Back to Journals » Cancer Management and Research » Volume 13
A Community Hospital-Based Study: A Prespecified Neutrophil Count with Adjuvant mFOLFOX6 Associated with Increased Delays, Increased G-CSF Use, and Reduced Dose Intensity
Authors Chiarotto JA, Dranitsaris G
Received 21 February 2021
Accepted for publication 28 April 2021
Published 19 May 2021 Volume 2021:13 Pages 4087—4094
DOI https://doi.org/10.2147/CMAR.S307713
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Seema Singh
James A Chiarotto,1 George Dranitsaris2
1Department of Medicine, The Scarborough Health Network, Toronto, Ontario, Canada; 2Augmentium Pharma Consulting, Inc., Toronto, Ontario, Canada
Correspondence: James A Chiarotto
Department of Medicine, The Scarborough Health Network, Centenary Site, 105-2863 Ellesmere Road, Scarborough, Ontario, M1E 5E9, Canada
Tel +1 416-284-8131 x4666
Fax +1 416-281-7228
Email [email protected]
Purpose: To look at how the absolute neutrophil count (ANC) is used by community oncologists as the main factor in ordering adjuvant mFOLFOX6 for colorectal cancer. This study reports on how this decision impacts chemotherapy delays, effects received dose intensity (RDI), and increases the use of granulocyte-colony-stimulating factor (G-CSF).
Methods: A retrospective chart review was conducted for all patients receiving adjuvant mFOLFOX6 for colorectal cancer at a two-site community hospital in Toronto, Ontario, Canada, between July 2013 and March 2019. Seven physicians treated 140 patients, who made 1636 clinic visits to receive 1461 cycles of prescribed chemotherapy.
Results: The mean ANC per physician associated with a decision to give chemotherapy ranged from 1.05× 109/L (95% CI 0.98– 1.13× 109/L) to 1.5x109/L with a decision to delay if the ANC was lower. Subsequent cycles were then supported by G-CSF with very similar ANC decision levels for dose delay. Physicians were more likely to prescribe chemotherapy with higher pretreatment ANC, r=0.3 (p< 0.000). G-CSF was used in 24.6% of cycles and usage had grown to 44.2% by the 12th cycle; physician use ranged from 0.36% to 54.2% of cycles. Secondary prophylaxis was the indication in 94.7% of cases. There was an inverse relationship between the frequency of G-CSF use and the RDI of continuous infusion 5FU, r=− 0.26 (p< 0.001). There were delays for 8.8% of visits for cycles not supported by G-CSF and, surprisingly, 15.9% of visits for cycles supported by G-CSF. Neutropenia caused 61.6% of delays for chemotherapy cycles not supported by G-CSF and 44.1% for cycles supported by G-CSF.
Conclusion: Physicians required a pretreatment ANC of 1.05– 1.5× 109/L before prescribing mFOLFOX6 chemotherapy. When ANC was low, a dose delay and secondary prophylaxis with G-CSF failed to consistently achieve the much sought after ANC. This then caused more delay, reduced RDI and increased expense for both patients and the system. Fewer delays, less G-CSF and increased RDI would have resulted with reduced reliance on ANC and adoption of chemotherapy dose reduction.
Keywords: colon cancer, G-CSF, dose intensity, dose delay, FOLFOX
Introduction
The adjuvant systemic treatment of early-stage colon and rectal cancer with FOLFOX chemotherapy improves survival.1 The low rate of chemotherapy-induced febrile neutropenia (CIFN) and the high rate of neutropenia and thrombocytopenia causing chemotherapy dose delay2 and reduced dose intensity3 have been described. However, the exact clinical thinking causing these outcomes has not.3 An effective, safe and economical strategy to deal with these adverse outcomes is needed.2
We have reported an approach of using adjuvant FOLFOX at low ANC and platelet counts.4 Neither dose delay nor G-CSF was used, but occasional minor dose modification allowed safe, on-time treatment with minimal treatment delay and excellent RDI. This method has also been used successfully in breast cancer.5,6
When using mFOLFOX6, a more common practice employs G-CSF for secondary prophylaxis of asymptomatic neutropenia.7 This strategy pharmacologically increases the ANC to the physician’s prespecified level allowing for chemotherapy. This is meant to reduce treatment delay and maintain RDI. However, success has not been confirmed with FOLFOX.
