13594
11. Montero-Vilchez T, Sierra-Sánchez A, Sanchez-Diaz M, et al. Mesenchymal stromal cell-conditioned medium for skin diseases: a systematic review. Front Cell Dev Biol. 2021;23:654210. doi:10.3389/fcell.2021.654210
12. Gowda A, Healey B, Ezaldein H, Merati M. A systematic review examining the potential adverse effects of microneedling. J Clin Aesthet Dermatol. 2021;14(1):45–54.
13. Zhou B, Xu Y, Guo S, et al. The effect of conditioned media of adipose-derived stem cells on wound healing after ablative fractional carbon dioxide laser resurfacing. Biomed Res Int. 2013;2013:519126. doi:10.1155/2013/519126
14. Sitohang IB, Sirait SA, Safira FD. Fractional carbon dioxide laser for treating hypertrophic scars: a systematic review of randomized trials. Australas J Dermatol. 2022;63(1):27–35. doi:10.1111/ajd.13730
15. Jung JH, Jin SG. Microneedle for transdermal drug delivery: current trends and fabrication. J Pharm Investig. 2021;51(5):503–517. doi:10.1007/s40005-021-00512-4
16. Majid I, Imran S. Efficacy and safety of fractional CO2 laser resurfacing in non-hypertrophic traumatic and burn scars. J Cutan Aesthet Surg. 2015;8(3):159–164. doi:10.4103/0974-2077.167276
17. Isik B, Gurel MS, Erdemir AT, Kesmezacar O. Development of skin aging scale by using dermoscopy. Skin Res Technol. 2013;19(2):69–74. doi:10.1111/srt.12033
18. Geisthoff U, Nguyen H, Lefering R, Maune S, Thangavelu K, Droege F. Trauma can induce telangiectasia in hereditary hemorrhagic telangiectasia. J Clin Med. 2020;9(5):1507. doi:10.3390/jcm9051507
19. Li L, Ngo HT, Hwang E, et al. Conditioned medium from human adipose-derived mesenchymal stem cell culture prevents UVB-induced skin aging in human keratinocytes and dermal fibroblasts. Int J Mol Sci. 2020;21(1):49. doi:10.3390/ijms21010049
20. Endly DC, Miller RA. Oily skin: a review of treatment options. J Clin Aesthet Dermatol. 2017;10(8):49–55.
21. Platsidaki E, Dessinioti C. Recent advances in understanding Propionibacterium acnes (Cutibacterium acnes) in acne. F1000Res. 2018;7:1953. doi:10.12688/f1000research.15659.1
22. Li B, Ren K, Yin X, She H, Liu H, Zhou B. Efficacy and adverse reactions of fractional CO2 laser for atrophic acne scars and related clinical factors: a retrospective study on 121 patients. J Cosmet Dermatol. 2022;21(5):1989–1997. doi:10.1111/jocd.14868
23. Pooja T, Gopal KV, Rao TN, Devi BG, Kumar SA. A randomized study to evaluate the efficacy fractional CO2 laser, microneedling and platelet rich plasma in post-acne scarring. Indian Dermatol Online J. 2020;11(3):349–354. doi:10.4103/idoj.IDOJ_370_19
24. Habbema L, Verhagen R, Van Hal R, Liu Y, Varghese B. Minimally invasive non-thermal laser technology using laser-induced optical breakdown for skin rejuvenation. J Biophotonics. 2012;5(2):194–199. doi:10.1002/jbio.201100083
25. Peng GL. Platelet-rich plasma for skin rejuvenation: facts, fiction, and pearls for practice. Facial Plast Surg Clin North Am. 2019;27(3):405–411. doi:10.1016/j.fsc.2019.04.006
26. Arshdeep, Kumaran, MS. Platelet-rich plasma in dermatology: boon or a bane? Indian J Dermatol Venereol Leprol. 2014;80(1):5–14. doi:10.4103/0378-6323.125467
27. Banihashemi M, Zabolinejad N, Salehi M, Alamdari ZH, Nakhaizadeh S. Platelet-rich plasma use for facial rejuvenation: a clinical trial and review of current literature. Acta Biomed. 2021;92(2):e2021187. doi:10.23750/abm.v92i2.9687
28. Dayan E, Burns AJ, Rohrich RJ, Theodorou S. The use of radiofrequency in aesthetic surgery. Plast Reconstr Surg Glob Open. 2020;8(8):e2861. doi:10.1097/GOX.0000000000002861
29. El-Domyati M, El-Ammawi TS, Medhat W, et al. Radiofrequency facial rejuvenation: evidence-based effect. J Am Acad Dermatol. 2011;64(3):524–535. doi:10.1016/j.jaad.2010.06.045
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.