In this study, the practice of community oncologists using mFOLFOX6 in the adjuvant setting is reviewed for pretreatment ANC values, leading to a decision to give chemotherapy, delay rates, RDI, and G-CSF use.
We will test the hypothesis that if a pretreatment ANC is below a physician’s prespecified level, the use of subsequent G-CSF as secondary prophylaxis will minimize chemotherapy dose delay and RDI will be maximized.
Materials and Methods
Study Design and Patient Selection
A retrospective chart review was carried out for every patient receiving mFOLFOX6 in the curative setting (oxaliplatin 85mg/m2 IV day 1, leucovorin 400 mg/m2 IV day 1, 5FU 400mg/m2 IV day 1 followed by 2400 mg/m2 over 46h) for two physicians at The Scarborough Health Network Centenary site, Toronto, Ontario, Canada, Department of Medicine, between July 2013 and July 2017 and for five physicians at the Scarborough Health Network General site, Toronto, Ontario, Canada, Department of Medicine, between March 2015 and March 2019. The author (JAC) was a physician at Centenary site. One reviewer at each site abstracted data from the electronic medical record (EMR). The Research Ethics Board, governing all sites of Scarborough Health Network, granted a waiver as per Tri-Council Policy Statement: Ethical Conduct for Research Involving Humans (2018), covering patient and physician data which were anonymized and protected.
All comers at both sites with pathology confirming early-stage colon or rectal cancer, as well as completely resected metastatic cases receiving mFOLFOX6 in the adjuvant setting in the above time frames were included. There was no institutional protocol mandating chemotherapy dose delay, dose modification, or the use of G-CSF based on lab values. The treating physician made all chemotherapy-related decisions independently. Patients with rectal cancer received neoadjuvant concurrent radiotherapy and capecitabine; therefore, a surgical stage was not assigned. All chemotherapy doses, patient morphology data, and treatment dates were gathered for unadjusted RDI calculation.8 RDI was calculated for oxaliplatin, 5FU bolus, and the 46-hour continuous infusion 5FU for each cycle of treatment. Complete blood count and renal and liver function tests were collected for each cycle. Analysis of physician documentation in the EMR clarified reasons for chemotherapy dose delay or was inferred if not explicitly stated. Reasons that were noted for dose delay were categorized in the following manner: neutropenia, thrombocytopenia, neutropenia and thrombocytopenia, renal dysfunction, diarrhea, fever, palmar-plantar erythrodysesthesia, and others.
The institutional protocol for each patient visit is to draw the CBC the day before planned treatment. Based on these results, the chemotherapy order is written for the next day. This two-visit system allows for the efficient use of chemotherapy clinic resources. On occasion, the assessment visit may be two or three days before planned treatment.
Any admission to hospital for chemotherapy-induced febrile neutropenia (CIFN)9 was captured and confirmed by hospital EMR.
Statistical Methods
Patient and clinical data prior to the start of chemotherapy were presented descriptively as means, medians, or proportions, with appropriate measures of variance (ie, 95% CI, SD, minimum, maximum). The chi squared test was used to evaluate categorical differences between subgroups. Spearman’s rank correlation coefficient or Spearman’s ρ was used to evaluate relationships between variables. All statistical analyses were performed using Stata release 16.0 (Stata Corp., College Station, Texas, USA).
Results
Patient Characteristics and Treatment Details
A total of 140 charts met the above criteria and were reviewed. The Centenary site supplied 61 charts and the General site supplied 79. The mean age was 63 years and 61% were male (Table 1). There were 1636 patient visits and 1461 cycles of mFOLFOX6 given. The median number of cycles of chemotherapy analyzed was 6 per patient as was the number of clinic visits per patient. The maximum number of cycles for a colon cancer patient was 12 and for a rectal cancer patient was 8. In 87.8% of cycles, the lab investigations were the day before treatment (Table 1). There were no deaths.
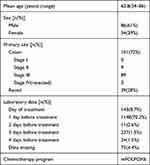 |
Table 1 Patient Demographics, Disease and Laboratory Characteristics |
Physician Characteristics
Seven physicians prescribed curative-intent mFOLFOX6: four males and three females. Six physicians were medical oncologists and one was a hematologist/oncologist. All physicians were trained in Canada. Three physicians had fewer than five years of independent practice experience and three had greater than 15 years (median 10 years).
The median number of patients treated was 18 (range 9–37). The median number of treatment cycles was 176 (range 88–302) (Table 2).
 |
Table 2 Data Associated with Specific Physicians |
Patient Visits and Chemotherapy Given
Treatment was delayed 10.7% of the time. For individual physicians, the range was 4.17% to 17.48% (p<0.000) (Table 2). The most common reason for dose delay was blood count values: neutropenia (54.8%), thrombocytopenia (21.1%) or a combination of both (2.3%) for a total of 78.2% of delays (Table 3).
 |
Table 3 Reasons for Dose Delay: Overall, Cycles without G-CSF, Cycles with G-CSF Support |
Cycles of chemotherapy given without G-CSF support were delayed 8.8% of the time. Cycles given with G-CSF support were delayed 15.9% of the time.
ANC Decision Levels
The mean day-before ANC below 1.5×109/L associated with a decision to give a chemotherapy treatment without dose delay or G-CSF showed a range from the lowest of 1.05×109/L (95% CI 0.98–1.13 x 109/L) for one physician to another physician who never gave chemotherapy if the ANC was below 1.5×109/L (Table 4).
 |
Table 4 Mean Lowest ANC Less Than 1.5×109/L to Give Chemotherapy without G-CSF or with G-CSF and Correlation of ANC to Decision to Give Chemotherapy for Each Physician |
Cycles supported by G-CSF were reviewed. The range of day-before ANC less than 1.5×109/L associated with a decision to give chemotherapy was from 1.15×109/L (95% CI 0.51–1.78×109/L) to the highest of 1.35×109/L (95% CI 1.29–1.41×109/L). The use of G-CSF did not alter the ANC levels that would lead to a decision to give chemotherapy by the physicians (Table 4).
Received Dose Intensity
The mean RDI for oxaliplatin for the entire sample was 85.3% (95% CI 83.9–86.7%). The RDI per provider ranged from 65.4% (95% CI 59.0–71.8%) to 90.3% (95% CI 87.0–93.6%). The RDI for bolus 5-FU was 93.3% (95% CI 92.3–94.2%) for the entire sample. The range per provider was 88.5% (95% CI 84.4–93.2%) to 97.9 (95% CI 95.3–100%). The RDI for continuous infusion 5FU for the entire sample was 93.0% (95% CI 92.7–94.4%). The range per provider was 87.4% (95% CI 82.9–91.8%) to 96.9% (95% CI 95.6–98.1%) (Table 2).
Correlation
Patients presenting with a higher ANC at assessment were more likely to receive treatment without delay (r=0.3, p<0.000) for the entire sample of physicians. For one physician, there was no correlation between ANC and decision to give chemotherapy (r= −0.02, p=0.7). For the other six physicians, there was a positive and statistically significant correlation (Table 4).
There was an inverse relationship between rate of G-CSF use and RDI of continuous infusion 5FU (r= −0.26, p<0.001).
G-CSF Usage
Overall, 24.6% (359/1461) of the cycles were supported by G-CSF and use per physician ranged from a low of 0.36% (1/276) of chemotherapy cycles to 54.2% (64/118) of cycles (Table 2).
G-CSF was used for primary prophylaxis in 5.3% (19/359) of cycles and for secondary prophylaxis in 94.7% (340/359). G-CSF use was 1.4% of cycles in cycle 1 and increased steadily in each cycle to a rate of 44.2% in cycle 12 (Figure 1).
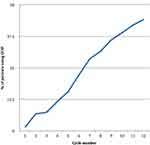 |
Figure 1 G-CSF use by cycle. |
Of the 359 cycles supported by G-CSF, delays were noted 17.7% of the time. Neutropenia (44.1%), thrombocytopenia (32.3%) or a combination (4.4%) accounted for 80.8% of the reasons (Table 3).
Two physicians used G-CSF on four patients several days prior to a cycle of chemotherapy in order to increase the ANC and deliver the next cycle of chemotherapy.
G-CSF Prescriptions
Typical usage is illustrated by one physician’s practice: filgrastim 300μg daily for seven days 37% of prescriptions, filgrastim 480μg daily for seven days 3% of prescriptions and pegylated filgrastim 6mg for 60% of prescriptions. For the other physicians, filgrastim 300μg daily was given for seven days, with rare five- and ten-day prescriptions, rare 480μg prescriptions and always after chemotherapy, except for occasional pretreatment use as stated above.
Once the decision to start G-CSF was made, it was continued for all subsequent cycles regardless of the ANC (Figure 1).
Platelet Decision Levels
Data for platelet counts causing dose delay were available for four physicians and ranged from 54×109/L (95% CI 48–60×109/L) to 65×109/L (95% CI 61–70×109/L) (Table 2).
Febrile Neutropenia
Four patients developed CIFN (2.8%). One event occurred on a cycle supported by G-CSF with a day-before ANC of 5.5×109/L. Another physician had a case with day-before ANC of 1.1×109/L. A third physician had two cases with day-before ANCs of 1.3 and 2.3×109/L.
Discussion
The focus of this study was to understand the decisions that lead to cycle delays and the consequences to RDI and whether G-CSF use can reduce their negative impact. This study showed that 10.7% of cycles were delayed and that in 78.2% of the cases the cause was asymptomatic neutropenia, asymptomatic thrombocytopenia or both.
In the Mosaic strategy,10 an ANC taken the same day of treatment of less than 1.5×109/L would mandate dose delay. Justification for this level as the decision point was not given. In this community practice, if lab values are relied on, the decision point is a day-before ANC of less than between 1.25×109/L and 1.5×109/L. We have shown a significant correlation between a patient’s day-before ANC and the decision to give chemotherapy. The higher the required ANC threshold for chemotherapy, the more likely a cycle will be delayed. In this study, a requirement of day-before ANC 1.5×109/L leads to a delay rate of 17.48%.
Our study showed that community oncologists were able to maintain excellent dose intensities,10 especially for the continuous infusion 5FU (Table 2). Most physicians accomplished this by dose delay if the ANC did not meet a prespecified level and the ongoing use of G-CSF to attempt to achieve that level in subsequent cycles and prevent further interruptions.
While the delay rate for cycles not supported by G-CSF was 8.8%, the delay rate for cycles supported by G-CSF was almost double (15.9%). We showed that G-CSF use did not affect the physician’s prespecified ANC decision point (Table 4). Our data showed that, surprisingly, G-CSF was only able to reduce the delays due to asymptomatic neutropenia from 61.6% to 44.1%. G-CSF support did not induce confidence in the oncologist to prescribe chemotherapy with a lower ANC, leading to a significant rate of delay for neutropenia.
Physicians who use G-CSF and do not reduce chemotherapy dose must contend with increasing rates of thrombocytopenia. Dose delays due to thrombocytopenia accounted for 32.3% of delays for cycles supported by G-CSF (Table 3).
Our study’s observed reduction in RDI for cycles supported by G-CSF (r= −0.26, p<0.001) was due to a combination of persistently high rates of neutropenia, increased rates of thrombocytopenia and the physician response of no dose reduction or change in the ANC required for chemotherapy. A reduction in RDI of FOLFOX with the use of G-CSF has also been reported.11
No clear guidelines exist to advise physicians on how to deal with asymptomatic neutropenia prior to a cycle of chemotherapy.12,13 The mFOLFOX6 regimen is low risk for CIFN13 and G-CSF is discouraged as primary prophylaxis.12 Using G-CSF for asymptomatic neutropenia is strongly discouraged.12 The efficacy of G-CSF for secondary prophylaxis has never been specifically evaluated, and most guidelines discuss the relative merits of dose modification or G-CSF. While dose modification is cheaper and safer, the reduction in RDI is feared to potentially compromise cancer-related outcomes.14 However, G-CSF has never been shown to improve cancer-related outcomes via maintaining RDI.12
Investigators have shown no link between RDI and survival in the adjuvant colon cancer setting,15 with the field even looking into shorter courses of treatment.16 The strongest evidence was retrospective and showed decreased survival at an adjusted RDI below 70%.17
The majority of the lab data were done the day before treatment. Some providers used two- or three-day-before data to make chemotherapy decisions (eg, Friday assessment for treatment on Monday) with the same prespecified pretreatment ANC level. It is known that the ANC can change leading up to the day of chemotherapy.18 The physicians did not account for this and may have made a different decision with a timelier ANC.
This study has documented the ongoing use of G-CSF for asymptomatic neutropenia for every subsequent cycle, regardless of the ANC. There are recommendations to evaluate risk factors for CIFN before administering each cycle of chemotherapy.13 However, whether to continue prophylactic G-CSF for the remaining cycles for asymptomatic neutropenia in a previous cycle regardless of ANC has not been addressed.
We have also described the practice of using G-CSF several days prior to a cycle of mFOLFOX6 in order to increase the ANC above a prespecified level to allow for chemotherapy administration.
In this data set, one may contrast two widely divergent strategies: using the lab values to guide chemotherapy decisions or not. One provider (JAC) did not have a prespecified ANC or platelet count that would mandate dose delay. This strategy has been previously described,4 resulting in high RDI of 96% and CIFN rate of 4.5% with a 2.2% delay rate for hematologic reasons. The 5FU continuous infusion RDI for the current study, 96.9% (95% CI 95.6–98.1%), is the highest of the physicians, the lowest delay rate 4.17%, and the lowest G-CSF use, 0.36%. One physician followed the Mosaic strategy for dose delay, which resulted in the lowest 5FU continuous infusion RDI in the sample at 87% (95% CI 82.9–91.8%), the highest delay rate, 17.5%, and highest G-CSF use, 54.2%. Neither strategy produced an episode of CIFN.
We have shown that surprisingly, G-CSF as prescribed by these community physicians for secondary prophylaxis, could not consistently achieve their prespecified ANC, resulting in treatment delay and reduced RDI. This would argue for a lower ANC for chemotherapy,19 or dose reduction of chemotherapy,4 since G-CSF is expensive and burdensome without improving RDI, preventing delay or CIFN. No validated ANC level has been established for safety.2,4,5,19 Despite this, we have shown that physicians in the community use this strategy.
Asymptomatic thrombocytopenia can be dealt with using dose reduction. Alternatively, we have shown previously that it is safe to proceed with treatment without dose delay.4
We recommend a cycle-by-cycle reassessment of whether G-CSF is needed, instead of automatically renewing the prescription, especially if the ANC is well above the threshold decision level.
This study adds to a growing and compelling literature calling for a change in the practice of using pretreatment ANC to regulate chemotherapy administration.4–6,19 We responded to the call to examine the clinical thinking leading to dose delay3 and G-CSF use.
Our study is small, retrospective and from a two-site community hospital. Reasons for dose delay were not confirmed with the treating physician but are assumed to be accurate. The pretreatment laboratory studies, whether one, two or three days before treatment, were analyzed as equivalent and may not be easily comparable to existing literature. While most G-CSF prescriptions were 300μg/day for seven days or pegylated filgrastim, alternate protocols may yield different results. We suggest a larger sample to confirm these findings.
We have shown in this study that the adoption of an arbitrary ANC leads to more dose delays, reduction in RDI, and increasing use of G-CSF. Additionally, the hypothesis that G-CSF can maintain this arbitrary ANC and prevent dose delay and reduction in RDI was not supported by the data. Each of these outcomes puts an increased strain on our patients and health systems.
Acknowledgments
The authors wish to thank Sujee Kulendran and Jeremy Wong for data abstraction and Pamela West for manuscript review.
Funding
This work was supported by a grant from the RS McLaughlin Durham Regional Cancer Center.
Disclosure
Dr. George Dranitsaris owns and is a consultant for Augmentium Pharma Consulting, Inc. The authors report no other conflicts of interest in this work.
References
1. André T, Boni C, Navarro M, et al. Improved overall survival with oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment in stage II or III colon cancer in the MOSAIC trial. J Clin Oncol. 2009;27:3109–3116. doi:10.1200/JCO.2008.20.6771
2. Kogan LG, Davis SL, Brooks GA. Treatment delays during FOLFOX chemotherapy in patients with colorectal cancer: a multicenter retrospective analysis. J Gastrointest Oncol. 2019;10(5):841–846. doi:10.21037/jgo.2019.07.03
3. Denduluri N, Patt DA, Wang Y, et al. Dose delays, dose reductions, and relative dose intensity in patients with cancer who received adjuvant or neoadjuvant chemotherapy in oncology practices. J Natl Compr Canc Netw. 2015;13:1383–1393. doi:10.6004/jnccn.2015.0166
4. Chiarotto JA, Dranitsaris G. FOLFOX chemotherapy can safely be given to neutropenic patients with early-stage colorectal cancer for higher dose intensity and fewer visits. Support Care Cancer. 2016;24:2533–2539. doi:10.1007/s00520-015-3059-0
5. Chiarotto JA, Dranitsaris G. Full-dose chemotherapy in early stage breast cancer regardless of absolute neutrophil count and without G-CSF does not increase chemotherapy-induced febrile neutropenia. Support Cancer Care. 2013;21:2727–2731. doi:10.1007/s00520-013-1851-2
6. Debled M, Houédé N, Madranges N, et al. Does chemotherapy-induced neutropenia result in a postponement of adjuvant or neoadjuvant regimens in breast cancer patients? Results of a retrospective analysis. BJC. 2007;97:1642–1647. doi:10.1038/sj.bjc.6604094
7. Gotfrit J, Marginean H, Maroun JA, et al. Chemotherapy-induced neutropenia with FOLFOX in the adjuvant treatment of colorectal cancer. J Clin Oncol. 2020;38(4_supp):38. doi:10.1200/JCO.2020.38.4_suppl.38
8. Balducci L, Mo M, Abella E, et al. Retrospective analysis of relative dose intensity in patients with non-Hodgkin lymphoma receiving CHOP-based chemotherapy and pegfilgrastim. Am J Clin Oncol. 2014;37:603–610. doi:10.1097/COC.0000000000000141
9. Freifeld AG, Bow EJ, Sepkowitz KA, et al. Clinical practice guidelines for the use of antimicrobial agents in neutropenic patients with cancer: 2010 update by the Infectious Disease Society of America. Clin Infect Dis. 2010;52(4):e56–e93.
10. André T, Boni C, Mounedji-Boudiaf L, et al. Oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment for colon cancer. NEJM. 2004;350:2343–2351. doi:10.1056/NEJMoa032709
11. Maroun JA, Marginean H. Hematologic toxicity with adjuvant FOLFOX in colon cancer. J Clin Oncol. 2016;34(15_suppl):e15062. doi:10.1200/JCO.2016.34.15_suppl.e15062
12. Smith TJ, Bohlke K, Lyman GH, et al. Recommendations for the use of WBC growth factors: American Society of Clinical Oncology practice guideline update. J Clin Oncol. 2015;33:3199–3212. doi:10.1200/JCO.2015.62.3488
13. Aapro MS, Bohlius J, Cameron DA, et al. Update of EORTC guidelines for the use of granulocyte-colony stimulating factor to reduce the incidence of chemotherapy-induced febrile neutropenia in adult patients with lymphoproliferative disorders and solid tumours. EJC. 2010;2010(47):8–32.
14. Veitch Z, Khan OF, Tilley D, et al. Impact of cumulative chemotherapy dose on survival with adjuvant FEC-D chemotherapy for breast cancer. J Natl Compr Canc Netw. 2019;17(8):957–967. doi:10.6004/jnccn.2019.7286
15. Smoragiewicz M, Javaheri KR, Yin Y, et al. Neutropenia and relative dose intensity on adjuvant FOLFOX chemotherapy are not associated with survival for resected colon cancer. J Gastrointest Cancer. 2014;45:460–465. doi:10.1007/s12029-014-9639-2
16. Grothey A, Sobrero A, Shields AF, et al. Duration of chemotherapy for stage III colon cancer. NEJM. 2018;378:1177–1188. doi:10.1056/NEJMoa1713709
17. Aspinall SL, Good CB, Zhao X, et al. Adjuvant chemotherapy for stage III colon cancer: relative dose intensity and survival among veterans. BMC Cancer. 2015;15:62–74. doi:10.1186/s12885-015-1038-y
18. Clemons MJ, Marshall E, Durig J, et al. A randomized phase-II study of BB-10010 (macrophage inflammatory protein-1?) in patients with advanced breast cancer receiving 5-fluorouracil, adriamycin, and cyclophosphamide chemotherapy. Blood. 1998;92:1532–1540. doi:10.1182/blood.V92.5.1532
19. Lalami Y, Klastersky J. Impact of chemotherapy-induced neutropenia (CIN) and febrile neutropenia (FN) on cancer treatment outcomes: an overview about well-established and recently emerging clinical data. Crit Rev Oncol Hematol. 2017;120:163–167. doi:10.1016/j.critrevonc.2017.11.005
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
